
Research Article
Austin J Nurs Health Care. 2017; 4(1): 1037.
The Effects of Pre-Intervention Training Provided through Therapeutic Play on the Anxiety of Pediatric Oncology Patients during Peripheral Catheterization
Orhan E1* and Yildiz S2
1Department of Nursing Education, Koc University Hospital, Turkey
2Department of Pediatric Nursing, Istanbul University,Turkey
*Corresponding author: Eda Orhan, Department of Nursing Education, Koc University Hospital, Istanbul, Turkey
Received: March 27, 2017; Accepted: May 02, 2017; Published: May 11, 2017
Abstract
Aim: The study aims to determine the effect of pre-intervention training provided through therapeutic play on reducing the anxiety of pediatric oncology patients during peripheral catheterization.
Materials and Methods: This experimental study was conducted between September 2012 and March 2013. The study included 40 children (20 in the control group and 20 in the experimental group) who were receiving treatment for hematological-oncological diseases in the Pediatric Hematology and Oncology Units of Istanbul University, Istanbul Faculty of Medicine and Cerrahpasa Faculty of Medicine, and in the Department of Pediatrics of the American Hospital. The children were randomly allocated into the experiment and control groups. The children were asked to complete an information form before the peripheral catheterization. The intervention was explained to the experimental group through therapeutic play. The data obtained through the State-Trait Anxiety Inventory for Children (STAIC) after the peripheral catheterization were analyzed using the Statistical Package for Social Science (SPSS) 21 package for Windows.
Findings: It was found that the mean trait anxiety scores were similar and remained moderate in the experimental and control groups; that there wasn’t any statistically difference between the groups (p<0.05); and that the mean state anxiety score of the experimental group was lower than that of the control group (Control: 43.40 ± 5.42, Experimental: 31.50 ± 4.73) and this difference was statistically significant (p<0.001). The state anxiety level was found to be reduced in all children - regardless of the variables - with the training provided through therapeutic play.
Conclusion: Children provided training with therapeutic play before the intervention can reduce their anxiety during procedures, such as peripheral catheterization. The use of therapeutic play made widespread in health institutions will decrease both pain and suffering in children.
Keywords: Therapeutic play; Education; Anxiety; Peripheral catheterization; Cancer; Child
Introduction
Pediatric cancer is a life-threatening chronic disease. Despite significant progress with medical treatment and in the rates of survival in the last 30 years, progress in terms of the children’s psychosocial problems remains inadequate. Therefore, the number of studies on the psychosocial status of children with cancer has increased in the last 15 years [1-3].
The worst aspect of cancer for children diagnosed with the disease is the repetitive and painful treatment procedures to which they are exposed. The frequent intravenous, subcutaneous or intramuscular interventions and insertion of port-catheters cause stress and fear in hospitalized children [4,5].
Nurses should be aware of the fact that any medical practice can be traumatic for children. In particular, school-age children may want to learn the reasons for the medical practices and tests and can ask questions about their disease, as they have an idea of body parts, organs and their functions. Nurses should inform the children about the treatment procedures, making sure that they understand them, and in doing so, choose the appropriate technique for the children’s developmental age, competence for coping with the disease and previous experience [3,6-8].
Nurses also should reduce the children’s anxiety or stress due to the experience they have in the hospital and prepare them for the next treatment procedures they will undergo [9]. One of the most appropriate methods for this is therapeutic play. Therapeutic play is a play technique which reduces the trauma due to the disease or being hospitalized, evaluates the children’s feelings and misunderstandings about the treatment or procedures, and is used for the children to develop positive coping methods before, during and after the events that cause stress [10,11].
Many case studies indicate the benefits of therapeutic play for hospitalized pediatric patients: Therapeutic play was emphasized to be important in meeting the psychosocial needs of pediatric oncology patients by O’Connor and Drennan in 2003, in developing a trust relationship between pediatric patients and healthcare personnel by Pan, Chiu, Shen and Chen in 2004, and in reducing the children’s negative feelings during bloodletting by Riberio and Sabates in 2001 [12].
Children can better understand the reasons for being hospitalized, having surgery or invasive procedures and learn to cope with the stress caused by these factors more effectively. Bandages, injectors without needles, gloves, masks or uniforms to be worn, or dolls on which children can practice, are among appropriate materials that can be used in dramatic play. Medical toys are useful to show medical and surgical operations to children and help them express their feelings [3,4].
Even in a hospital, a child’s world is a world of playing. Enabling hospitalized children to play has become one of the necessities of nursing. In particular, pediatric nurses should know the importance of playing, research this subject and be able to use play while they are giving care to hospitalized children [2,8].
This study aims to determine the effect of pre-intervention training provided through therapeutic play on reducing the anxiety of pediatric oncology patients during peripheral catheterization.
The hypotheses of this study
H0: There is no difference between the postprocedural state anxiety levels of children who receive, or do not receive training through therapeutic play.
H1: The postprocedural state anxiety level is lower in the children who receive training through therapeutic play.
H2: The postprocedural state anxiety level is higher in the children who do not receive training through therapeutic play.
The independent variables of this study were the training provided to the children through therapeutic play and the introductory information on the children (age, gender, education, etc.), and the dependent variable was the score on the State-Trait Anxiety Inventory for Children.
Materials and Methods
The permissions were obtained from the Clinical Research Ethics Committee of Istanbul University, Cerrahpasa Faculty of Medicine Dean’s Office (No: B.30.2.ÎST.0.30.90.00/26699, Date: 9.10.2012), the Pediatric Hematology and Oncology Unit of Cerrahpasa Faculty of Medicine, Department of Pediatrics (No: 31553, Date: 1.11.2012), Istanbul Faculty of Medicine, Oncology Institute (No: B.30.2.IST.0.53.00.00/2901, Date: 10.17.2012), and the American Hospital, Directorate of Nursing Services (No: 13092012-35, Date: 13.09.2012) before the study. The parents and children were informed about the aim, plan and duration of the study using the Informed Consent Form before the study, and then included in the study if they volunteered to participate.
This experimental study was conducted with pediatric oncology patients in the Pediatric Hematology and Oncology Units of Istanbul University, Istanbul Faculty of Medicine and the Cerrahpasa Faculty of Medicine, and in the Department of Pediatrics of American Hospital to analyze the effect of the training provided through therapeutic play before peripheral catheterization on the anxiety level of children aged between 8 and 12 who receive treatment in pediatric hematology-oncology units. The study population consisted of 40 pediatric patients receiving treatment in these units for hematologicaloncological diseases. The sample size was calculated through power analysis at 95% confidence interval and with ± 5% margin of error in line with the literature [1,12], and determined to be 34 patients: 17 in the control group and 17 in the experimental group. All were children (n=40) who were receiving treatment in these units during the dates of this study and met the inclusion criteria, considering the possible participant losses. The children were randomly allocated into the experimental and control groups.
Inclusion criteria
The participants must have been;
- Aged between 8 and 12,
- Receiving treatment through peripheral vascular access, and
- At the mental development level to be able answers the questions.
Data Collection Tools
The data were collected using the information form for Children prepared by the researcher to obtain introductory information on the children and the State-Trait Anxiety Inventory for Children to assess the children’s anxiety level. During the intervention, the children were provided training using the Chemo Duck toy and a training booklet.
Information form for children
The form includes 18 questions on the children’s age, gender, literacy, number of siblings, health coverage, diagnosis, duration of the diagnosis, knowing the diagnosis, having surgery and the type of the surgery, existence of physical disability and its type, having chemotherapy and number of cycles, having radiotherapy and the number of sessions, and the number of monthly laboratory tests and hospitalizations. The form was administered as a pilot study to the first 10 cases.
The training booklet
The Vascular Access Training and Coloring Book prepared by the researcher provides information about the definition, intended use and benefits of peripheral vascular access through caricaturized drawings and gives the children a chance to color them while reading the information. The drawings have caricaturized by the researcher and the information has referenced and translated in Turkish from The Chemo Duck website [13] (Figure 1).

Figure 1: Pages from the training booklet in Turkish.
The State-Trait Anxiety Inventory for Children (STAIC)
This inventory was developed by Spielberger in 1973 and adapted into Turkish language by Seniz Özusta in 1995. It can be administered to children aged between 9 and 12 and was tested for validity and reliability. The STAIC has two subscales, each including 20 items, along with reverse statements. The Cronbach’s alpha coefficient was found to be 0.82 for the state anxiety subscale, and 0.81 for the trait anxiety subscale, in a study conducted with 615 healthy children.
State anxiety subscale
The children are asked to select one of three choices about how they feel at that very moment. The subscale includes 20 items assessing feelings about state-anxiety such as stress, anger, or panic. Half of the items reflect uneasiness, panic and stress, and the other half reflect the intensity of these feelings. The maximum score of the items is 3 (showing the intense existence of these feelings), and the minimum score is 1 (showing non-existence of these feelings). The minimum and maximum total scores of the state anxiety scale are 20 and 60, respectively. The STAIC can be administered to an individual or to a group.
Trait anxiety subscale
The children are asked to select the most appropriate choice according to how they generally feel themselves about the occurrence frequency of the situation given in the items. Each item is answered as “almost never,” “sometimes,” and “often,” where “often” yields the maximum item score of 3, and “almost never” yields the minimum item score of 1. The minimum and maximum total scores of the trait anxiety scale are 20 and 60, respectively [14].
The chemo duck toy
The toy, used as a training material for therapeutic play in this study, was developed by an entrepreneur mother named Lu Sipos in the USA. It is produced in Spring Hill, USA and used to train pediatric oncology patients in the hospital around the city under the name of “Gabe’s Chemoduck Program”. The Chemo Duck toy has a port catheter site, a bandanna, an arm bandage, and surgical scrubs. The children have an opportunity to practice the treatment or procedures in the hospital as they play with this toy (Figure 2, Reference: https:// chemoduck.org/about/). The permission of Lu Sipos was obtained via e-mail to use the toy and 10 toys were sent to be used in this study (Figure 3).

Figure 2: Chemoduck Toy, (Reference: https://chemoduck.org/about/ ).

Figure 3: Permission of using the Chemoduck Toy for the research.
Data Collection
The children were included in the study after their parents were informed about the study using the Informed Consent Form and they approved the participation of their children. The data were collected by the researcher.
The Information form for children was administered as a pilot study to the first 10 cases with 14 questions.
The experimental group
Before the intervention, the Information form was completed by the patients in between 10-15 minutes. After filling the information form the researcher explained to the patients what peripheral vascular access is, why it is used, its benefits and how it is applied, in line with the Vascular Access Training and Coloring Book. After the explanation the researcher performed with the patients using peripheral vascular access materials (intravenous catheter without needle, injectors without needles, cotton and bandage) on the chemo duck toy. The materials were used under the control of the researcher and the patients were not hurt in any way. The training took minimum 20 minutes and maximum 30 minutes depending on the children’s level of understanding; answering their questions about the procedure. Catheterization was applied to the children by the nurse of the unit after the training. The STAIC form was filled by the researcher by asking the children the questions, one by one when the peripheral catheterization finished (Figure 4).
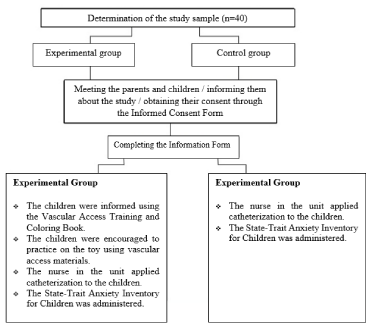
Figure 4: The study flow chart.
Control group
Before the intervention, the information form was completed by the patients in between 10-15 minutes. Catheterization was applied to the children by the nurse in that unit. The STAIC was then completed by the researcher by asking the children the questions, one by one.
Data Analysis
The data were analyzed using the Statistical Package for the Social Science (SPSS) 21 package for Windows. Mean, standard deviation, frequency and percentage distribution were used for statistics. The chi-square and Fisher’s exact probability tests were used to compare the frequencies and percentages between the groups. The T test for comparison of two groups was used to compare the normally distributed variable means. The significance level was accepted p=0.05 in the interpretations.
Findings
There wasn’t any statistically difference between the sociodemographic characteristics of the experimental and control groups such as age, gender, literacy rate, number of siblings and having a health coverage (Table 1: p>0.05). The socioeconomic characteristics were similar in the experimental and control groups.
Groups
Control (n=20)
Experimental (n=20)
X2
p
Characteristics
n
%
n
%
Age
8-10 years
8
40.0
7
35.0
0.11
0.74
11-12 years
12
60.0
13
65.0
Gender
Female
5
25.0
6
30.0
0.13
0.72
Male
15
75.0
14
70.0
Literacy
Yes
19
95.0
19
95.0
Fisher
1.00
No
1
5.0
1
5.0
Number of Siblings
None or 1 sibling
10
50.0
7
35.0
0.92
0.34
2 or more siblings
10
50.0
13
65.0
Health Coverage
SSI
16
84.2
17
65.0
0.23
0.63
Private Insurance
4
15.8
3
35.0
Table 1: Sociodemographic characteristics (n=40).
The diagnosis distribution of the children showed that tumor cancer was predominant; most children had been diagnosed with cancer within the last one year. The majority of the children had had surgical operations. No statistically significant difference was found between the diagnostic characteristics of the experimental and control groups (Table 2; p>0.05); the groups had similar characteristics.
Groups
Control (n=20)
Experimental (n=20)
X2
p
Characteristics
n
%
n
%
Diagnosis
Leukemia
5
25.0
5
25.0
3.9
0.1
Tumor
13
65.0
8
40.0
7
38
Lymphoma
2
10.0
7
35.0
Duration of the Diagnosis
Newly diagnosed / 1 year
15
75.0
12
60.0
1.03
0.311
2 years or more
5
25.0
8
40.0
Knowing the Diagnosis
Knowing
14
70.0
15
75.0
0.13
0.723
Not knowing
6
30.0
5
25.0
Table 2: Comparison of the groups in terms of diagnosis (n=40).
The children who had had surgery were predominant in the control group (60%) and equal in the experimental group (50%). Physical disability level was low in both the experimental and the control group. No statistically significant difference was found between the experimental and control groups in terms of these variables (Table 3; p>0.05).
Groups
Control (n=20)
Experimental (n=20)
X2
p
Characteristics
n
%
n
%
Having Surgery
Yes
12
60.0
10
50.0
0.40
0.525
No
8
40.0
10
50.0
Type of Surgery
Did not have
8
40.0
10
50.0
Tumor resection
8
40.0
5
25.0
0.40
0.525
Biopsy
4
20.0
5
25.0
Physical Disability
Yes
3
15.0
4
20.0
0.17
0.677
No
17
85.0
16
80.0
Table 3: Comparison of the groups in terms of having surgery and physical disability (n=40).
All patients in the experimental and control groups werec receiving chemotherapy. Most of the patients had received three or more chemotherapy cycles both in experimental and control groups. In addition, most of them also receive radiotherapy. The children had laboratory tests once or twice a month. Most of the children in the experimental (65%) and control (55%) groups were hospitalized for once or twice a month. No statistically significant difference was found between the experimental and control groups in terms of these variables (Table 4; p>0.05).
Groups
Control (n=20)
Experimental (n=20)
X2
p
Characteristics
n
%
n
%
Receiving Chemotherapy
Yes
20
100
20
100
0
1
No
0
0.0
0
0.0
Number of Cycles
1 or 2 cycles
7
35
3
15
2.13
0.144
3 or more cycles
13
65
17
85
Receiving Radiotherapy
Yes
11
55
15
75
1.76
0.185
No
9
45
5
25
Number of Radiotherapy
Not Receiving
10
45
15
75
1 day
1
5.3
0
0
2.67
0.263
3 or more days
8
49.7
5
25
Monthly Laboratory Test
Having Laboratory Tests
1 or 2 times
10
50
13
65
3 or more times
10
50
7
35
0.92
0.337
Number of Monthly Hospitalization
1 or 2 times
11
55
13
65
0.42
0.52
3 or more times
9
45
7
35
Table 4: The distribution and comparison of the disease characteristics.
The mean trait anxiety score was 35.40 ± 5.71 in the control group and 33.55 ± 7.08 in the experimental group. The statistical assessment showed no significant difference between the groups (t= 0.910; p= 0.369). The mean state anxiety score was 43.40 ± 5.42 in the control group and 31.50 ± 4.73 in the experimental group. The state anxiety score of the experimental group was significantly lower than that of the control group (Table 5; p= 0.000).
Experimental
Control
Variables
Min.-Max.
n
Mean
±SD
n
Mean
±SD
t
p
Trait Anxiety Score
20-60
20
33.55
7.08
20
35.40
5.71
0.910
0.369
State Anxiety Score
20-60
20
31.50
4.73
20
43.40
5.42
7.40
0.000
Table 5: Comparison of the Mean State-Trait Anxiety Scores.
Discussion and Conclusion
It was found that the mean trait anxiety scores were similar and remained moderate in the experimental and control groups and there wasn’t any statistically significant difference between the groups. The state anxiety score of the control group was statistically significantly higher than that of the experimental group (Table 5). This shows that the training provided to the children through therapeutic play before the procedure reduced the state anxiety level of the children caused by venous catheterization. Many studies and publications support this result.
These studies highlight that it is critical to prepare and inform the children before they undergo painful procedures, such as catheterization [15-17]. A research conducted with children aged between 7 and 12 reported that the training through therapeutic play before a surgical operation, reduced their postoperative anxiety levels [12]. A study conducted with 142 children in 2012 reported that informing children before bloodletting reduced their anxiety after the procedure [18]. A study conducted with 33 children aged between 5 and 12 also showed that a medical game played before examination reduced the children’s anxiety levels [19]. The most important difference of this research from previous studies is the sample type studied on pediatric oncology patients as explained children who had chronical disease. Another study of Chen et al. (2013) conducted with 19 children aged between 3 and 15 who had brain tumors revealed that preparation through therapeutic play before radiotherapy reduced the children’s anxiety level. The sample type is similar with the study of Chen et al. but our study based on a larger sample size and the peripheric catheterization as a medical procedure is more painful than radiotherapy.
In the beginning of the study;
The H0 explained the possibility of indifference between postprocedural state anxiety levels of children who receive, or do not receive training through therapeutic play. The H1explainedthe postprocedural state anxiety level is lower in the children who receive training through therapeutic play and the H2 explained the postprocedural state anxiety level is higher in the children who do not receive training through therapeutic play.
Based on the research results;
- The H1and the H2 was supported.
- The H0 wasn’t supported based on the research results showed that the postprocedural state anxiety level is lower in the children who do not receive training through therapeutic play.
- The method of training through pre-intervention therapeutic play be used to reduce the children’s state anxiety and negative reactions during invasive procedures such as catheterization;
- Awareness of the pediatric nurses be raised on the importance of therapeutic play and informing children before medical procedures;
- The use of the method of training through therapeutic play in different medical interventions and age groups be supported by other evidence-based studies.
It is recommended that:
The Limitations of the Study
The research first planned with pediatric oncology patients who have port catheters. However due to the lack of sufficient numbers meeting, the researcher changed the sample criteria to pediatric oncology patients who had peripheral IV catheters.
It was planned to use the State-Trait Anxiety Inventory for Children before the intervention and was used in the first three cases; however, it took longtime for patients as filling the information form and STAI, explanation of the procedure and practicing catheterization with the toy took a total of one hour delay on medication. In addition, the duration of case collection was extended due to the difficulty of finding pediatric oncology patients aged between 8 and 12 who received chemotherapy through a peripheral line.
During the research it was paid regard to the state anxiety score, because the trait anxiety is variable from person to person.
Because of difficulty of control environmental factors, the similarity of socioeconomic characteristics in the experimental and control groups was featured.
References
- Gariépy N, Howe N. The therapeutic power of play: examining the play of young children with leukaemia. Child: Care, Health & Development. 2003; 29: 523-537.
- Haiat H, Bar-Mor G, Shochat M. The World of the Child: A World of Play Even in the Hospital. Journal of Pediatric Nursing. 2003; 18: 209-214.
- Ball JW, Bindler RC, Cowen KJ. Child Health Nursing-Partnering With Children and Families, 2nd Edn, Pearson Education; New Jersey, USA. 2010.
- Slifer KJ, Tucker CL, Dahlquist LM. Helping Children and Caregivers Cope with Repeated Invasive Procedures: How are we doing? Journal of Clinical Psychology in Medical Settings. 2002; 9: 131-152.
- Breiner SM. Preparation of the Pediatric Patient for Invasive Procedures. Journal of Infusion Nursing. 2009; 32: 252-256.
- James SR, Ashwill JW, Droske SC. Nursing Care of Children-Principles and Practice, 2nd Edn, W.B. Saunders Company, USA. 2002.
- LeRoy S, Chair M, Elixson EM, Cochair M, O’Brien P, Tong E, et al. Recommendations for Preparing Children and Adolescents for Invasive Cardiac Procedures. Journal of the American Health Association. 2003; 108: 2550-2564.
- Kuguoglu S, Tanir MK. Gelisim Dönemlerine Göre Oyunun Terapötik Kullanimi, Ege Üniversitesi Hemsirelik Yüksek Okulu Dergisi. 2006; 22: 293- 304.
- Maia EB, Ribeiro CA, Borba RI. Understanding Nurses’ Awareness as to the Use of Therapeutic Play in Child Care. 2011; 45: 839-846.
- Çavusoglu H. Çocuk Sagligi Hemsireligi. Bizim Büro Basimevi, Ankara. 2002; 64.
- Altay NC. Çocuklarda Ameliyat Öncesi Hazirlik. Saglik Bilimleri Fakültesi Hemsirelik Dergisi. 2008; 68-76.
- Li HC, Lopez V. Effectiveness and Appropriateness of Therapeutic Play Intervention in Preparing Children for Surgery: A Randomized Controlled Trial Study. JSPN. 2008; 13: 63-73.
- Gabe’s My Heart Company. I’m Still Me! - A Fun Activity Book for Kids with Living with Cancer. 2008.
- Özusta S. Çocuklar için Durumluk Sürekli Kaygi Envanterinin uyarlama, Geçerlik ve Güvenirlik Çalismasi. Türk Psikoloji Dergisi. 1995; 10: 32-44.
- Cohen LL, Lemanek K, Blount RL, Dahlquist LM, Lim SC, Palermo TM, et al. Evidence-Based Assessment of Pediatric Pain. Journal of Pediatric Psychology. 2008; 33: 939-955.
- Savaser S, Yildiz S, Gözen D, Balci S, Mutlu B, Çaglar S. Hemsireler Için Çocuk Sagligi ve Hastaliklari Ögrenim Rehberi. Istanbul: Îstanbul Tip Kitabevi. 2009.
- Hudges T. Providing Information to Children Before and During Venepuncture. Nurs Child Young People. 2012; 24: 23-28.
- Balci S, Mutlu B. Çocuklarda Venöz Kan Örnegi Alirken Olusan Agriyi Azaltmada Balon Sisirme ve Öksürme Yöntemlerinin Etkisi (Doktora Tezi), Îstanbul Üniversitesi Saglik Bilimleri Enstitüsü- Çocuk Sagligi ve Hastaliklari Hemsireligi Anabilim Dali/Çocuk Sagligi ve Hastaliklari Hemsireligi Programi. 2012.
- Nader S, Reif MH, Thoma SJ. Play and Video Effects on Mood and Procedure Behaviors in School-Aged Children Visiting the Pediatrician. Clinical Pediatrics. 2013; 52: 929-935.