
Review Article
Ann Nutr Disord & Ther. 2015;2(1): 1019.
Oxidative Stress, Nutritional Antioxidants, and Testosterone Secretion in Men
Glade MJ1* and Smith K2
1The Nutrition Doctor, Kailuα-Kona, USA
2Progressive Laboratories Inc, USA
*Corresponding author: Glade MJ, The Nutrition Doctor, Suite 406, 78-7100 Kamehameha III Road, Kailuα-Kona, HI 9674, USA
Received: December 02, 2014; Accepted: February 23, 2015; Published: February 25, 2015
Abstract
The biochemistry of testosterone synthesis within the Leydig cells of the human testes is well characterized. Reliance on the mitochondrial electron transfer system for the energy to drive testosterone synthesis exposes Leydig cell mitochondria to oxidative stress. Leydig cells experiencing oxidative stress exhibit reduced activities of antioxidant enzymes, increased lipid peroxidation, reductions in mitochondrial membrane potential required for testosterone synthesis, and reduced expression of the StAR steroidogenic acute regulatory (StAR) protein, culminating in inhibition of the synthesis and secretion of testosterone.
Evidence obtained from in vitro, laboratory, and animal experiments, and from human trials, provides strong support for the hypothesis that reducing oxidative stress releases Leydig cells from oxidative inhibition of testosterone synthesis and can improve testosterone status. Selected dietary antioxidants (e.g., the phytonutrients in pomegranates, phosphatidylserine, vitamin C, vitamin E, α-lipoic acid, zinc, and selenium) can contribute safely to oxidative stress reduction and enhanced androgenic status in otherwise healthy adult males. In this era of science-based medical decision-making, addressing oxidative stress and its potential role in undermining testosterone status deserves closer scrutiny.
Keywords: Low testosterone; Leydig cells; Oxidative stress; Antioxidants; Phosphatidylserine; Pomegranates
Abbreviations
SHBG: Steroid Hormone Binding Globulin; DHT: Dihydrotestosterone; the STAR protein: The Steroidogenic Acute Regulatory Protein; ERK1/2: Extracellular Signal-Regulated Kinase 1/2; LH: Luteinizing Hormone; AMP: Adenosine Monophosphate; 5’-Adenylic Acid; cAMP: Cyclic AMP; P450scc, CYP11A1: Cytochrome P450cholesterol side-chain cleavage enzyme; NADPH: Reduced Nicotine Adenine Diphosphonucleotide; Cytochrome P450 17α-hydroxylase/17, 20-lyase: CYP17A1 hydroxylase and CYP17A1 lyase; 3β-HSD, HSD3B2: 3β-hydroxysteroid dehydrogenase/Δ5→Δ4 isomerase; 17β-HSD, HSD17B3: 17β-hydroxysteroid dehydrogenase; DHEA: Dehydroepiandrosterone; SRD5A1, SRD5A2, SRD5A3: Short-Chain Dehydrogenase/Reductases; GnRH: Gonadotropin- Releasing Hormone; MrOS: The Osteoporotic Fractures in Men Study; BACH: The Boston Area Community Health Survey; CRP: C-Reactive Protein; TNF-α: Tumor Necrosis Factor-α; MIP1α: Macrophage Inflammatory Protein 1α; MIP1β: Macrophage Inflammatory Protein 1β; ROS: Reactive Oxygen Species; SO: ·O2 -, Superoxide; H2O2: Hydrogen Peroxide; ·OH-: Hydroxy Radical; ·NO: Nitric Oxide; ·ROO-: Peroxyl Radical; ·ONOO-, Peroxynitrite; ·1O2: Singlet Oxygen; HOCl: Hypochlorous Acid; SOD: Superoxide Dismutase; GPx: Glutathione Peroxidase; MDA: Malondialdehyde; PON1: Paraoxonase 1; PON2: Paraoxonase 2; CCl4: Carbon Tetrachloride; CYP1A2: Cytochrome P450 Isozyme CYP1A2; CYP3A: Cytochrome P450 Isozyme CYP3A:GR; Glutathione Reductase; MAM: Mitochondriα-Associated Membrane; DHA: Docosahexaenoic Acid; Akt: Protein Kinase B; mTOR2: Mammalian Target of Rapamycin-2; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; DHA: Dehydroascorbate; HO-1: Heme Oxygenase-1; Nrf2: Nuclear Factor-Erythroid 2-Related Factor 2
Testosterone Synthesis and Metabolism
Testosterone appears in the human circulation either free from binding to any carrier molecules, bound to albumin, or bound to steroid hormone binding globulin (SHBG). Bound and unbound forms can be measured and serum testosterone concentrations are expressed as either free testosterone, biologically active testosterone (unbound testosterone plus testosterone bound to albumin), or total testosterone (unbound testosterone plus testosterone bound to SHBG plus testosterone bound to albumin) [1,2]. Circulating testosterone concentrations, which determine the availability of testosterone to the tissues, reflect the net balance between testicular synthesis and secretion of testosterone and the conversion of circulating testosterone into 17β-estradiol, dihydrotestosterone (DHT), and excretory metabolites.
Testosterone Synthesis
The biochemistry of testosterone synthesis within the Leydig cells of the human testes is well characterized [3,4]. Testosterone anabolism in men is proportional to the plasma concentration of pituitary-derived luteinizing hormone (LH) [5] and is triggered by binding of LH to its plasma membrane receptors on Leydig cells of the testes [6] and subsequent activation of intracellular signaling cascades that increase the conversion of adenosine monophosphate (AMP) to cyclic AMP (cAMP) and initiate the de novo synthesis of testosterone [7]. Testosterone biosynthesis begins with the activation of the steroidogenic acute regulatory protein (the StAR protein) by extracellular signal-regulated kinase 1/2 (ERK1/2) in response to the LH-induced increase in intracellular cAMP concentration (Figure 1) [8]. The StAR protein is a component of a transmembrane multiprotein complex (the transduceosome) that catalyzes the rate-limiting step in steroid biosynthesis [9], the translocation of cholesterol from the cytoplasm to cytochrome P450cholesterol side-chain cleavage enzyme (P450scc; CYP11A1) embedded within the inner mitochondrial membrane [9- 13].
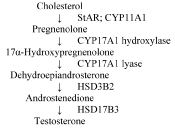
Figure 1: The Δ5 testosterone synthesizing pathway in men (see text for full names of enzymes).
Three sequential reactions catalyzed by CYP11A1 convert cholesterol into pregnenolone, utilizing energy provided by mitochondrial reduced nicotine adenine diphosphonucleotide (NADPH) [3,4]. Pregnenolone diffuses from the mitochondria into adjacent smooth endoplasmic reticulum, where cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17A1 hydroxylase and CYP17A1 lyase), 3β-hydroxysteroid dehydrogenase/Δ5→Δ4 isomerase (3β-HSD; the HSD3B2 isoform in human Leydig cells), and 17β-hydroxysteroid dehydrogenase (17β-HSD; the HSD17B3 isoform in human Leydig cells) drive the predominant "Δ5" testosterone synthesizing pathway in men [3,4,14,15].
A minor "Δ4" pathway converts a small amount of pregnenolone into testosterone following the conversion of pregnenolone into progesterone by the isomerase activity of HSD3B2 (Figure 2) [16].

Figure 2: The Δ4 testosterone synthesizing pathway in men (see text for full names of enzymes).
The CYP17A1 enzyme complex catalyzes the NADPH-dependent conversion of pregnenolone into 17α-hydroxypregnenolone and the subsequent conversion of 17α-hydroxypregnenolone into dehydroepiandrosterone (DHEA). HSD3B2 then catalyzes the 2-step conversion of DHEA into androstenedione; this reaction is ratelimiting for the entire pathway of de novo testosterone biosynthesis from pregnenolone [3,4] and the expression of HSD3B2 is upregulated by LH [12-17]. HSD17B3 then catalyzes the NADPH-dependent conversion of androstenedione into testosterone. Testosterone is metabolized to DHT in Leydig cells and peripheral tissues by several types of 5α-reductase enzymes (short-chain dehydrogenase/ reductases; SRD5A1, SRD5A2 and SRD5A3; Figure 3); SRD5A2 has particularly high affinity for testosterone [3,4,14].
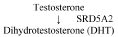
Figure 3: Testosterone conversion to DHT (see text for the full name of SRD5A2).
The Aromatase Complex, Testosterone, and 17β-Estradiol
Circulating testosterone is converted into 17β-estradiol by the aromatase enzyme complex embedded within the membranes of the endoplasmic reticulum of testicular Leydig and Sertoli cells, spermatocytes, prostate epithelial cells, bone, adipose tissue, and other organs in men (Figure 4) [17-21]. The aromatase complex also converts androstenedione to mildly estrogenic estrone, which can then be converted into 17β-estradiol by HSD17B3 (Figure 4) [3,4,18].
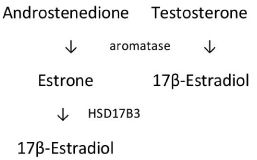
Figure 4: Testosterone and androstenedione conversion to 17β-estradiol (see text for full name of HSD17B3).
The rates of conversion of androstenedione and testosterone into 17β-estradiol are determined by both substrate availability and the level of aromatase expression and activity [22-25]. Testicular and prostatic aromatase activities account for only 15% to 20% of the 17β-estradiol in the circulation, while aromatase activity in nontesticular and nonprostatic tissues accounts for about 80% to 85% of the estrogens (predominantly 17β-estradiol) circulating in the adult male [26]. However, increased aromatase complex activity (as in obesity) increases the production of 17β-estradiol from circulating testosterone, increasing the serum total and free 17β-estradiol concentrations and reducing the serum total and free testosterone concentrations; conversely, decreased aromatase complex activity (such as that accompanying significant weight loss) decreases the production of 17β-estradiol from testosterone, decreasing the serum total and free 17β-estradiol concentrations and maintaining greater serum total and free testosterone concentrations [18,24-30].
An elevated ratio of serum total 17β-estradiol concentration to serum total testosterone concentration stimulates the externalization of phosphatidylserine on the plasma membranes of human Leydig cells and initiates premature engulfment of healthy Leydig cells by testicular macrophages, reducing the amount of testosterone synthesizing tissue [31-33]. Elevated ratios of serum total 17β-estradiol concentration to serum total testosterone concentration are associated with "estrogenic erectile dysfunction," a disorder characterized by loss of libido and erectile impairment [34,35]. In addition, postprandial insulin sensitivity and the rate of postprandial glucose clearance decrease as the ratio of serum total 17β-estradiol concentration to serum total testosterone concentration increases [36].
Circulating Androgens, Age, and Male Sexual Function
Partly because their whole-body aromatase activity is greater and, therefore, older men convert testosterone to 17β-estradiol faster than younger men [23], aging is associated with increased serum total 17β-estradiol concentration [35,37] and decreased serum total testosterone concentration [22,35,37-52]. In many countries and among many ethnic groups, men more than 40 years old experience annual declines of between 0.5% and 2.5% in mid-morning serum concentrations of free testosterone, biologically active testosterone, and total testosterone [22,35,37-55]. This decline is caused by agingassociated attenuation of hypothalamic secretion of gonadotropinreleasing hormone (GnRH), resulting from hypothalamic hypersensitivity to the negative feedback inhibition of LH and testosterone on GnRH secretion, and a blunted response of testicular Leydig cells to testosterone-stimulating LH [56-62]. Together these changes downregulate testosterone production in the testes and decrease testosterone secretion into the circulation [57-62].
These declines carry clinical relevance. Male sexual performance and function are dependent upon testosterone adequacy [63-73], while relative testosterone inadequacy reduces male sexual desire, function, performance, and potency [63-67]. The strength, duration, and quality of erections, as well as the frequency of successful intercourse, are proportional to the serum free testosterone concentration [63-67,73,74] and successful erectile function may require a serum free testosterone concentration of at least 7 nmol/L (1 nmol/L = 28.8 ng/dL) [63,64,66]. In parallel with the aging-associated decline in circulating testosterone concentrations, the percentage of men who experience declining interest in sexual activity, impaired sexual performance, or moderate to severe erectile failure increases with increasing age after the 40th year [75].
"Testosterone Deficiency" and "Low Normal Testosterone"
Disordered testosterone physiology occurs along a continuum of severity. "Testosterone deficiency" reflects classical frank endocrine deficiency and is associated with clinical symptoms of hypogonadism, such as incomplete or delayed sexual development, sexual disorders, breast discomfort, gynecomastia, loss of body hair, reduced need for shaving, very small or shrinking testes, inability to father children, low or zero sperm count, loss of height, predisposition to fracture, low bone mineral density, hot flushes, and sweats [68]. Although definitions of "testosterone deficiency" vary, mid-morning serum total testosterone concentrations less than 7 nmol/L to 10.5 nmol/L are considered to reflect "testosterone deficiency" [68,76-78]. A less severe disorder of testosterone physiology, a condition of "low normal testosterone" (also called "Leydig cell impairment" [69]), is associated with physiological conditions that may include reductions in energy, motivation, initiative, self-confidence, concentration and memory, sleep quality, muscle bulk and strength; diminished physical or work performance; feeling sad or blue; depressed mood or dysthymia; mild anemia; and increased body fat and body mass index [68]. A diagnosis of "low normal testosterone" is considered to be consistent with a mid-morning serum total testosterone concentration between 7 nmol/L to 10.5 nmol/L and 12.2 nmol/L to 14 nmol/L [68,76-78].
Consequences of Low Normal Serum Testosterone Concentration
Low normal serum testosterone concentrations jeopardize men’s health [79-88]. For example, in several prospective studies, remaining life expectancy was directly correlated with the initial serum total testosterone concentration [79], while the risk for premature death from any cause was inversely correlated with the initial serum total testosterone concentration [80-85]. In addition, during the 20-year prospective observational Rancho Bernardo Study of men aged 50 to 91 years living in southern California, men with initial serum total testosterone concentrations less than 8.4 nmol/L were at significantly greater risk for premature death from any cause, death from any cancer, death from any form of cardiovascular disease, or death from respiratory disease [86]. The results of a metα-analysis of the combined results of 11 previously-published epidemiologic observational studies indicated that the risk for suffering premature death from any cause was 35% greater among men with serum total testosterone concentrations less than 14 nmol/L [88].
Cardiovascular and cerebrovascular health are compromised by low normal serum testosterone concentrations [81,82,86,87,89-97]. For example, in the 5-year prospective observational Health in Men study of men aged 70 years or older in Australia, the combined risk for first stroke or first transient ischemic attack was doubled in men with initial serum total testosterone concentrations less than 11.7 nmol/L [89]. In a cross-sectional study of men with hypertension but free of evidence of clinical atherosclerosis in Athens, Greece, the risk for suffering either stroke, transient ischemic attack, peripheral artery disease, coronary artery disease, or premature death from cardiovascular disease was more than tripled in men with serum total testosterone concentrations less than 14 nmol/L [90]. In other studies, the rate of thickening of the carotid artery wall [91] and the risk for developing severe aortic atherosclerosis [92] were inversely correlated with the serum total testosterone concentration. The results of a metα-analysis of the combined results of 54 previouslypublished cross-sectional observational studies and 7 double-blind randomized placebo-controlled clinical trials indicated that the risk for developing any form of cardiovascular disease was inversely correlated with the serum total testosterone concentration [93].
Low normal serum testosterone concentrations also may be detrimental to skeletal integrity [49,98,99]. In a cross-sectional study of Swedish men aged 69 to 80 years, bone mineral densities of the hip, femoral trochanter, and spine were positively correlated with the serum free testosterone concentration [49]. However, a more important threat to men’s health is the relationship between low normal serum testosterone concentrations and functional physical disability [100-104]. For example, in the cross-sectional Toledo Study for Healthy Aging, the risk for frailty (the presence of at least 3 of the following: weakness, slowness, low activity, exhaustion, sarcopenia) was inversely correlated with the serum total testosterone concentration [103]. In a 4.5-year prospective cohort study of 5994 ambulatory men aged 65 years or older conducted within the Osteoporotic Fractures in Men (MrOS) study at six U.S. clinical centers, performance on a test of functional physical ability (ability to sit and stand rapidly without assistance) was inversely correlated with the serum total testosterone concentration [104].
Cognitive functioning also may be impaired in men with low normal serum testosterone concentrations [50,105]. For example, the 50- to 91-year old male participants in the Baltimore Longitudinal Study of Aging exhibited significant correlations between their initial serum total testosterone concentrations and measures of visual memory, verbal memory, and visuospatial functioning measured 10 years later [50].
Immune system functioning and inflammation also may be adversely affected by low normal serum testosterone concentrations [106-110]. In the cross-sectional Boston Area Community Health (BACH) survey of men aged 30 to 79 years residing in the Boston area, the serum total testosterone concentration was inversely correlated with the plasma concentration of C-reactive protein (CRP) [107], a plasma protein that is secreted during inflammation, stimulates the activation of immune system defense responses, and produces a persistently elevated degree of chronic systemic inflammation [108]. Consistent with that report, in a case-control study of hypogondal and eugonadal young men, the serum total testosterone concentration was inversely correlated with the plasma concentrations of the pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α) and macrophage inflammatory proteins 1α and 1β (MIP1α and MIP1β) [109].
Oxidative Stress, Leydig Cells, and Testosterone Secretion
Healthy cellular metabolism requires the generation of metabolic energy within mitochondria without the production of collateral oxidative damage caused by the oxidizing byproducts of metabolism, including reactive oxygen species (ROS) such as superoxide (SO; ·O2 -); ), hydrogen peroxide (H2O2), hydroxy radicals (·OH-), nitric oxide (·NO), peroxyl radicals (·ROO-), peroxynitrite (·ONOO-), singlet oxygen (·1O2), and hypochlorous acid (HOCl) [111-115]. About 2% to 3% of these ROS escape endogenous antioxidant mechanisms to oxidize cellular and circulating lipids, proteins, and nucleic acids [113-119]. ROS and other oxidizing molecules generated by environmental insults (e.g., ultraviolet irradiation, cigarette smoke, and air pollutants [120-123]), drugs [123], and ethanol [124] add to local and systemic oxidative stress.
Dependence on the mitochondrial electron transfer system for the energy to drive testosterone synthesis exposes Leydig cell mitochondria to oxidative stress [3,4], and the generation of ROS within Leydig cells increases when testosterone synthesis is stimulated [6,125]. Leydig cells exposed to oxidative stress exhibit reduced activities of antioxidant enzymes (e.g., catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPx)) [61,62,126,127], reduced intracellular glutathione content [61,62,128,129], increased secretion of the malondialdehyde (MDA) product of lipid peroxidation [61,62,127,128], increased oxidative modification of DNA [6], reductions in the mitochondrial membrane potential required for testosterone synthesis [130,131], and reduced expression of the StAR protein [130]. Oxidatively damaged Leydig cells are less sensitive to LH, with fewer LH receptors expressed per cell, and exhibit impaired activation of the StAR protein, reduced activities of several enzymes of the testosterone biosynthetic pathway (CYP11A1, 3β-HSD, CYP17A1 hydroxylase, CYP17A1 lyase, 17β-HSD), and inhibition of testosterone synthesis [61,62,127,129,132-134]. In contrast, a reduction in systemic oxidative stress induced by lifelong daily episodes of running reduces oxidative stress within Leydig cells and increases the rate of testosterone secretion in mice [135].
The aging-associated declines in testosterone production and circulating testosterone concentrations [56-62] are at least in part the consequences of cumulative oxidative stress within Leydig cells [136,137]. With advancing age, Leydig cells exhibit increasing degrees of intracellular lipid peroxidation [136,137] and decreasing expression of SOD, GPx, and glutathione [136-138].
This evidence provides strong support for the hypotheses that 1) systemic oxidative stress inhibits testosterone synthesis in Leydig cells and 2) reducing systemic oxidative stress releases testosterone synthesis from oxidative inhibition and improves testosterone status (Figure 5). Consistent with these hypotheses, several nutritional antioxidants (e.g., the phytonutrients in pomegranates, phosphatidylserine, vitamin C, vitamin E, α-lipoic acid, zinc, and selenium) have been observed to contribute to a reduction in systemic and local oxidative stress, stimulation or reversal of inhibition of testosterone synthesis, and enhancement of androgenic status.

Figure 5: Hypothetical scheme illustrating oxidative inhibition of testosterone synthesis and its prevention by nutritional antioxidants.
Pomegranates, Punicalagins, Ellagitannins, Anthocyanidins, Anthocyanins, Proanthocyanidins, Procyani
Pomegranates and their extracts contain a large number of nutritional antioxidants, including punicalagins, hydrolyzable ellagitannins (ellagic esters of glucose), anthocyanins, ascorbic acid, and α-tocopherol [139-145]. Punicalagins and ellagitannins are large polyphenols (Figure 6) that are stable at stomach pH and pass into the small intestine unchanged [142,146], although a small fraction are hydrolyzed within the neutral pH environment of the human small intestine, forming combinations of ellagic and gallic acids [139,146- 148]. Nonetheless, most large pomegranate polyphenols proceed to the large intestine, where microbial metabolism converts them into the smaller urolithins (Figure 7): urolithin A (3,8-dihydroxy-6Hdibenzo[ b,d]pyran-6-one), urolithin B (3-hydroxy-6H-dibenzo[b,d] pyran-6-one), urolithin C (3,8,9-trihydroxy-6H-dibenzo[b,d]pyran- 6-one) and urolithin D (3,8,9,10-tetrahydroxy-6H-dibenzo[b,d] pyran-6-one) [139,144,147,149-153]. Ellagic acid (Figure 8) and the urolithins are absorbed intact into the human bloodstream [144,147,151,153-155].
Figure 6: Punicalagin. By Markwdck (Own work) [CC0], via Wikimedia Commons. Downloaded from https://upload.wikimedia.org/wikipedia/commons/9/91/Punicalagin1.png on December 22, 2014.
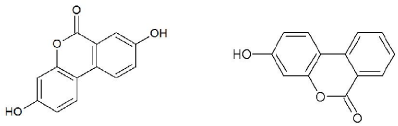
Figure 7: Left, urolithin A*; right, urolithin B#. *By NotWith (Own work) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons. Reprinted under the Creative Commons Attribution-Share Alike 3.0 Unported license from https://commons.wikimedia.org/wiki/ File:Urolithin_A.png on December 22, 2014. #By NotWith (Own work) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons. Downloaded from https://upload.wikimedia.org/wikipedia/commons/f/fa/Urolithin_B.png on December 22, 2014.
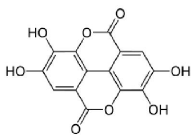
Figure 8: Ellagic acid. By Yikrazuul (Own work) [Public domain], via Wikimedia Commons. Downloaded from https://upload.wikimedia.org/wikipedia/commons/d/da/Ellagic_acid.svg on December 22, 2014.
Pomegranate juice and methanolic and aqueous extracts of pomegranate fruits also contain mixtures of polyphenolic anthocyanidins (200 mg to 400 mg per L) [141,143,156]. Anthocyanidins are natural pigments responsible for the blue, purple, red and orange colors of many fruits and vegetables; the chromophore of 8 conjugated double bonds carrying a positive charge on the heterocyclic oxygen ring is responsible for the intense redorange to blue-violet color produced by anthocyanidins under acidic conditions [157,158]. Of the more than 650 known anthocyanidins [157,158], pelargonidin, cyanidin, delphinidin, petunidin, peonidin, and malvidin (Figure 9) account for 90% of all dietary anthocyanidins [159], and 13 others (Table 1) account for most of the other 10% [160].
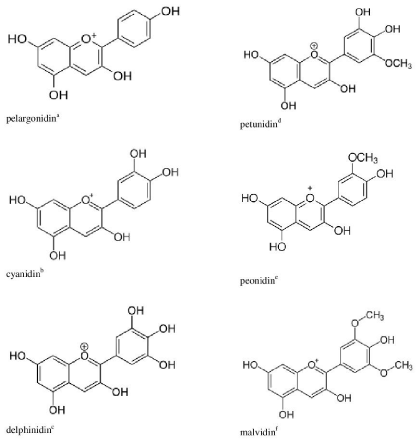
Figure 9: The 6 principal anthocyanidins. aBy Pelargonidin.png: Edgar181 derivative work: Shakiestone (this file was derived from: Pelargonidin.png) [Public domain], via Wikimedia Commons). Downloaded from https://upload. wikimedia.org/wikipedia/commons/1/1a/Pelargonidin.svg on December 22, 2014. bBy NEUROtiker (Own work) [Public domain], via Wikimedia Commons. Downloaded from https://upload.wikimedia.org/wikipedia/commons/8/8b/ Cyanidin.svg on December 22, 2014. cBy Yikrazuul (Own work) [Public domain], via Wikimedia Commons. Downloaded from https://upload. wikimedia.org/wikipedia/commons/5/5e/Delphinidin.svg on December 22,2014. dBy Yikrazuul (Own work) [Public domain], via Wikimedia Commons. Downloaded from https://upload.wikimedia.org/wikipedia/commons/1/1b/ Petunidin.svg on December 22, 2014. eBy Edgar181 (Own work) [Public domain], via Wikimedia Commons. Downloaded from https://upload. wikimedia.org/wikipedia/commons/e/eb/Peonidin.png on December 22, 2014. fBy NEUROtiker (Own work) [Public domain], via Wikimedia Commons. Downloaded from https://upload.wikimedia.org/wikipedia/commons/d/d7/ Malvidin.svg on December 22, 2014.
Carbon Position
Anthocyanidin
3
5
6
7
3’
4’
5’
Apigeninidin
H
OH
H
OH
H
OH
H
Aurantinidin
OH
OH
OH
OH
H
OH
H
Capensinidin
OH
OCH3
H
OH
OCH3
OH
OCH3
Europinidin
OH
OCH3
H
OH
OCH3
OH
OH
Hirsutidin
OH
OH
H
OCH3
OCH3
OH
OCH3
6-Hydroxycyanidin
OH
OH
OH
OH
OH
OH
H
6-Hydroxydelphinidin
OH
OH
OH
OH
OH
OH
OH
Luteolinidin
H
OH
H
OH
OH
OH
H
5-Methylcyanidin
OH
OCH3
H
OH
OH
OH
H
Pulchellidin
OH
OCH3
H
OH
OH
OH
OH
Riccionidin A
H
H
OH
OH
H
OH
H
Rosinidin
OH
OH
H
OCH3
OCH3
OH
H
Tricetinidin
H
OH
H
OH
OH
OH
OH
Table 1: Structural variation among 13 minor anthocyanidins [160].
Anthocyanidins are unstable to light and are water-insoluble so that they usually do not occur in their free state but most often are linked to sugars, which provide stability and water solubility [158]. These glycosides are called anthocyanins [158]. The anthocyanins occurring in pomegranate juice in the largest amounts contain glucose that is covalently bound to the ring oxygen and are cyanidin- 3-glucoside (chrysanthemin), cyanidin-3,5-diglucoside, delphinidin- 3-glucoside (myrtillin), pelargonidin-3-glucoside (callistephin) and malvidin-3-glucoside (oenin) [145,159]. Anthocyanins are stable at stomach pH and, following their passage into the intestinal tract, most are absorbed into the bloodstream, although with low efficiency [159].
Pomegranates also contain small amounts of proanthocyanidins (polymers of flavan-3-ols such as cyanidin, catechin, and epicatechin) and procyanidins (polymers containing only epicatechin units) [161,162]. Cyanidin, catechin and epicatechin are extremely similar structurally (Figure 10) and procyanidins (Figure 11) are the most abundant type of proanthocyanidins in plants [161]. Humans absorb the epicatechin and catechin monomers from dietary proanthocyanidins and procyanidins with very low effiicency, producing plasma concentrations that are directly proportional to intake [163-166].
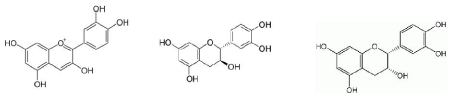
Figure 10: Left, cyanidina; Center, catechinb; Right, epicatechinc. aBy NEUROtiker (Own work) [Public domain], via Wikimedia Commons. Downloaded from https://upload.wikimedia.org/wikipedia/commons/8/8b/ Cyanidin.svg on December 22, 2014. bBy Edgar181 (Own work) [Public domain], via Wikimedia Commons. Downloaded from https://upload.wikimedia. org/wikipedia/commons/4/44/%28%2B%29-Catechin.png on December 22, 2014. cBy selfmade by Shaddack, 23 October 2005 (Own work) [Public domain], via Wikimedia Commons. Downloaded from https://upload.wikimedia. org/wikipedia/commons/2/20/Epicatechin.png on December 22, 2014.

Figure 11: Procyanidin B2. By n/a [Public domain], via Wikimedia Commons. Downloaded from https://upload.wikimedia.org/wikipedia/commons/e/eb/Structure_of_Procyanidin_B2.png on December 22, 2014.
Antioxidant activity
Pomegranate polyphenols exhibit strong antioxidant potency [141,167-176]. The individual pomegranate polyphenols and their metabolites, including punicalagin, ellagic acid, gallic acid, and the urolithins, express antioxidant activities proportional to the number of hydrolyzable hydroxyl groups that are present [139-141,173,177- 186]. Dietary supplementation with pomegranate polyphenols upregulates the expression and activities of paraoxonases 1 (PON1) [187] and 2 (PON2) [188], endogenous antioxidants that buffer the intracellular (PON2) and extracellular (PON1) environments from oxidizing conditions [187,188] and increase the systemic antioxidant capacity and reduce systemic oxidative stress in men [140,187,189- 191].
Anthocyanidins and their glucosides contain multiple binding sites for unpaired free radical electrons [158,160,192] which confer potent antioxidant activity [159,192-198]. Following anthocyanin consumption, serum ORAC is directly proportional to the amount of anthocyanins consumed [159,199,200]. This increase in circulating antioxidant capacity is accompanied by increased circulating glutathione concentration [201]; decreased circulating concentrations of oxidized glutathione, oxidized proteins, and lipid peroxides [201]; and less oxidative damage to DNA in circulating white blood cells [201].
Antioxidant activity appears to be a general characteristic of proanthocyanidins [202]. In human studies, the consumption of 200 mg to 466 mg of total proanthocyanidins daily for several weeks has reduced the plasma concentration of oxidized LDL particles [203] and has increased the resistance of freshly-harvested LDL particles to ex vivo oxidation [166].
Activity against oxidative inhibition of testosterone synthesis
Pomegranate polyphenols downregulate the expression of hepatic CYP1A2 and CYP3A, slowing the rate of oxidative conversion of carbon tetrachloride (CCl4) into the trichloromethyl radical that reacts with O2 to produce the lipid peroxidizing trichloromethyl peroxyl radical and subsequent lipid peroxidation [204,205]. Although CCl4 also reduces the testicular activities of the antioxidants, glutathione, GPx, glutathione reductase (GR), SOD, and catalase, while inhibiting LH-stimulated testosterone synthesis, these effects are prevented by the concurrent consumption of pomegranate polyphenols [206]. Consistent with these reports, the pomegranate polyphenol, ellagic acid, has prevented adriamycin-induced testicular lipid peroxidation and inhibition of testosterone synthesis [207]. These animal data indicate that pomegranate polyphenols reduce toxin-induced oxidative stress and deinhibit testosterone synthesis within the testes.
Antiaromatase activity
The addition of either urolithin B or 8-O-methylurolithin B (a product of hepatic processing of urolithin B [147,148]) to the culture medium of aromatase-overexpressing MCF-7aro estrogen receptor-positive breast cancer cells attenuated the stimulatory effect of androstenedione on aromatase complex activity [148]. 50% inhibition of androstenedione stimulation of aromatase complex activity was obtained with physiologically relevant concentrations of 8-O-methylurolithin B (2.5 μM) and urolithin B (5 μM). A classification system that compares the inhibitory potency of phytonutrients to the potency of aromatase complex-inhibiting drugs has been suggested [208]; according to these standards, urolithin B, at plasma concentrations that are achieved through the consumption of pomegranate polyphenols [147,151,153], is a "strong" inhibitor of the aromatase complex and contributes to the maintenance of serum testosterone concentrations [148,208].
Phosphatidylserine
Phosphatidylserine is the major acidic phospholipid in human membranes and constitutes 2% to 20% of the total lipid mass of human plasma and intracellular membranes [209-211]. Within the human testes, phosphatidylserine constitutes 1% to 2% of total tissue weight [211] and is a basic structural component of endoplasmic reticulum, nuclear envelopes, Golgi apparati, inner (cytosolic) leaflets of plasma membranes, and outer mitochondrial membranes [209- 217].
Phosphatidylserine is synthesized within mitochondriaassociated membrane (MAM) domains of the endoplasmic reticulum, almost entirely through the phosphatidylserine synthase 1-catalyzed substitution of serine for choline on docosahexaenoic acid (DHA)- enriched phosphatidylcholine [218-225]. In addition, a small amount of phosphatidylserine results from the replacement of ethanolamine with serine on phosphatidylethanolamine by phosphatidylserine synthase 2 [219-222,226], an enzyme whose expression is the greatest within testicular tissues [225,227].
The amount of oral phosphatidylserine that is taken up and incorporated into human cell membranes increases as phosphatidylserine intake increases [228-232]. Exogenous phosphatidylserine is transported from the plasma membrane’s outer leaflet to its inner leaflet by a phosphatidylserine-specific ATPdependent aminophospholipid translocase ("flippase") [219,222,229- 236]. Maintenance of transmembrane phosphatidylserine asymmetry is critical to cell survival; in contrast, increased outer leaflet phosphatidylserine content is a required signal for the irreversible initiation of phagocytic engulfment of apoptotic cells in many cell types, including testicular cells [237-245]. In order to avoid inappropriately initiating apoptosis, healthy cells devote up to 8% of all ATP consumption to maintaining transmembrane phosphatidylserine asymmetry [230,246].
Testicular cells are enriched in phosphatidylserine [210] and require phosphatidylserine for the performance of normal testicular functions, including testosterone synthesis [247,248] and spermatogenesis [238]. In Leydig cells, testosterone synthesis is stimulated by phosphatidylserine through a sequence in which phosphatidylserine induces the translocation of cytosolic Akt (protein kinase B) to the plasma membrane and interacts directly with Akt to alter its conformation and allow it to be activated via phosphorylation by mTOR2 [249,250]. Phosphatidylserine-dependent activation of Akt is followed by Akt activation of protein kinase C [248-254], which participates in signaling pathways that culminate in testosterone synthesis through the primary "Δ5" pathway (pregnenolone → 17α-hydroxypregnenolone → dehydroepiandrosterone → androstenedione → testosterone). Phosphatidylserine also stimulates the isomerase activity of HSD3B2 in the testes, increasing testosterone synthesis through the alternate "Δ4" pathway (pregnenolone → progesterone → androstenedione → testosterone) [247,255,256]. By participating in the initiation of androgenic signaling cascades and through direct stimulation of the rate-limiting HSD3B2 enzyme, while not affecting the activities of any other "Δ5" or "Δ4" pathway enzymes (including 5α-reductase), phosphatidylserine can exert a substantial positive influence on testosterone status [3,4,247-257].
These androgenic responses to the presence of phosphatidylserine can be triggered in men. In a double-blind, randomized, placebocontrolled study, healthy men with initially "desirable" resting plasma free testosterone concentrations and participating in a prescribed exercise regimen added 600 mg of phosphatidylserine to their daily diets for 10 days [258]. Supplemental phosphatidylserine produced a 60% greater increase in resting plasma free testosterone concentration than was associated with the ingestion of placebo.
The antioxidant activity of phosphatidylserine contributes to its potentiating effect on Leydig cell testosterone production [131]. The antioxidant effects of phosphatidylserine are ubiquitous; human neurons cultured in the presence of phosphatidylserine (25 μM) exhibit significant reductions in electric shock-induced ROS production [260], rats fed phosphatidylserine exhibit upregulated antioxidant enzyme activities in the brain (SOD and catalase) and liver (SOD and GPx) [259], and the capacity of human high-density lipoprotein (HDL) particles to prevent the spontaneous oxidation of circulating low-density lipoprotein (LDL) particles [260,261] is proportional to the phosphatidylserine content of the HDL particles [261].
Vitamin C
Vitamin C is an essential nutrient that must be supplied through the diet because humans lack the enzyme, gulonolactone oxidase, and cannot synthesize vitamin C de novo [262]. Vitamin C is known to be an electron donor for a number of human enzymes that participate in hydroxylation reactions [263]. The best known of these reactions is the posttranslational hydroxylation of peptide-bound proline and lysine residues during formation and cross-linking of mature collagen [262,263]. In these reactions, vitamin C (as ascorbate) reactivates the enzymes by reducing the metal sites of prolyl (iron) and lysyl (copper) hydroxylases [262].
Ascorbate, the dominant form of vitamin C in humans [262], contains 2 enolic hydrogen atoms that provide electrons that are available for nonenzymatic transfer to biological oxidants [264] and provide the basis for the antioxidant properties of vitamin C [264]. Ascorbate readily scavenges (reduces) reactive oxygen and nitrogen species, including hydroxyl, peroxyl, nitroxyl, ferryl, thyl, α-tocopheryl, ·O2- ·ONOO-, and nitroxide radicals: ·1O2: hypochlorite: and H2O2 generated from ·O2 - [264-270]. Oxidized ascorbate can be reduced back to ascorbate by transfer of its free radical electron to another receptor molecule (such as reduced glutathione, α-lipoic acid, or another molecule of oxidized ascorbate) or can be further oxidized to become dehydroascorbate (DHA) [263-271]. In turn, DHA can be recycled to ascorbate or can be converted into the excretory end product, 2,3-diketogulonate [264,267-271]
In humans, the total antioxidant capacity of the circulation is positively correlated with daily vitamin C intake [272,273] and is increased by dietary supplementation with vitamin C [274]. Supplemental vitamin C reduces systemic oxidative stress (reflected in deceased whole-body lipid peroxidation) [275] and large amounts of intracellular ascorbate provide protection against collateral oxidative damage secondary to generation of ROS during mitochondrial respiration [276]. The uptake of vitamin C by tissues, including the Leydig cells of the testes, is proportional to vitamin C intake [277- 280]
Oral vitamin C increases LH secretion by isolated pituitary cells in the absence of hypothalamic LH releasing hormone [281] and stimulates testosterone synthesis [278,282] and increased serum total testosterone concentrations in otherwise unmanipulated healthy male rats. The concurrent consumption of vitamin C has prevented the inhibition of testicular antioxidant enzymes, the increase in the formation of lipid peroxidation products and oxidized protein carbonyls, and the suppression of testosterone synthesis commonly observed in laboratory animals exposed to cadmium [283], lead [284], cyclophosphamide [285-287], or arsenic [288,289]. In addition, these environmental toxins downregulate the activities of testicular HSD3B2 and HSD17B3 [283,285-291]; this effect is prevented by concurrent consumption of vitamin C [283,285,288,289], suggesting that vitamin C may directly upregulate testosterone synthesis in addition to protecting the synthetic pathway from oxidative inhibition.
Antiaromatase activity
The safety of vitamin C consumption is not in doubt. The consumption of 2500 mg daily for 7 days has been harmless [292] and a detailed systematic comparison of 29 published human clinical trials in which subjects had consumed supplemental vitamin C in amounts ranging from a single bolus of 4000 mg to daily consumption of 10000 mg for up to 210 days determined that the scientific evidence clearly substantiates that the daily intake of 2000 mg of supplemental vitamin C (in addition to that provided by the diet) indefinitely is safe and short-term intakes of up to 4000 mg daily are without adverse effects other than occasional bowel intolerance in some individuals [293]. In addition, the Cochrane Collaboration examination of the data obtained from 2490 subjects who consumed more than 1000 mg of supplemental vitamin C daily revealed no adverse effects that could be attributed to the vitamin [294]. Even intravenous injection of an amount of vitamin C sufficient to increase plasma vitamin C concentrations by approximately 2500% (to over 1200 μM) was without adverse effect [295].
Two reports have generated concern that intakes of vitamin C greater than the RDA could promote urolithiasis in susceptible individuals [296,297]. In a short, 6-day study, the consumption of 2000 mg of supplemental vitamin C by individuals with histories of prior kidney stone formation produced a significant increase in the urinary excretion of oxalate, a putative biomarker of urolithogenic risk [296]. However, no signs of crystal formation were observed. In addition, the diagnostic reliability of increased urinary oxalate excretion as a direct biomarker of increased risk for kidney stone formation has been challenged in recent years [298-300].
Furthermore, when the risk of symptomatic kidney stones was studied for 14 years prospectively in a cohort of 85,557 women with no prior history of kidney stones (the Nurses’ Health Study), vitamin C intake was not associated with increased risk for developing kidney stones (RR, daily vitamin C intake greater than 1500 mg compared to daily vitamin C intake less than 250 mg [301]. Similarly, a 6-year prospective study of the relationship between the intake of vitamin C and the risk of symptomatic kidney stones performed in a cohort of 45,251 men aged 40 to 75 years old and with no prior history of kidney calculi (the Health Professionals Follow-Up Study) determined that vitamin C intake was not associated with increased risk for developing kidney stones [302].
A re-evaluation of the Health Professionals Follow-Up Study data after an additional 8 years of observation initially reported that compared to the effects of the consumption of less than 90 mg of vitamin C daily, the daily consumption of 1000 mg or more significantly increased the relative risk of forming a kidney stone by 41% [296]. Although seemingly impressive, this would increase an individual’s likelihood of forming a kidney stone sometime during their lifetime from 4.8% to 6.7%. However, these investigators compared vitamin C intakes that can be achieved only through dietary supplementation to frankly deficient intakes; if correct, their conclusion implies that vitamin C deficiency is somehow protective against urolithiasis. Further analysis revealed that there was no increase in risk when the effects of the consumption of 1000 mg or more of supplemental vitamin C was compared to the effects of no supplementation with vitamin C [303]. In contrast, when the effects of the daily consumption of 218 mg or more of vitamin C from food were compared to the effects of the consumption of 105 mg or less of vitamin C from food, the relative risk for kidney stone formation increased significantly by 31%, suggesting that a confounding factor consumed with vitamin C-rich foods and beverages, and not vitamin C, is urolithogenic. Together, these updated findings confirm the safety of vitamin C consumption.
It should be noted that it has been reported that healthy human peripheral blood white blood cells exposed to supraphysiologic concentrations of vitamin C (in the order of 1000 μM) exhibited impaired ability to synthesize DNA or phagocytose pathogens [304]. However, this concentration is 5- to 15-fold greater than the highest concentrations ever reported in human blood following oral vitamin C intake and has been equaled for only a few minutes following direct intravenous injection 1000 mg or more of ascorbate [292,295,305]. The findings in this report [304] cannot be considered representative of the safety of dietary supplementation with vitamin C.
Vitamin E
Vitamin E (α-tocopherol) is highly bioavailable in humans [306- 313] and is the most powerful chain-breaking lipid-soluble dietary antioxidant [314]. In addition, the rate-limiting enzyme in the synthesis of glutathione, γ-glutamyl-cysteinyl synthetase, requires vitamin E as a cofactor [315]. The presence of vitamin E in the culture medium has attenuated iron-induced lipid peroxidation in cultured Leydig cells [316].
While vitamin E deficiency impairs the cAMP response to LH, thereby desensitizing the Leydig cell to LH and reducing the testosterone synthetic response to LH stimulation [317], Leydig cell responsiveness to LH is proportional to the amount of vitamin E to which the cells are exposed [316] and supplemental vitamin E administered to healthy male rats has increased the testosterone synthetic response to LH stimulation [318]. Consistent with these reports, dietary supplementation with vitamin E (483 mg daily for 8 weeks) has produced a 20% increase in testosterone synthesis in healthy men [319].
Chronic forced swimming exercise increases systemic oxidative stress in the Leydig cells of rats, as reflected in increased intracellular lipid peroxidation and downregulation of glutathione, SOD, GPx, and catalase [282,320,321]. In these cells, the increase in oxidative stress is accompanied by inhibition of testosterone synthesis [282,320,321]. The activities of testicular antioxidant enzymes also are inhibited, increasing intracellular oxidative stress, and testosterone synthesis is impaired, in rats administered cadmium [283,322,323] or chromium VI [324] and in mice administered sodium azide [325]. In addition, chromium VI inhibits the activities of HSD3B2 and HSD17B3, contributing to the impairment of testosterone synthesis [324]. In all of these model systems, concurrent dietary supplementation with vitamin E prevents both the increase in oxidative stress and the inhibition of testosterone synthesis [283,320,322-325].
Vitamin C and Vitamin E
The Leydig cells of rats fed the polychlorinated biphenyl, Arochlor-1254, exhibit decreased activities of antioxidant enzymes, increased generation of H2O2, lipid peroxidation products and other ROS, and inhibition of the StAR protein, P450scc, HSD3B2, and testosterone synthesis [326-333]. The combined concurrent consumption of supplemental vitamin C and vitamin E attenuates or prevents these negative effects on testosterone status [330,331].
α-Lipoic Acid
α-Lipoic acid (5-(1,2-dithiolan-3-yl)-pentanoic acid; thioctic acid) is a disulfide compound that is synthesized within human mitochondria by lipoic acid synthase [334,335]. Exogenous (dietary) α-lipoic acid is absorbed into the human circulation and contributes to the human α-lipoic acid pool [336,337]. The most important dietary sources of α-lipoic acid are spinach and broccoli [338].
Mitochondrial enzymes of oxidative metabolism that require α-lipoic acid as a cofactor include pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, branched-chain α-ketoacid dehydrogenase, and the glycine cleavage system that converts glycine into pyruvate [339]. α-Lipoic acid must interact with these enzymes in order for aerobic metabolism to proceed [339]. In addition to its cofactor roles, α-lipoic acid penetrates both cell membranes and aqueous compartments throughout the body, allowing α-lipoic acid to act as a multi-purpose nonenzymatic antioxidant that protects mitochondria and surrounding cellular elements from oxidation by the free radicals produced by mitochondria during oxidative metabolism [335,340,341]. The sulfhydryl groups on the α-lipoic acid molecule provide strong antioxidant potency, directly exchanging free protons for free radical electrons in lipophillic environments (e.g., biological membranes) and exchanging free protons with hydroxyl ions and water during the deactivating reduction of free radical electrons in aqueous environments (e.g., biological fluids) [340]. During these reactions, α-lipoic acid becomes reduced to dihydrolipoic acid, an intermediate that retains some antioxidant potency before being either β-oxidized or S-methylated into excretory metabolites [337,338].
In addition to reducing oxidizing compounds, α-lipoic acid can recycle (reduce) other nonenzymatic antioxidants after they have become oxidized, prompting the descriptor, "antioxidant of antioxidants" [342-344]. α-Lipoic acid also stimulates increased activities of endogenous antioxidant enzymes, including SOD, catalase, GPx, and heme oxygenase-1 (HO-1) [342-353]. Conversely, elevated systemic oxidative stress downregulates the expression of lipoic acid synthase, increasing the need for dietary α-lipoic acid in order to forestall retardation of aerobic energy production [354].
An elevated level of systemic oxidative stress inhibits Leydig cell synthesis of testosterone [61,62,127,129,134]. Elevated oxidative stress caused by exposure to cadmium [291], cyclophosphamide [355], acrylamide [356], bis-n-butyl phthalate [357], or bisphenol-A (BPA) [358,359] inhibits the activities of testicular and Leydig cell glutathione, GPx, GR, SOD, catalase, HSD3B2, and HSF17B3, increases intracellular lipid peroxidation, and attenuates testosterone synthesis. Dietary supplementation of rats exposed to these agents with α-lipoic acid has prevented or reversed these detrimental effects on testosterone status [291,355,356,358,359]. Long-term intense forced swimming exercise also produces inhibition of antioxidant enzymes, elevated oxidative stress, inhibition of HSD3B3 and HSD17B3, and impaired testosterone synthesis within the testes of rats; concurrent consumption of α-lipoic acid attenuates or prevents these damaging consequences of exercise-induced oxidative stress [360].
Dietary α-lipoic acid is safe. In a multicenter, randomized, double-blind, placebo-controlled clinical trial, men and women with symptomatic diabetic sensorimotor polyneuropathy consumed either placebo or α-lipoic acid (600 mg daily, 1200 mg daily, or 1800 mg daily) for 5 weeks [361]. Among these subjects, the incidence of gastrointestinal upset was increased slightly by 1200 mg and 1800 mg of α-lipoic acid daily, although no other adverse reactions were observed. In contrast, supplementation with 1800 mg of α-lipoic acid daily for 7 months did not precipitate adverse reactions in men and women with advanced multi-site polyneuropathies accompanying diabetes [362]. In addition to the absence of adverse reactions during these trials [361,362], the safety of exogenous α-lipoic acid was demonstrated by the absence of adverse reactions during a study in which men and women with metabolically stable diabetes complicated by symptomatic diabetic sensorimotor polyneuropathy received intravenous injections of 600 mg of α-lipoic acid (5 injections per week for 3 weeks) [363].
Zinc
Chronic zinc deprivation generally results in a low intracellular zinc concentration [364,365], increased intracellular oxidative stress [366-369], and increased sensitivity to oxidative stress [365,366]. An abundance of zinc may protect protein sulfhydryls and DNA from oxidation, or reduce the rate of hydroxyl radical formation from H2O2, through the antagonism of redox-active transition metals, such as iron and copper [226,368-372]. Protection of protein sulfhydryl groups from oxidative modification may involve reduction of sulfhydryl reactivity through direct binding of zinc to the sulfhydryl groups of cysteine residues, steric hindrance as a result of zinc binding to some other protein site in close proximity to the sulfhydryl group, or a conformational change in the protein resulting from zinc binding to some other site on the protein [226,368].
An increase in the intracellular concentration of free zinc ions following their oxidative stress-induced release from intracellular metallothionein releases the nuclear transcription factor, nuclear factor-erythroid 2-related factor 2 (Nrf2), from repression by Kelchlike ECH-associated protein 1 (Keap1) [373]; in turn, derepressed Nrf2 stimulates the expression of the Gclc gene that codes for glutamate cysteine ligase, the rate-limiting enzyme in the synthetic pathway for glutathione, resulting in increased de novo synthesis of glutathione [374-377].
Increased intracellular oxidative stress is associated with inhibition of testosterone synthesis by Leydig cells [61,62,127,129,132- 134,366,378]. Conversely, zinc supplementation can prevent oxidative stress-induced inhibition of testicular antioxidant enzyme activities (with increased formation of lipid peroxidation products and oxidative modification of DNA) and of testosterone synthesis [378,379].
Selenium
Selenium expresses its biological activity after its incorporation into the twenty-first amino acid, selenocysteine, and the geneticallycoded insertion of selenocysteine into the amino acid sequence of selenoproteins during translation of mRNA [380-386]. The abundance of individual selenocysteine-containing selenoproteins is directly responsive to dietary selenium availability [387-393].
At physiological pH, selenocysteine within selenoproteins is fully ionized and acts as a very efficient redox catalyst [380,385,394,395]. Several families of selenoproteins that act as redox enzymes and exploit the chemical properties of selenium have been identified, including the six selenium-dependent antioxidant GPx enzymes that catalyze the removal of hydroperoxides and peroxynitrites by glutathione [380,383-385,396,397]. The selenium-dependent thioredoxin reductases also catalyze the regeneration of ascorbic acid from oxidized dehydroascorbic acid (thereby contributing to intracellular antioxidation) [380,398]. Dietary supplementation with selenium increases intracellular thioredoxin reductase activity and increases the efficiency of regeneration of ascorbic acid [399].
By fostering increased systemic oxidative stress, dietary selenium deficiency impairs the ability of Leydig cells to synthesize testosterone in response to LH [400]. Conversely, supplemental selenium has been reported to attenuate or prevent the oxidative stress-induced inhibition of testosterone synthesis associated with exposure to cadmium [290,291], sodium azide [325], or di(2-ethylhexyl) phthalate [401,402].
Improving Testosterone Status does not Increase the Risk for Prostate Disease
Because the normal growth of prostate tissue, as well as the progression of certain intermediate and high-risk prostate cancers, are dependant on the presence of testosterone, it has been feared that improvement in testosterone status might increase the risk for the development of benign prostatic hyperplasia or prostate cancer [77]. Fortunately, the available scientific evidence confirms that testosterone has no impact on prostate health in adult men, at any age, in the absence of pre-existing prostate cancer [56,71,76,77,403,404]. Metα-analyses of the results of previously published studies have demonstrated that the risk for developing prostate cancer is independent of the serum concentrations of total or bioavailable testosterone [56,405] and that testosterone replacement therapy has no detrimental effect on prostate health [73]. In joint statements released in 2010 [76] and 2013 [77], the International Endocrine Aspects of Male Sexual Dysfunctions Committee and the Endocrine Subcommittee of the Standards Committee of the International Society for Sexual Medicine concluded that increasing circulating testosterone concentrations with exogenous testosterone has no effect on the risk for the development of prostate cancer, the progression of prostate cancer, the appearance of prostate-related symptoms, the development of benign prostate hyperplasia, or the serum prostatespecific antigen concentration.
Conclusion
Healthy serum testosterone concentrations in aging men promote health and longevity. This androgenic hormone is so important to maintenance of health that even "low normal testosterone" is associated with reductions in energy, motivation, initiative, selfconfidence, concentration, memory, sleep quality, muscle bulk, and strength; diminished physical or work performance; feeling sad or depressed; mild anemia; and increased body fat and body mass index.
A growing body of evidence demonstrates that aging often is accompanied by excessive oxidative stress and enhanced oxidative damage within Leydig cells. In addition, oxidizing environmental insults add to local and systemic oxidative stress and contribute to alterations the redox environment of the aging Leydig cells. Oxidatively damaged Leydig cells exhibit decreased responsiveness to LH, with inhibition of testosterone synthesis. These observations support the hypothesis that ROS play an important role in age-related reductions in Leydig cell testosterone production.
Conversely, antioxidant defenses can be augmented by dietary supplementation with specific antioxidant and mitochondrial protective nutrients that reduce cell-wide oxidative damage, support redox balance within Leydig cells, release Leydig cells from oxidative inhibition of testosterone synthesis, and increase the rate of testosterone secretion. The available scientific evidence provides strong support for the hypothesis that reducing oxidative stress can safely improve testosterone status.
References
- Morales A, Collier CP, Clark AF. A critical appraisal of accuracy and cost of laboratory methodologies for the diagnosis of hypogonadism: the role of free testosterone assays. Can J Urol. 2012; 19: 6314-6318.
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999; 84: 3666-3672.
- Ye L, Su ZJ, Ge RS. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules. 2011; 16: 9983-10001.
- Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011; 32: 81-151.
- Iranmanesh A, Lawson D, Veldhuis JD. Glucose ingestion acutely lowers pulsatile LH and basal testosterone secretion in men. Am J Physiol Endocrinol Metab. 2012; 302: E724-730.
- Beattie MC, Chen H, Fan J, Papadopoulos V, Miller P, Zirkin BR. Aging and luteinizing hormone effects on reactive oxygen species production and DNA damage in rat Leydig cells. Biol Reprod. 2013; 88: 100.
- Nishimura K, Matsumiya K, Tsujimura A, Koga M, Kitamura M, Okuyama A. Association of selenoprotein P with testosterone production in cultured Leydig cells. Arch Androl. 2001; 47: 67-76.
- Poderoso C, Converso DP, Maloberti P, Duarte A, Neuman I, Galli S, et al. A mitochondrial kinase complex is essential to mediate an ERK1/2-dependent phosphorylation of a key regulatory protein in steroid biosynthesis. PLoS One. 2008; 3: e1443.
- Crivello JF, Jefcoate CR. Intracellular movement of cholesterol in rat adrenal cells. Kinetics and effects of inhibitors. J Biol Chem. 1980; 255: 8144-8151.
- Arakane F, Kallen CB, Watari H, Foster JA, Sepuri NB, Pain D, et al. The mechanism of action of steroidogenic acute regulatory protein (StAR). StAR acts on the outside of mitochondria to stimulate steroidogenesis. J Biol Chem. 1998; 273: 16339-16345.
- Duarte A, Poderoso C, Cooke M, Soria G, Cornejo Maciel F, Gottifredi V, et al. Mitochondrial fusion is essential for steroid biosynthesis. PLoS One. 2012; 7: e45829.
- Midzak A, Rone M, Aghazadeh Y, Culty M, Papadopoulos V. Mitochondrial protein import and the genesis of steroidogenic mitochondria. Mol Cell Endocrinol. 2011; 336: 70-79.
- Miller WL, Bose HS. Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res. 2011; 52: 2111-2135.
- Penning TM, Jin Y, Rizner TL, Bauman DR. Pre-receptor regulation of the androgen receptor. Mol Cell Endocrinol. 2008; 281: 1-8.
- Mulder E, Lamers-Stahlhofen GJ, van der Molen HJ. Isolation and characterization of 17β-hydroxysteroid dehydrogenase from human erythrocytes. Biochem J. 1972; 127: 649-659.
- Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest. 1969; 48: 2191-2201.
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010; 365: 1517-1535.
- Stocco C. Tissue physiology and pathology of aromatase. Steroids. 2012; 77: 27-35.
- Jiang W, Ghosh D. Motion and flexibility in human cytochrome p450 aromatase. PLoS One. 2012; 7: e32565.
- Ghosh D, Griswold J, Erman M, Pangborn W. X-ray structure of human aromatase reveals an androgen-specific active site. J Steroid Biochem Mol Biol. 2010; 118: 197-202.
- Conley A, Hinshelwood M. Mammalian aromatases. Reproduction. 2001; 121: 685-695.
- Dhindsa S, Furlanetto R, Vora M, Ghanim H, Chaudhuri A, Dandona P. Low estradiol concentrations in men with subnormal testosterone concentrations and type 2 diabetes. Diabetes Care. 2011; 34: 1854-1859.
- Lakshman KM, Kaplan B, Travison TG, Basaria S, Knapp PE, Singh AB, et al. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab. 2010; 95: 3955-3964.
- Advani A, Johnson SJ, Nicol MR, Papacleovoulou G, Evans DB, Vaikkakara S, et al. Adult-onset hypogonadotropic hypogonadism caused by aberrant expression of aromatase in an adrenocortical adenocarcinoma. Endocr J. 2010; 57: 651-656.
- Castro B, Sánchez P, Torres JM, Preda O, del Moral RG, Ortega E. Bisphenol A exposure during adulthood alters expression of aromatase and 5α-reductase isozymes in rat prostate. PLoS One. 2013; 8: e55905.
- Merlotti D, Gennari L, Stolakis K, Nuti R. Aromatase activity and bone loss in men. J Osteoporos. 2011; 2011: 230671.
- Hero M, Ankarberg-Lindgren C, Taskinen MR, Dunkel L. Blockade of oestrogen biosynthesis in peripubertal boys: effects on lipid metabolism, insulin sensitivity, and body composition. Eur J Endocrinol. 2006; 155: 453-460.
- Lapauw B, T'Sjoen G, Mahmoud A, Kaufman JM, Ruige JB. Short-term aromatase inhibition: effects on glucose metabolism and serum leptin levels in young and elderly men. Eur J Endocrinol. 2009; 160: 397-402.
- Mauras N, O'Brien KO, Klein KO, Hayes V. Estrogen suppression in males: metabolic effects. J Clin Endocrinol Metab. 2000; 85: 2370-2377.
- Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab. 2009; 94: 4785-4792.
- Yu W, Zheng H, Lin W, Tajima A, Zhang Y, Zhang X, et al. Estrogen promotes Leydig cell engulfment by macrophages in male infertility. J Clin Invest. 2014; 124: 2709-2721.
- Strauss L, Kallio J, Desai N, Pakarinen P, Miettinen T, Gylling H, et al. Increased exposure to estrogens disturbs maturation, steroidogenesis, and cholesterol homeostasis via estrogen receptor a in adult mouse Leydig cells. Endocrinology. 2009; 150: 2865-2872.
- Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. J Endocrinol. 2013; 217: R25-45.
- Srilatha B, Adaikan PG. Endocrine milieu and erectile dysfunction: is oestradiol-testosterone imbalance, a risk factor in the elderly? Asian J Androl. 2011; 13: 569-573.
- Srilatha B, Adaikan PG, Chong YS. Relevance of oestradiol-testosterone balance in erectile dysfunction patients' prognosis. Singapore Med J. 2007; 48: 114-118.
- Phillips GB. Relationship between serum sex hormones and glucose, insulin and lipid abnormalities in men with myocardial infarction. Proc Natl Acad Sci U S A. 1977; 74: 1729-1733.
- Gennari L, Merlotti D, Martini G, Gonnelli S, Franci B, Campagna S, Lucani B. Longitudinal association between sex hormone levels, bone loss, and bone turnover in elderly men. J Clin Endocrinol Metab. 2003; 88: 5327-5333.
- Simon D, Preziosi P, Barrett-Connor E, Roger M, Saint-Paul M, Nahoul K, et al. The influence of aging on plasma sex hormones in men: the Telecom Study. Am J Epidemiol. 1992; 135: 783-791.
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005; 26: 833-876.
- Nakhai Pour HR, Grobbee DE, Muller M, Emmelot-Vonk M, van der Schouw YT. Serum sex hormone and plasma homocysteine levels in middle-aged and elderly men. Eur J Endocrinol. 2006; 155: 887-893.
- Andersson AM, Jensen TK, Juul A, Petersen JH, Jørgensen T, Skakkebaek NE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab. 2007; 92: 4696-4705.
- van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000; 85: 3276-3282.
- Alvarado LC. Do evolutionary life-history trade-offs influence prostate cancer risk? a review of population variation in testosterone levels and prostate cancer disparities. Evol Appl. 2013; 6: 117-133.
- Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998; 147: 750-754.
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001; 86: 724-731.
- Bhasin S, Pencina M, Jasuja GK, Travison TG, Coviello A, Orwoll E, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. J Clin Endocrinol Metab. 2011; 96: 2430-2439.
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: Longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002; 87: 589-598.
- Lapauw B, Goemaere S, Zmierczak H, Van Pottelbergh I, Mahmoud A, Taes Y, et al. The decline of serum testosterone levels in community-dwelling men over 70 years of age: descriptive data and predictors of longitudinal changes. Eur J Endocrinol. 2008; 159: 459-468.
- Mellström D, Johnell O, Ljunggren O, Eriksson AL, Lorentzon M, Mallmin H, et al. Free testosterone is an independent predictor of BMD and prevalent fractures in elderly men: MrOS Sweden. J Bone Miner Res. 2006; 21: 529-535.
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002; 87: 5001-5007.
- Svartberg J, Midtby M, Bønaa KH, Sundsfjord J, Joakimsen RM, Jorde R. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromsø Study. Eur J Endocrinol. 2003; 149: 145-152.
- Araujo AB, O'Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004; 89: 5920-5926.
- Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, et al. Age-associated changes in hypothalamic–pituitary–testicular function in middle-aged and older men are modified by weight change and lifestyle factors: Longitudinal results from the European Male Ageing Study. Euro J Endocrinol. 2013; 168: 445-455.
- St Sauver JL, Jacobson DJ, McGree ME, Girman CJ, Klee GG, Lieber MM, et al. Associations between longitudinal changes in serum estrogen, testosterone, and bioavailable testosterone and changes in benign urologic outcomes. Am J Epidemiol. 2011; 173: 787-796.
- Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, et al. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008; 93: 2737-2745.
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006; 60: 762-769.
- Veldhuis JD. Recent insights into neuroendocrine mechanisms of aging of the human male hypothalamic-pituitary-gonadal axis. J Androl. 1999; 20: 1-17.
- Veldhuis JD, Iranmanesh A, Mulligan T, Pincus SM. Disruption of the young-adult synchrony between luteinizing hormone release and oscillations in follicle-stimulating hormone, prolactin, and nocturnal penile tumescence (NPT) in healthy older men. J Clin Endocrinol Metab. 1999; 84: 3498-3505.
- Luboshitzky R, Shen-Orr Z, Herer P. Middle-aged men secrete less testosterone at night than young healthy men. J Clin Endocrinol Metab. 2003; 88: 3160-3166.
- Mulligan T, Iranmanesh A, Johnson ML, Straume M, Veldhuis JD. Aging alters feed-forward and feedback linkages between LH and testosterone in healthy men. Am J Physiol. 1997; 273: R1407-1413.
- Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004; 88: 61-67.
- Chen H, Ge RS, Zirkin BR. Leydig cells: From stem cells to aging. Mol Cell Endocrinol. 2009; 306: 9-16.
- Seftel AD, Mack RJ, Secrest AR, Smith TM. Restorative increases in serum testosterone levels are significantly correlated to improvements in sexual functioning. J Androl. 2004; 25: 963-972.
- Martínez-Jabaloyas JM, Queipo-Zaragozá A, Pastor-Hernández F, Gil-Salom M, Chuan-Nuez P. Testosterone levels in men with erectile dysfunction. BJU Int. 2006; 97: 1278-1283.
- Gray PB, Singh AB, Woodhouse LJ, Storer TW, Casaburi R, Dzekov J, et al. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. J Clin Endocrinol Metab. 2005; 90: 3838-3846.
- Greco EA, Spera G, Aversa A. Combining testosterone and PDE5 inhibitors in erectile dysfunction: basic rationale and clinical evidences. Eur Urol. 2006; 50: 940-947.
- Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006; 91: 4335-4343.
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010; 95: 2536-2559.
- Yeap BB, Hyde Z, Norman PE, Chubb SA, Golledge J. Associations of total testosterone, sex hormone-binding globulin, calculated free testosterone, and luteinizing hormone with prevalence of abdominal aortic aneurysm in older men. J Clin Endocrinol Metab. 2010; 95: 1123-1130.
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O'Neill TW. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010; 363: 123-135.
- Bassil N, Alkaade S, Morley JE. The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag. 2009; 5: 427-448.
- Gades NM, Jacobson DJ, McGree ME, St Sauver JL, Lieber MM, Nehra A, et al. The associations between serum sex hormones, erectile function, and sex drive: the Olmsted County Study of Urinary Symptoms and Health Status among Men. J Sex Med. 2008; 5: 2209-2220.
- Tsujimura A. The Relationship Between Testosterone Deficiency and Men’s Health. World J Mens Health. 2013; 31: 126-135.
- El-Sakka AI. Impact of the association between elevated oestradiol and low testosterone levels on erectile dysfunction severity. Asian J Androl. 2013; 15: 492-496.
- Laumann EO, Waite LJ. Sexual dysfunction among older adults: prevalence and risk factors from a nationally representative U.S. probability sample of men and women 57-85 years of age. J Sex Med. 2008; 5: 2300-2311.
- Buvat J, Maggi M, Gooren L, Guay AT, Kaufman J, Morgentaler A, et al. Endocrine aspects of male sexual dysfunctions. J Sex Med. 2010; 7: 1627-1656.
- Buvat J, Maggi M, Guay A, Torres LO. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J Sex Med. 2013; 10: 245-284.
- Nehra A, Jackson G, Miner M, Billups KL, Burnett AL, Buvat J, et al. The Princeton III Consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc. 2012; 87: 766-778.
- Lehtonen A, Huupponen R, Tuomilehto J, Lavonius S, Arve S, Isoaho H, et al. Serum testosterone but not leptin predicts mortality in elderly men. Age Ageing. 2008; 37: 461-464.
- Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Almeida OP, et al. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014; 99: E9-18.
- Hyde Z, Norman PE, Flicker L, Hankey GJ, Almeida OP, McCaul KA, et al. Low free testosterone predicts mortality from cardiovascular disease but not other causes: the Health in Men Study. J Clin Endocrinol Metab. 2012; 97: 179-189.
- Jankowska EA, Rozentryt P, Ponikowska B, Hartmann O, Kustrzyckα-Kratochwil D, Reczuch K, et al. Circulating estradiol and mortality in men with systolic chronic heart failure. JAMA. 2009; 301: 1892-1901.
- Tivesten A, Vandenput L, Labrie F, Karlsson MK, Ljunggren O, Mellström D, et al. Low serum testosterone and estradiol predict mortality in elderly men. J Clin Endocrinol Metab. 2009; 94: 2482-2488.
- Ohlsson C, Barrett-Connor E, Bhasin S, Orwoll E, Labrie F, Karlsson MK, et al. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J Am Coll Cardiol. 2011; 58: 1674-1681.
- Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007; 116: 2694-2701.
- Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008; 93: 68-75.
- Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010; 96: 1821-1825.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and metα-analysis. J Clin Endocrinol Metab. 2011; 96: 3007-3019.
- Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, Jamrozik K, et al. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009; 94: 2353-2359.
- Vlachopoulos C, Ioakeimidis N, Terentes-Printzios D, Aznaouridis K, Rokkas K, Aggelis A, et al. Plasma total testosterone and incident cardiovascular events in hypertensive patients. Am J Hypertens. 2013; 26: 373-381.
- van den Beld AW, Bots ML, Janssen JA, Pols HA, Lamberts SW, Grobbee DE. Endogenous hormones and carotid atherosclerosis in elderly men. Am J Epidemiol. 2003; 157: 25-31.
- Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002; 87: 3632-3639.
- Corona G, Rastrelli G, Monami M, Guay A, Buvat J, Sforza A, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a metα-analytic study. Eur J Endocrinol. 2011; 165: 687-701.
- Park BJ, Shim JY, Lee YJ, Lee JH, Lee HR. Inverse relationship between bioavailable testosterone and subclinical coronary artery calcification in non-obese Korean men. Asian J Androl. 2012; 14: 612-615.
- Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004; 109: 2074-2079.
- Page ST, Mohr BA, Link CL, O'Donnell AB, Bremner WJ, McKinlay JB. Higher testosterone levels are associated with increased high-density lipoprotein cholesterol in men with cardiovascular disease: Results from the Massachusetts Male Aging Study. Asian J Androl. 2008; 10: 193-200.
- Oskui PM, French WJ, Herring MJ, Mayeda GS, Burstein S, Kloner RA. Testosterone and the cardiovascular system: a comprehensive review of the clinical literature. J Am Heart Assoc. 2013; 2: e000272.
- Chin KY, Imα-Nirwana S. Sex steroids and bone health status in men. Int J Endocrinol. 2012; 2012: 208719.
- Lorentzon M, Swanson C, Andersson N, Mellström D, Ohlsson C. Free testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD Study. J Bone Miner Res. 2005; 20: 1334-1341.
- Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, Ulloor J, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab. 2010; 95: 2790-2799.
- Cawthon PM, Ensrud KE, Laughlin GA, Cauley JA, Dam TT, Barrett-Connor E, et al. Sex hormones and frailty in older men: the Osteoporotic Fractures in Men (MrOS) Study. J Clin Endocrinol Metab. 2009; 94: 3806-3815.
- Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SA, et al. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab. 2010; 95: 3165-3172.
- Carcaillon L, Blanco C, Alonso-Bouzón C, Alfaro-Acha A, Garciα-García FJ, Rodriguez-Mañas L. Sex differences in the association between serum levels of testosterone and frailty in an elderly population: The Toledo Study for Healthy Aging. PLoS One. 2012; 7: e32401.
- LeBlanc ES, Wang PY, Lee CG, Barrett-Connor E, Cauley JA, Hoffman AR, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab. 2011; 96: 3855-3863.
- Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry. 2008; 65: 283-289.
- Vodo S, Bechi N, Petroni A, Muscoli C, Aloisi AM. Testosterone-induced effects on lipids and inflammation. Mediators Inflamm. 2013; 2013: 183041.
- Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB. Association of sex hormones and C-reactive protein levels in men. Clin Endocrinol (Oxf). 2010; 72: 527-533.
- Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004; 279: 48487-48490.
- Bobjer J, Katrinaki M, Tsatsanis C, Lundberg Giwercman Y, Giwercman A. Negative association between testosterone concentration and inflammatory markers in young men: A nested cross-sectional study. PLoS One. 2013; 8: e61466.
- Helaly MA, Daoud E, El-Mashad N. Does the serum testosterone level have a relation to coronary artery disease in elderly men? Curr Gerontol Geriatr Res. 2011; 2011: 791765.
- Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004; 18: 357-368.
- Bogenhagen D, Clayton DA. The number of mitochondrial deoxyribonucleic acid genomes in mouse L and human HeLa cells. Quantitative isolation of mitochondrial deoxyribonucleic acid. J Biol Chem. 1974; 249: 7991-7995.
- Wickens AP. Ageing and the free radical theory. Respir Physiol. 2001; 128: 379-391.
- Harman D. Aging: overview. Ann N Y Acad Sci. 2001; 928: 1-21.
- Naskalski JW, Bartosz G. Oxidative modifications of protein structures. Adv Clin Chem. 2000; 35: 161-253.
- Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013; 1: 244-257.
- Beckman KB, Ames BN. Oxidative decay of DNA. J Biol Chem. 1997; 272: 19633-19636.
- Henle ES, Linn S. Formation, prevention, and repair of DNA damage by iron/hydrogen peroxide. J Biol Chem. 1997; 272: 19095-19098.
- Jacobs AT, Marnett LJ. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc Chem Res. 2010; 43: 673-683.
- Berneburg M, Grether-Beck S, Kürten V, Ruzicka T, Briviba K, Sies H, et al. Singlet oxygen mediates the UVA-induced generation of the photoaging-associated mitochondrial common deletion. J Biol Chem. 1999; 274: 15345-15349.
- Berneburg M, Lowe JE, Nardo T, Araújo S, Fousteri MI, Green MH, et al. UV damage causes uncontrolled DNA breakage in cells from patients with combined features of XP-D and Cockayne syndrome. EMBO J. 2000; 19: 1157-1166.
- Berneburg M, Plettenberg H, Medve-König K, Pfahlberg A, Gers-Barlag H, Gefeller O, et al. Induction of the photoaging-associated mitochondrial common deletion in vivo in normal human skin. J Invest Dermatol. 2004; 122: 1277-1283.
- Halliwell B, Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect. 1994; 102 Suppl 10: 5-12.
- Lieber CS. Herman Award Lecture, 1993: A personal perspective on alcohol, nutrition, and the liver. Am J Clin Nutr. 1993; 58: 430-442.
- Tai P, Ascoli M. Reactive Oxygen Species (ROS) play a critical role in the cAMP-induced activation of Ras and the phosphorylation of ERK1/2 in Leydig cells. Mol Endocrinol. 2011; 25: 885-893.
- Chang MS, Kim WN, Yang WM, Kim HY, Oh JH, Park SK. Cytoprotective effects of Morinda officinalis against hydrogen peroxide-induced oxidative stress in Leydig TM3 cells. Asian J Androl. 2008; 10: 667-674.
- Gautam DK, Misro MM, Chaki SP, Sehgal N. H2O2 at physiological concentrations modulates Leydig cell function inducing oxidative stress and apoptosis. Apoptosis. 2006; 11: 39-46.
- Chen H, Zhou L, Lin CY, Beattie MC, Liu J, Zirkin BR. Effect of glutathione redox state on Leydig cell susceptibility to acute oxidative stress. Mol Cell Endocrinol. 2010; 323: 147-154.
- Chen H, Pechenino AS, Liu J, Beattie MC, Brown TR, Zirkin BR. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old brown Norway rats. Endocrinology. 2008; 149: 2612-2619.
- Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003; 144: 2882-2891.
- Allen JA, Shankara T, Janus P, Buck S, Diemer T, Hales KH, et al. Energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology. 2006; 147: 3924-3935.
- Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008; 1: 15-24.
- Zhou L, Beattie MC, Lin CY, Liu J, Traore K, Papadopoulos V, Zirkin BR. Oxidative stress and phthalate-induced down-regulation of steroidogenesis in MA-10 Leydig cells. Reprod Toxicol. 2013; 42: 95-101.
- Othman AI, El-Missiry MA, Koriem KM, El-Sayed AA. Alfα-lipoic acid protects testosterone secretion pathway and sperm quality against 4-tert-octylphenol induced reproductive toxicity. Ecotoxicol Environ Saf. 2012; 81: 76-83.
- Chigurupati S, Son TG, Hyun DH, Lathia JD, Mughal MR, Savell J, Li SC. Lifelong running reduces oxidative stress and degenerative changes in the testes of mice. J Endocrinol. 2008; 199: 333-341.
- Luo L, Chen H, Trush MA, Show MD, Anway MD, Zirkin BR. Aging and the brown Norway rat Leydig cell antioxidant defense system. J Androl. 2006; 27: 240-247.
- Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004; 88: 61-67.
- Luo L, Chen H, Trush MA, Show MD, Anway MD, Zirkin BR. Aging and the brown Norway rat Leydig cell antioxidant defense system. J Androl. 2006; 27: 240-247.
- Mertens-Talcott SU, Jilmα-Stohlawetz P, Rios J, Hingorani L, Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum l.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem. 2006; 54: 8956-8961.
- Tzulker R, Glazer I, Bar-Ilan I, Holland D, Aviram M, Amir R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J Agric Food Chem. 2007; 55: 9559-9570.
- Gil MI, Tomás-Barberán FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000; 48: 4581-4589.
- Colombo E, Sangiovanni E, Dell'agli M. A review on the anti-inflammatory activity of pomegranate in the gastrointestinal tract. Evid Based Complement Alternat Med. 2013; 2013: 247145.
- Elfalleh W, Hannachi H, Tlili N, Yahia Y, Nasri N, Ferchichi A. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J Med Plants Res. 2012; 6: 4724-4730.
- Heber D. Pomegranate ellagitannins. Benzie IFF, Wachtel-Galor S, editors. 2nd edn. In: Herbal Medicine: Biomolecular and Clinical Aspects. CRC Press. 2011.
- Viladomiu M, Hontecillas R, Lu P, Bassaganyα-Riera J. Preventive and prophylactic mechanisms of action of pomegranate bioactive constituents. Evid Based Complement Alternat Med. 2013; 2013: 789764.
- Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee RP, et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007; 55: 7732-7737.
- Seeram NP, Henning SM, Zhang Y, Suchard M, Li Z, Heber D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J Nutr. 2006; 136: 2481-2485.
- Adams LS, Zhang Y, Seeram NP, Heber D, Chen S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev Res. 2010; 3: 108-113.
- Yoshida T, Amakura Y, Yoshimura M. Structural features and biological properties of ellagitannins in some plant families of the order Myrtales. Int J Mol Sci. 2010; 11: 79-106.
- Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013; 18: 1818-1892.
- Seeram NP, Zhang Y, McKeever R, Henning SM, Lee RP, Suchard MA, et al. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J Med Food. 2008; 11: 390-394.
- Vicinanza R, Zhang Y, Henning SM, Heber D. Pomegranate juice metabolites, ellagic acid and urolithin A, synergistically inhibit androgen-independent prostate cancer cell growth via distinct effects on cell cycle control and apoptosis. Evid Based Complement Alternat Med. 2013; 2013: 247504.
- Espín JC, Larrosa M, Garcíα-Conesa MT, Tomás-Barberán F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med. 2013; 2013: 270418.
- Gonzalez-Sarrías A, Giménez-Bastida JA, Garcíα-Conesa MT, Gómez-Sánchez MB, Garcíα-Talavera NV, Gil-Izquierdo A, et al. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res. 2010; 54: 311-322.
- Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, Seeram N. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006; 12: 4018-4026.
- Turkyilmaz M, Tagi S, Dereli U, Özkan M. Effects of various pressing programs and yields on the antioxidant activity, antimicrobial activity, phenolic content and colour of pomegranate juices. Food Chem. 2013; 138: 1810-1818.
- Wallace TC. Anthocyanins in cardiovascular disease. Adv Nutr. 2011; 2: 1-7.
- de Pascual-Teresa S, Moreno DA, Garcíα-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010; 11: 1679-1703.
- Mazza GJ. Anthocyanins and heart health. Ann Ist Super Sanita. 2007; 43: 369-374.
- Welch CR, Wu Q, Simon JE. Recent advances in anthocyanin analysis and characterization. Curr Anal Chem. 2008; 4: 75-101.
- Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008; 269: 378-387.
- Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture. USDA Database for the Proanthocyanidin Content of Selected Foods. Beltsville, MD: U.S. Department of Agriculture. 2006.
- Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005; 81: 230S-242S.
- Rein D, Lotito S, Holt RR, Keen CL, Schmitz HH, Fraga CG. Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status. J Nutr. 2000; 130: 2109S-2114S.
- Wang JF, Schramm DD, Holt RR, Ensunsa JL, Fraga CG, Schmitz HH, et al. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J Nutr. 2000; 130: 2115S-9S.
- Wan Y, Vinson JA, Etherton TD, Proch J, Lazarus SA, Kris-Etherton PM. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am J Clin Nutr. 2001; 74: 596-602.
- Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, et al. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem. 2008; 56: 1415-1422.
- Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010; 9: 3.
- Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010; 9: 3.
- Rosenblat M, Volkova N, Attias J, Mahamid R, Aviram M. Consumption of polyphenolic-rich beverages (mostly pomegranate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum's ability to attenuate macrophage cholesterol accumulation. Food Funct. 2010; 1: 99-109.
- Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005; 46: 1239-1247.
- Aviram M, Rosenblat M. Pomegranate protection against cardiovascular diseases. Evid Based Complement Alternat Med. 2012; 2012: 382763.
- Wang Y, Zhang H, Liang H, Yuan Q. Purification, antioxidant activity and protein-precipitating capacity of punicalin from pomegranate husk. Food Chem. 2013; 138: 437-443.
- Rosenblat M, Volkova N, Coleman R, Aviram M. Pomegranate byproduct administration to apolipoprotein E-deficient mice attenuates atherosclerosis development as a result of decreased macrophage oxidative stress and reduced cellular uptake of oxidized low-density lipoprotein. J Agric Food Chem. 2006; 54: 1928-1935.
- Shiner M, Fuhrman B, Aviram M. Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPAR gamma and AP-1 pathway activation. Atherosclerosis. 2007; 195: 313-321.
- Waly MI, Al-Rawahi AS, Al Riyami M, Al-Kindi MA, Al-Issaei HK, Farooq SA, et al. Amelioration of azoxymethane induced-carcinogenesis by reducing oxidative stress in rat colon by natural extracts. BMC Complement Altern Med. 2014; 14: 60.
- Kulkarni AP, Mahal HS, Kapoor S, Aradhya SM. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J Agric Food Chem. 2007; 55: 1491-1500.
- Shukla M, Gupta K, Rasheed Z, Khan KA, Haqqi TM. Bioavailable constituents/metabolites of pomegranate (Punica granatum L) preferentially inhibit COX2 activity ex vivo and IL-1ß-induced PGE2 production in human chondrocytes in vitro. J Inflamm. 2008; 5: 9.
- Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007; 109: 177-206.
- Elfalleh W, Tlili N, Nasri N, Yahia Y, Hannachi H, Chaira N, et al. Antioxidant capacities of phenolic compounds and tocopherols from Tunisian pomegranate (Punica granatum) fruits. J Food Sci. 2011; 76: C707-713.
- Ito H, Li P, Koreishi M, Nagatomo A, Nishida N, Yoshida T. Ellagitannin oligomers and a neolignan from pomegranate arils and their inhibitory effects on the formation of advanced glycation end products. Food Chem. 2014; 152: 323-330.
- Mena P, Vegara S, Martí N, Garcíα-Viguera C, Saura D, Valero M. Changes on indigenous microbiota, colour, bioactive compounds and antioxidant activity of pasteurised pomegranate juice. Food Chem. 2013; 141: 2122-2129.
- Fawole OA, Makunga NP, Opara UL. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement Altern Med. 2012; 12: 200.
- Aqil F, Munagala R, Vadhanam MV, Kausar H, Jeyabalan J, Schultz DJ, Gupta RC. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res Int. 2012; 49: 345-353.
- Chen B, Tuuli MG, Longtine MS, Shin JS, Lawrence R, Inder T, et al. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am J Physiol Endocrinol Metab. 2012; 302: E1142-E1152.
- Bialonska D, Kasimsetty SG, Khan SI, Ferreira D. Urolithins, intestinal microbial metabolites of Pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J Agric Food Chem. 2009; 57: 10181-10186.
- Rock W, Rosenblat M, Miller-Lotan R, Levy AP, Elias M, Aviram M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J Agric Food Chem. 2008; 56: 8704-8713.
- Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, et al. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem. 2001; 276: 44444-44449.
- Basu A, Newman ED, Bryant AL, Lyons TJ, Betts NM. Pomegranate polyphenols lower lipid peroxidation in adults with type 2 diabetes but have no effects in healthy volunteers: a pilot study. J Nutr Metab. 2013; 2013: 708381.
- Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R, et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000; 71: 1062-1076.
- Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, Dornfeld L, et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intimα-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004; 23: 423-433.
- Galvano F, La Fauci L, Vitaglione P, Fogliano V, Vanella L, Felgines C. Bioavailability, antioxidant and biological properties of the natural free-radical scavengers cyanidin and related glycosides. Ann Ist Super Sanita. 2007; 43: 382-393.
- Fukumoto LR, Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem. 2000; 48: 3597-3604.
- Kolodziej H, Kayser O, Kiderlen AF, Ito H, Hatano T, Yoshida T, et al. Proanthocyanidins and related compounds: Antileishmanial activity and modulatory effects on nitric oxide and tumor necrosis factor-α-release in the murine macrophage-like cell line RAW 264.7. Biol Pharm Bull. 2001; 24: 1016-1021.
- Noda Y, Kaneyuki T, Mori A, Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. J Agric Food Chem. 2002; 50: 166-171.
- Ichiyanagi T, Hatano Y, Matsugo S, Konishi T. Kinetic comparisons of anthocyanin reactivities towards 2,2'-azobis(2-amidinopropane) (AAPH) radicals, hydrogen peroxide and tert-buthylhydroperoxide by capillary zone electrophoresis. Chem Pharm Bull. 2004; 52: 434-438.
- Kano M, Takayanagi T, Harada K, Makino K, Ishikawa F. Antioxidative activity of anthocyanins from purple sweet potato, Ipomoera batatas cultivar Ayamurasaki. Biosci Biotechnol Biochem. 2005; 69: 979-988.
- Ding M, Feng R, Wang SY, Bowman L, Lu Y, Qian Y, et al. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J Biol Chem. 2006; 281: 17359-17368.
- Mazza G, Kay CD, Cottrell T, Holub BJ. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J Agric Food Chem. 2002; 50: 7731-7737.
- Mazza G, Kay CD. Bioactivity, absorption, and metabolism of anthocyanins. Lattanzio V, Daayf F, editors. In: Recent Advances in Polyphenols Research. Vol. I. Blackwell Publishing Ltd., 2008; 228-262.
- Spormann TM, Albert FW, Rath T, Dietrich H, Will F, Stockis JP, et al. Anthocyanin/polyphenolic-rich fruit juice reduces oxidative cell damage in an intervention study with patients on hemodialysis. Cancer Epidemiol Biomarkers Prev. 2008; 17: 3372-3380.
- Calderón AI, Wright BJ, Hurst WJ, van Breemen RB. Screening antioxidants using LC-MS: case study with cocoa. J Agric Food Chem. 2009; 57: 5693-5699.
- Sano A, Uchida R, Saito M, Shioya N, Komori Y, Tho Y, et al. Beneficial effects of grape seed extract on malondialdehyde-modified LDL. J Nutr Sci Vitaminol (Tokyo). 2007; 53: 174-182.
- Turk G, Ceribasi S, Sönmez M, Ciftçi M, Yüce A, Güvenç M, et al. Ameliorating effect of pomegranate juice consumption on carbon tetrachloride-induced sperm damages, lipid peroxidation, and testicular apoptosis. Toxicol Ind Health. 2013.
- Faria A, Monteiro R, Azevedo I, Calhau C. Pomegranate juice effects on cytochrome P450S expression: in vivo studies. J Med Food. 2007; 10: 643-649.
- Al-Olayan EM, El-Khadragy MF, Metwally DM, Abdel Moneim AE. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement Altern Med. 2014; 14: 164.
- Ceribasi AO, Sakin F, Türk G, Sönmez M, Atessahin A. Impact of ellagic acid on adriamycin-induced testicular histopathological lesions, apoptosis, lipid peroxidation and sperm damages. Exp Toxicol Pathol. 2012; 64: 717-724.
- Balunas MJ, Kinghorn AD. Natural compounds with aromatase inhibitory activity: an update. Planta Med. 2010; 76: 1087-1093.
- van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008; 9: 112-124.
- Coniglio JG, Grogan WM Jr, Rhamy RK. Lipids of human testes removed at orchidectomy. J Reprod Fertil. 1974; 41: 67-73.
- Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1968; 9: 570-579.
- Kobayashi T, Beuchat MH, Chevallier J, Makino A, Mayran N, Escola JM, et al. Separation and characterization of late endosomal membrane domains. J Biol Chem. 2002; 277: 32157-32164.
- Hayes LW, Jungalwala FB. Synthesis and turnover of cerebrosides and phosphatidylserine of myelin and microsomal fractions of adult and developing rat brain. Biochem J. 1976; 160: 195-204.
- Voelker DR. Disruption of phosphatidylserine translocation to the mitochondria in baby hamster kidney cells. J Biol Chem. 1985; 260: 14671-14676.
- Voelker DR. Phosphatidylserine translocation to the mitochondrion is an ATP-dependent process in permeabilized animal cells. Proc Natl Acad Sci U S A. 1989; 86: 9921-9925.
- Voelker DR. Characterization of phosphatidylserine synthesis and translocation in permeabilized animal cells. J Biol Chem. 1990; 265: 14340-14346.
- Omori T, Mihara H, Kurihara T, Esaki N. The distribution of phosphatidyl-D-serine in the rat. Biosci Biotechnol Biochem. 2010; 74: 1953-1955.
- Kuge O, Saito K, Nishijima M. Control of phosphatidylserine synthase II activity in Chinese hamster ovary cells. J Biol Chem. 1999; 274: 23844-23849.
- Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res. 2005; 44: 207-234.
- Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondriα-associated membranes. J Biol Chem. 2000; 275: 34534-34540.
- Vance JE, Tasseva G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim Biophys Acta. 2013; 1831: 543-554.
- Schenkel LC, Bakovic M. Formation and regulation of mitochondrial membranes. Int J Cell Biol. 2014; 2014: 709828.
- Osman C, Voelker DR, Langer T. Making heads or tails of phospholipids in mitochondria. J Cell Biol. 2011; 192: 7-16.
- Voelker DR. Organelle biogenesis and intracellular lipid transport in eukaryotes. Microbiol Rev. 1991; 55: 543-560.
- Garcia MC, Ward G, Ma YC, Salem N Jr, Kim HY. Effect of docosahexaenoic acid on the synthesis of phosphatidylserine in rat brain in microsomes and C6 glioma cells. J Neurochem. 1998; 70: 24-30.
- Kimura AK, Kim HY. Phosphatidylserine synthase 2: high efficiency for synthesizing phosphatidylserine containing docosahexaenoic acid. J Lipid Res. 2013; 54: 214-222.
- Sturbois-Balcerzak B, Stone SJ, Sreenivas A, Vance JE. Structure and expression of the murine phosphatidylserine synthase-1 gene. J Biol Chem. 2001; 276: 8205-8212.
- Rosadini G, Sannita WG, Nobili F, Cenacchi T. Phosphatidylserine: quantitative EEG effects in healthy volunteers. Neuropsychobiology. 1990; 24: 42-48.
- Martin OC, Pagano RE. Transbilayer movement of fluorescent analogs of phosphatidylserine and phosphatidylethanolamine at the plasma membrane of cultured cells. Evidence for a protein-mediated and ATP-dependent process(es). J Biol Chem. 1987; 262: 5890-5898.
- Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: Relation to shape changes. Proc Natl Acad Sci USA. 1984; 81: 3751-3755.
- Tsakiris S, Deliconstantinos G. Influence of phosphatidylserine on (Na+ + K+)-stimulated ATPase and acetylcholinesterase activities of dog brain synaptosomal plasma membranes. Biochem J. 1984; 220: 301-307.
- Nishijima M, Kuge O, Akamatsu Y. Phosphatidylserine biosynthesis in cultured Chinese hamster ovary cells. I. Inhibition of de novo phosphatidylserine biosynthesis by exogenous phosphatidylserine and its efficient incorporation. J Biol Chem. 1986; 261: 5784-5789.
- Daleke DL. Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res. 2003; 44: 233-242.
- Daleke DL. Phospholipid flippases. J Biol Chem. 2007; 282: 821-825.
- Yamaji-Hasegawa A, Tsujimoto M. Asymmetric distribution of phospholipids in biomembranes. Biol Pharm Bull. 2006; 29: 1547-1553.
- Sebastian TT, Baldridge RD, Xu P, Graham TR. Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim Biophys Acta. 2012; 1821: 1068-1077.
- Shiratsuchi A, Umeda M, Ohba Y, Nakanishi Y. Recognition of phosphatidylserine on the surface of apoptotic spermatogenic cells and subsequent phagocytosis by Sertoli cells of the rat. J Biol Chem. 1997; 272: 2354-2358.
- Nakanishi Y, Shiratsuchi A. Phagocytic removal of apoptotic spermatogenic cells by Sertoli cells: mechanisms and consequences. Biol Pharm Bull. 2004; 27: 13-16.
- Condorelli R, Calogero AE, La Vignera S. Relationship between testicular volume and conventional or nonconventional sperm parameters. Int J Endocrinol. 2013; 2013: 145792.
- Kawasaki Y, Nakagawa A, Nagaosa K, Shiratsuchi A, Nakanishi Y. Phosphatidylserine binding of class B scavenger receptor type I, a phagocytosis receptor of testicular Sertoli cells. J Biol Chem. 2002; 277: 27559-27566.
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995; 182: 1545-1556.
- Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 2003; 302: 1560-1563.
- Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J Biol Chem. 2001; 276: 1071-1077.
- Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001; 155: 649-659.
- Sambrano GR, Steinberg D. Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: Role of membrane phosphatidylserine. Proc Natl Acad Sci USA. 1995; 92: 1396-1400.
- Purdon AD, Rapoport SI. Energy requirements for two aspects of phospholipid metabolism in mammalian brain. Biochem J. 1998; 335: 313-318.
- Cooke GM. Identification and mechanism of action of phospholipids capable of modulating rat testicular microsomal 3β-hydroxysteroid dehydrogenase-isomerase activity in vitro. Biol Reprod. 1989; 41: 438-445.
- Papadopoulos V, Carreau S, Drosdowsky MA. Effect of phorbol ester and phospholipase C on LH-stimulated steroidogenesis in purified rat Leydig cells. FEBS Lett. 1985; 188: 312-316.
- Huang BX, Akbar M, Kevala K, Kim HY. Phosphatidylserine is a critical modulator for Akt activation. J Cell Biol. 2011; 192: 979-992.
- Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: A positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005; 102: 10858-10863.
- Takai Y, Kishimoto A, Iwasa Y, Kawahara Y, Mori T, Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979; 254: 3692-3695.
- Bocckino SB, Wilson PB, Exton JH. Phosphatidate-dependent protein phosphorylation. Proc Natl Acad Sci USA. 1991; 88: 6210-6213.
- Ree AH, Hansson V, Walaas SI, Eskild W, Taskén KA. Calcium/phospholipid-dependent protein kinases in rat Sertoli cells: Regulation of androgen receptor messenger ribonucleic acid. Biol Reprod. 1999; 60: 1257-1262.
- Shirai Y, Saito N. Activation mechanisms of protein kinase C: maturation, catalytic activation, and targeting. J Biochem. 2002; 132: 663-668.
- Rasmussen MK, Ekstrand B, Zamaratskaia G. Regulation of 3β-hydroxysteroid dehydrogenase/Δ⁵-Δ⁴ isomerase: a review. Int J Mol Sci. 2013; 14: 17926-17942.
- Cooke GM, Robaire B. Phospholipases modulate the rat testicular androgen biosynthetic pathway in vitro. Biol Reprod. 1988; 39: 329-339.
- Cooke GM, Robaire B. Modulation of epididymal delta 4-steroid 5 alphα-reductase activity in vitro by the phospholipid environment. J Biol Chem. 1985; 260: 7489-7495.
- Starks MA, Starks SL, Kingsley M, Purpura M, Jäger R. The effects of phosphatidylserine on endocrine response to moderate intensity exercise. J Int Soc Sports Nutr. 2008; 5: 11.
- Chaung HC, Chang CD, Chen PH, Chang CJ, Liu SH, Chen CC. Docosahexaenoic acid and phosphatidylserine improves the antioxidant activities in vitro and in vivo and cognitive functions of the developing brain. Food Chem. 2013; 138: 342-347.
- Kontush A, Chantepie S, Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler Thromb Vasc Biol. 2003; 23: 1881-1888.
- Camont L, Lhomme M, Rached F, Le Goff W, Nègre-Salvayre A, Salvayre R, et al. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: Relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler Thromb Vasc Biol. 2013; 33: 2715-2723.
- Food and Nutrition Board, Institute of Medicine. vitamin C. In: Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids: A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. National Academy of Sciences, National Academy Press, Washington DC. 2000; 95-185.
- Mandl J, Szarka A, Bánhegyi G. Vitamin C: Update on physiology and pharmacology. Br J Pharmacol. 2009; 157: 1097-1110.
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989; 86: 6377-6381.
- Iwata N, Okazaki M, Xuan M, Kamiuchi S, Matsuzaki H, Hibino Y. Orally administrated ascorbic acid suppresses neuronal damage and modifies expression of SVCT2 and GLUT1 in the brain of diabetic rats with cerebral ischemiα-reperfusion. Nutrients. 2014; 6: 1554-1577.
- Liu X, Zhang Y, Li J, Wang D, Wu Y, Li Y, et al. Cognitive deficits and decreased locomotor activity induced by single-walled carbon nanotubes and neuroprotective effects of ascorbic acid. Int J Nanomedicine. 2014; 9: 823-839.
- Lutsenko EA, Cárcamo JM, Golde DW. Vitamin C prevents DNA mutation induced by oxidative stress. J Biol Chem. 2002; 277: 16895-16899.
- Niki E. Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am J Clin Nutr. 1991; 54: 1119S-1124S.
- Stadtman ER. Ascorbic acid and oxidative inactivation of proteins. Am J Clin Nutr. 1991; 54: 1125S-1128S.
- Ueno M, Nyui M, Nakanishi I, Anzai K, Ozawa T, Matsumoto K, et al. Scavenging of reactive oxygen species induced by hyperthermia in biological fluid. J Clin Biochem Nutr. 2014; 54: 75-80.
- Wilson JX. The physiological role of dehydroascorbic acid. FEBS Lett. 2002; 527: 5-9.
- Hermsdorff HH, Barbosa KB, Volp AC, Puchau B, Bressan J, Zulet MÁ, et al. Vitamin C and fibre consumption from fruits and vegetables improves oxidative stress markers in healthy young adults. Br J Nutr. 2012; 107: 1119-1127.
- Romeu M, Aranda N, Giralt M, Ribot B, Nogues MR, Arija V. Diet, iron biomarkers and oxidative stress in a representative sample of Mediterranean population. Nutr J. 2013; 12: 102.
- Cao G, Russell RM, Lischner N, Prior RL. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J Nutr. 1998; 128: 2383-2390.
- Block G, Jensen CD, Morrow JD, Holland N, Norkus EP, Milne GL, et al. The effect of vitamins C and E on biomarkers of oxidative stress depends on baseline level. Free Radic Biol Med. 2008; 45: 377-384.
- KC S, Cárcamo JM, Golde DW. Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury. FASEB J. 2005; 19: 1657-1667.
- Moger WH. Uptake and release of ascorbic acid by rat Leydig cells in vitro. J Androl. 1987; 8: 398-402.
- Sönmez M, Türk G, Yüce A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology. 2005; 63: 2063-2072.
- Carr AC, Bozonet SM, Pullar JM, Simcock JW, Vissers MC. Human skeletal muscle ascorbate is highly responsive to changes in vitamin C intake and plasma concentrations. Am J Clin Nutr. 2013; 97: 800-807.
- Paidi MD, Schjoldager JG, Lykkesfeldt J, Tveden-Nyborg P. Chronic vitamin C deficiency promotes redox imbalance in the brain but does not alter sodium-dependent vitamin C transporter 2 expression. Nutrients. 2014; 6: 1809-1822.
- Karanth S, Yu WH, Walczewska A, Mastronardi CA, McCann SM. Ascorbic acid stimulates gonadotropin release by autocrine action by means of NO. Proc Natl Acad Sci U S A. 2001; 98: 11783-11788.
- Vijayprasad S, Bb G, Bb N. Effect of vitamin C on male fertility in rats subjected to forced swimming stress. J Clin Diagn Res. 2014; 8: HC05-08.
- Sen Gupta R, Sen Gupta E, Dhakal BK, Thakur AR, Ahnn J. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol Cells. 2004; 17: 132-139.
- Ayinde OC, Ogunnowo S, Ogedegbe RA. Influence of vitamin C and vitamin E on testicular zinc content and testicular toxicity in lead exposed albino rats. BMC Pharmacol Toxicol. 2012; 13: 17.
- Das UB, Mallick M, Debnath JM, Ghosh D. Protective effect of ascorbic acid on cyclophosphamide-induced testicular gametogenic and androgenic disorders in male rats. Asian J Androl. 2002; 4: 201-207.
- Ghosh D, Das UB, Misro M. Protective role of alphα-tocopherol-succinate (provitamin-E) in cyclophosphamide induced testicular gametogenic and steroidogenic disorders: A correlative approach to oxidative stress. Free Radic Res. 2002a; 36: 1209-1218.
- Ghosh D, Das UB, Ghosh S, Mallick M, Debnath J. Testicular gametogenic and steroidogenic activities in cyclophosphamide treated rat: A correlative study with testicular oxidative stress. Drug Chem Toxicol. 2002b; 25: 281-292.https://www.ncbi.nlm.nih.gov/pubmed/17188728
- Chang SI, Jin B, Youn P, Park C, Park JD, Ryu DY. Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicol Appl Pharmacol. 2007; 218: 196-203.
- Mukhopadhyay PK, Dey A, Mukherjee S, Pradhan NK. The effect of coadministration of α-tocopherol and ascorbic acid on arsenic trioxide-induced testicular toxicity in adult rats. J Basic Clin Physiol Pharmacol. 2013; 24: 245-253.
- Abarikwu SO, Iserhienrhien BO, Badejo TA. Rutin- and selenium-attenuated cadmium-induced testicular pathophysiology in rats. Hum Exp Toxicol. 2013; 32: 395-406.
- El-Maraghy SA, Nassar NN. Modulatory effects of lipoic acid and selenium against cadmium-induced biochemical alterations in testicular steroidogenesis. J Biochem Mol Toxicol. 2011; 25: 15-25.
- Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996; 93: 3704-3709.
- Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005; 81: 736-745.
- Douglas RM, Hemila H, Chalker E, Treacy B. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews 2007; CD000980.
- Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004; 140: 533-537.
- Massey LK, Liebman M, Kynast-Gales SA. Ascorbate increases human oxaluria and kidney stone risk. J Nutr. 2005; 135: 1673-1677.
- Taylor EN, Stampfer MJ, Curhan GC. Dietary factors and the risk of incident kidney stones in men: new insights after 14 years of follow-up. J Am Soc Nephrol. 2004; 15: 3225-3232.
- Porowski T, Zoch-Zwierz W, Wasilewska A, Spotyk A, Konstantynowicz J. Normative data on the Bonn Risk Index for calcium oxalate crystallization in healthy children. Pediatr Nephrol. 2007; 22: 514-520.
- Laube N, Hergarten S, Hoppe B, Schmidt M, Hesse A. Determination of the calcium oxalate crystallization risk from urine samples: the BONN Risk Index in comparison to other risk formulas. J Urol. 2004; 172: 355-359.
- Kavanagh JP, Laube N. Why does the Bonn Risk Index discriminate between calcium oxalate stone formers and healthy controls? J Urol. 2006; 175: 766-770.
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol. 1999; 10: 840-845.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J Urol. 1996; 155: 1847-1851.
- Lanman TH, Ingalls TH. Vitamin C deficiency and wound healing: an experimental and clinical study. Ann Surg. 1937; 105: 616-625.
- Bergman M, Salman H, Djaldetti M, Fish L, Punsky I, Bessler H. In vitro immune response of human peripheral blood cells to vitamins C and E. J Nutr Biochem. 2004; 15: 45-50.
- Johnston CS, Cox SK. Plasmα-saturating intakes of vitamin C confer maximal antioxidant protection to plasma. J Am Coll Nutr. 2001; 20: 623-627.
- Meydani SN, Barklund MP, Liu S, Meydani M, Miller RA, Cannon JG, et al. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr. 1990; 52: 557-563.
- Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997; 277: 1380-1386.
- Meydani SN, Meydani M, Rall LC, Morrow F, Blumberg JB. Assessment of the safety of high-dose, short-term supplementation with vitamin E in healthy older adults. Am J Clin Nutr. 1994; 60: 704-709.
- Meydani SN, Meydani M, Blumberg JB, Leka LS, Pedrosa M, Diamond R, et al. Assessment of the safety of supplementation with different amounts of vitamin E in healthy older adults. Am J Clin Nutr. 1998; 68: 311-318.
- Baker H, Handelman GJ, Short S, Machlin LJ, Bhagavan HN, Dratz EA, et al. Comparison of plasma α and γ tocopherol levels following chronic oral administration of either all-rac-α-tocopheryl acetate or RRR-α-tocopheryl acetate in normal adult male subjects. Am J Clin Nutr. 1986; 43: 382-387.
- Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, et al. Human plasma and tissue α-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998; 67: 669-684.
- Horwitt MK, Elliott WH, Kanjananggulpan P, Fitch CD. Serum concentrations of α-tocopherol after ingestion of various vitamin E preparations. Am J Clin Nutr. 1984; 40: 240-245.
- Jialal I, Grundy SM. Effect of combined supplementation with α-tocopherol, ascorbate, and β carotene on low-density lipoprotein oxidation. Circulation. 1993; 88: 2780-2786.
- Meydani SN, Han SN, Wu D. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol Rev. 2005; 205: 269-284.
- Rimbach G, Moehring J, Huebbe P, Lodge JK. Gene-regulatory activity of α-tocopherol. Molecules. 2010; 15: 1746-1761.
- Chen H, Liu J, Luo L, Baig MU, Kim JM, Zirkin BR. Vitamin E, aging and Leydig cell steroidogenesis. Exp Gerontol. 2005; 40: 728-736.
- Akazawa N, Mikami S, Kimura S. Effects of vitamin E deficiency on the hormone secretion of the pituitary-gonadal axis of the rat. Tohoku J Exp Med. 1987; 152: 221-229.
- Sönmez M, Yüce A, Türk G. The protective effects of melatonin and Vitamin E on antioxidant enzyme activities and epididymal sperm characteristics of homocysteine treated male rats. Reprod Toxicol. 2007; 23: 226-231.
- Umeda F, Kato K, Muta K, Ibayashi H. Effect of vitamin E on function of pituitary-gonadal axis in male rats and human subjects. Endocrinol Jpn. 1982; 29: 287-292.
- Manna I, Jana K, Samanta PK. Intensive swimming exercise-induced oxidative stress and reproductive dysfunction in male wistar rats: protective role of α-tocopherol succinate. Can J Appl Physiol. 2004; 29: 172-185.
- Manna I, Jana K, Samanta PK. Effect of different intensities of swimming exercise on testicular oxidative stress and reproductive dysfunction in mature male albino Wistar rats. Indian J Exp Biol. 2004; 42: 816-822.
- Jahan S, Zahra A, Irum U, Iftikhar N, Ullah H. Protective effects of different antioxidants against cadmium induced oxidative damage in rat testis and prostate tissues. Syst Biol Reprod Med. 2014a; 60: 199-205.
- Jahan S, Khan M, Ahmed S, Ullah H. Comparative analysis of antioxidants against cadmium induced reproductive toxicity in adult male rats. Syst Biol Reprod Med. 2014; 60: 28-34.
- Chandra AK, Chatterjee A, Ghosh R, Sarkar M. Vitamin E-supplementation protect chromium (VI)-induced spermatogenic and steroidogenic disorders in testicular tissues of rats. Food Chem Toxicol. 2010; 48: 972-979.
- El-Shenawy NS, Al-Harbi MS, Hamza RZ. Effect of vitamin E and selenium separately and in combination on biochemical, immunological and histological changes induced by sodium azide in male mice. Exp Toxicol Pathol. 2015; 67: 65-76.
- Elumalai P, Krishnamoorthy G, Selvakumar K, Arunkumar R, Venkataraman P, Arunakaran J. Studies on the protective role of lycopene against polychlorinated biphenyls (Aroclor 1254)-induced changes in StAR protein and cytochrome P450 scc enzyme expression on Leydig cells of adult rats. Reprod Toxicol. 2009; 27: 41-45.
- Krishnamoorthy G, Venkataraman P, Arunkumar A, Vignesh RC, Aruldhas MM, Arunakaran J. Ameliorative effect of vitamins (α-tocopherol and ascorbic acid) on PCB (Aroclor 1254) induced oxidative stress in rat epididymal sperm. Reprod Toxicol. 2007; 23: 239-245.
- Krishnamoorthy G, Selvakumar K, Venkataraman P, Elumalai P, Arunakaran J. Lycopene supplementation prevents reactive oxygen species mediated apoptosis in Sertoli cells of adult albino rats exposed to polychlorinated biphenyls. Interdiscip Toxicol. 2013; 6: 83-92.
- Murugesan P, Senthilkumar J, Balasubramanian K, Aruldhas MM, Arunakaran J. Impact of polychlorinated biphenyl Aroclor 1254 on testicular antioxidant system in adult rats. Hum Exp Toxicol. 2005; 24: 61-66.
- Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Studies on the protective role of vitamin C and E against polychlorinated biphenyl (Aroclor 1254)--induced oxidative damage in Leydig cells. Free Radic Res. 2005; 39: 1259-1272.
- Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Effects of vitamins C and E on steroidogenic enzymes mRNA expression in polychlorinated biphenyl (Aroclor 1254) exposed adult rat Leydig cells. Toxicology. 2007; 232: 170-182.
- Murugesan P, Balaganesh M, Balasubramanian K, Arunakaran J. Effects of polychlorinated biphenyl (Aroclor 1254) on steroidogenesis and antioxidant system in cultured adult rat Leydig cells. J Endocrinol. 2007; 192: 325-338.
- Murugesan P, Muthusamy T, Balasubramanian K, Arunakaran J. Polychlorinated biphenyl (Aroclor 1254) inhibits testosterone biosynthesis and antioxidant enzymes in cultured rat Leydig cells. Reprod Toxicol. 2008; 25: 447-454.
- Morikawa T, Yasuno R, Wada H. Do mammalian cells synthesize lipoic acid? Identification of a mouse cDNA encoding a lipoic acid synthase located in mitochondria. FEBS Lett. 2001; 498: 16-21.
- Hagen TM, Ingersoll RT, Lykkesfeldt J, Liu J, Wehr CM, Vinarsky V, et al. (R)-α-lipoic acid-supplemented old rats have improved mitochondrial function, decreased oxidative damage, and increased metabolic rate. FASEB J. 1999; 13: 411-418.
- Keith DJ, Butler JA, Bemer B, Dixon B, Johnson S, Garrard M, et al. Age and gender dependent bioavailability of R- and R,S-α-lipoic acid: a pilot study. Pharmacol Res. 2012; 66: 199-206.
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alphα-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009; 1790: 1149-1160.
- Gomes MB, Negrato CA. Alphα-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr. 2014; 6: 80.
- Hassan BH, Cronan JE. Protein-protein interactions in assembly of lipoic acid on the 2-oxoacid dehydrogenases of aerobic metabolism. J Biol Chem. 2011; 286: 8263-8276.
- Szeląg M, Mikulski D, Molski M. Quantum-chemical investigation of the structure and the antioxidant properties of α-lipoic acid and its metabolites. J Mol Model. 2012; 18: 2907-2916.
- Ruktanonchai U, Bejrapha P, Sakulkhu U, Opanasopit P, Bunyapraphatsara N, Junyaprasert V, et al. Physicochemical characteristics, cytotoxicity, and antioxidant activity of three lipid nanoparticulate formulations of α-lipoic acid. AAPS PharmSciTech. 2009; 10: 227-234.
- Wang Y, Dong W, Ding X, Wang F, Wang Y, Chen X, et al. Protective effect of α-lipoic acid on islet cells co-cultured with 3T3L1 adipocytes. Exp Ther Med. 2012; 4: 469-474.
- Wang L, Wu CG, Fang CQ, Gao J, Liu YZ, Chen Y, et al. The protective effect of α-Lipoic acid on mitochondria in the kidney of diabetic rats. Int J Clin Exp Med. 2013; 6: 90-97.
- Hagen TM, Liu J, Lykkesfeldt J, Wehr CM, Ingersoll RT, Vinarsky V, et al. Feeding acetyl-L-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci U S A. 2002; 99: 1870-1875.
- Feng B, Yan XF, Xue JL, Xu L, Wang H. The protective effects of α-lipoic acid on kidneys in type 2 diabetic Goto-Kakisaki rats via reducing oxidative stress. Int J Mol Sci. 2013; 14: 6746-6756.
- Jia L, Liu Z, Sun L, Miller SS, Ames BN, Cotman CW, et al. Acrolein, a toxicant in cigarette smoke, causes oxidative damage and mitochondrial dysfunction in RPE cells: protection by (R)-α-lipoic acid. Invest Ophthalmol Vis Sci. 2007; 48: 339-348.
- Goraca A, Skibska B. Beneficial effect of α-lipoic acid on lipopolysaccharide-induced oxidative stress in bronchoalveolar lavage fluid. J Physiol Pharmacol. 2008; 59: 379-386.
- Kim HY, Oi Y, Kim M, Yokozawa T. Protective effect of lipoic acid against methylglyoxal-induced oxidative stress in LLC-PK(1) cells. J Nutr Sci Vitaminol (Tokyo). 2008; 54: 99-104.
- Voloboueva LA, Liu J, Suh JH, Ames BN, Miller SS. (R)-α-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci. 2005; 46: 4302-4310.
- Akpinar D, Yargiçoğlu P, Derin N, Alicigüzel Y, Ağar A. The effect of lipoic acid on antioxidant status and lipid peroxidation in rats exposed to chronic restraint stress. Physiol Res. 2008; 57: 893-901.
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, Liu RM, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004; 101: 3381-3386.
- Bilska A, Wlodek L. Lipoic acid - the drug of the future? Pharmacol Rep. 2005; 57: 570-577.
- Inman DM, Lambert WS, Calkins DJ, Horner PJ. α-Lipoic acid antioxidant treatment limits glaucomα-related retinal ganglion cell death and dysfunction. PLoS One. 2013; 8: e65389.
- Padmalayam I, Hasham S, Saxena U, Pillarisetti S. Lipoic acid synthase (LASY): a novel role in inflammation, mitochondrial function, and insulin resistance. Diabetes. 2009; 58: 600-608.
- Motawi TM, Sadik NA, Refaat A. Cytoprotective effects of DL-α-lipoic acid or squalene on cyclophosphamide-induced oxidative injury: an experimental study on rat myocardium, testicles and urinary bladder. Food Chem Toxicol. 2010; 48: 2326-2336.
- Lebda M, Gad S, Gaafar H. Effects of lipoic acid on acrylamide induced testicular damage. Mater Sociomed. 2014; 26: 208-212.
- El-Beshbishy HA, Mariah RA, Al-Azhary NM, Aly HA, Ozbak HA, Baghdadi HH. Influence of lipoic acid on testicular toxicity induced by bi-n-butyl phthalate in rats. Food Chem Toxicol. 2014; 71: 26-32.
- El-Beshbishy HA, Aly HA, El-Shafey M. Lipoic acid mitigates bisphenol A-induced testicular mitochondrial toxicity in rats. Toxicol Ind Health. 2013; 29: 875-887.
- Nanjappa MK, Simon L, Akingbemi BT. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol Reprod. 2012; 86: 135, 1-12.
- Jana K, Samanta PK, Manna I, Ghosh P, Singh N, Khetan RP, et al. Protective effect of sodium selenite and zinc sulfate on intensive swimming-induced testicular gamatogenic and steroidogenic disorders in mature male rats. Appl Physiol Nutr Metab. 2008; 33: 903-914.
- Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, et al. Oral treatment with α-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006; 29: 2365-2370.
- Ziegler D, Hanefeld M, Ruhnau KJ, Hasche H, Lobisch M, Schütte K, et al. Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid: A 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. α-Lipoic Acid in Diabetic Neuropathy. Diabetes Care. 1999; 22: 1296-1301.
- Ametov AS, Barinov A, Dyck PJ, Hermann R, Kozlova N, Litchy WJ, et al. The sensory symptoms of diabetic polyneuropathy are improved with α-lipoic acid: the SYDNEY trial. Diabetes Care. 2003; 26: 770-776.
- Ho E, Ames BN. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFκ B, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc Natl Acad Sci U S A. 2002; 99: 16770-16775.
- Ho E, Courtemanche C, Ames BN. Zinc deficiency induces oxidative DNA damage and increases p53 expression in human lung fibroblasts. J Nutr. 2003; 133: 2543-2548.
- Aimo L, Oteiza PI. Zinc deficiency increases the susceptibility of human neuroblastoma cells to lead-induced activator protein-1 activation. Toxicol Sci. 2006; 91: 184-191.
- Sullivan JF, Jetton MM, Hahn HK, Burch RE. Enhanced lipid peroxidation in liver microsomes of zinc-deficient rats. Am J Clin Nutr. 1980; 33: 51-56.
- Powell SR. The antioxidant properties of zinc. J Nutr. 2000; 130: 1447S-54S.
- Mackenzie GG, Keen CL, Oteiza PI. Zinc status of human IMR-32 neuroblastoma cells influences their susceptibility to iron-induced oxidative stress. Dev Neurosci. 2002; 24: 125-133.
- Oteiza PI, Olin KL, Fraga CG, Keen CL. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J Nutr. 1995; 125: 823-829.
- Oteiza PI, Adonaylo VN, Keen CL. Cadmium-induced testes oxidative damage in rats can be influenced by dietary zinc intake. Toxicology. 1999; 137: 13-22.
- Zago MP, Oteiza PI. The antioxidant properties of zinc: interactions with iron and antioxidants. Free Radic Biol Med. 2001; 31: 266-274.
- McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci U S A. 2010; 107: 18838-18843.
- Cortese MM, Suschek CV, Wetzel W, Kröncke KD, Kolb-Bachofen V. Zinc protects endothelial cells from hydrogen peroxide via Nrf2-dependent stimulation of glutathione biosynthesis. Free Radic Biol Med. 2008; 44: 2002-2012.
- Cortese-Krott MM, Suschek CV, Wetzel W, Kröncke KD, Kolb-Bachofen V. Nitric oxide-mediated protection of endothelial cells from hydrogen peroxide is mediated by intracellular zinc and glutathione. Am J Physiol Cell Physiol. 2009; 296: C811-820.
- Feng W, Benz FW, Cai J, Pierce WM, Kang YJ. Metallothionein disulfides are present in metallothionein-overexpressing transgenic mouse heart and increase under conditions of oxidative stress. J Biol Chem. 2006; 281: 681-687.
- Omata Y, Salvador GA, Supasai S, Keenan AH, Oteiza PI. Decreased zinc availability affects glutathione metabolism in neuronal cells and in the developing brain. Toxicol Sci. 2013; 133: 90-100.
- Omu AE, Al-Azemi MK, Al-Maghrebi M, Mathew CT, Omu FE, Kehinde EO, et al. Molecular basis for the effects of zinc deficiency on spermatogenesis: An experimental study in the Sprague-Dawley rat model. Indian J Urol. 2015; 31: 57-64.
- Amara S, Abdelmelek H, Garrel C, Guiraud P, Douki T, Ravanat JL, et al. Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J Reprod Dev. 2008; 54: 129-134.
- Institute of Medicine. Selenium. In: Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press, Washington, DC. 2000; 284-324.
- Stadtman TC. Biosynthesis and function of selenocysteine-containing enzymes. J Biol Chem. 1991; 266: 16257-16260.
- Hatfield DL, Gladyshev VN. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2002; 22: 3565-3576.
- Burk RF, Hill KE, Motley AK. Selenoprotein metabolism and function: evidence for more than one function for selenoprotein P. J Nutr. 2003; 133: 1517S-20S.
- Diwadkar-Navsariwala V, Diamond AM. The link between selenium and chemoprevention: a case for selenoproteins. J Nutr. 2004; 134: 2899-2902.
- Gromer S, Eubel JK, Lee BL, Jacob J. Human selenoproteins at a glance. Cell Mol Life Sci. 2005; 62: 2414-2437.
- Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigó R, et al. Characterization of mammalian selenoproteomes. Science. 2003; 300: 1439-1443.
- Bermano G, Nicol F, Dyer JA, Sunde RA, Beckett GJ, Arthur JR, et al. Tissue-specific regulation of selenoenzyme gene expression during selenium deficiency in rats. Biochem J. 1995; 311 : 425-430.
- Sun QA, Wu Y, Zappacosta F, Jeang KT, Lee BJ, Hatfield DL, et al. Redox regulation of cell signaling by selenocysteine in mammalian thioredoxin reductases. J Biol Chem. 1999; 274: 24522-24530.
- Sun QA, Su D, Novoselov SV, Carlson BA, Hatfield DL, Gladyshev VN. Reaction mechanism and regulation of mammalian thioredoxin/glutathione reductase. Biochemistry. 2005; 44: 14528-14537.
- Vendeland SC, Beilstein MA, Yeh JY, Ream W, Whanger PD. Rat skeletal muscle selenoprotein W: cDNA clone and mRNA modulation by dietary selenium. Proc Natl Acad Sci U S A. 1995; 92: 8749-8753.
- Yeh JY, Beilstein MA, Andrews JS, Whanger PD. Tissue distribution and influence of selenium status on levels of selenoprotein W. FASEB J. 1995; 9: 392-396.
- Yeh JY, Vendeland SC, Gu Q, Butler JA, Ou BR, Whanger PD. Dietary selenium increases selenoprotein W levels in rat tissues. J Nutr. 1997; 127: 2165-2172.
- Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999; 13: 1169-1183.
- Beckett GJ, Arthur JR. Selenium and endocrine systems. J Endocrinol. 2005; 184: 455-465.
- Köhrle J, Jakob F, Contempré B, Dumont JE. Selenium, the thyroid, and the endocrine system. Endocr Rev. 2005; 26: 944-984.
- Klotz LO, Kröncke KD, Buchczyk DP, Sies H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J Nutr. 2003; 133: 1448S-51S.
- Saito Y, Yoshida Y, Akazawa T, Takahashi K, Niki E. Cell death caused by selenium deficiency and protective effect of antioxidants. J Biol Chem. 2003; 278: 39428-39434.
- May JM, Cobb CE, Mendiratta S, Hill KE, Burk RF. Reduction of the ascorbyl free radical to ascorbate by thioredoxin reductase. J Biol Chem. 1998; 273: 23039-23045.
- Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999; 20: 1657-1666.
- Behne D, Weiler H, Kyriakopoulos A. Effects of selenium deficiency on testicular morphology and function in rats. J Reprod Fertil. 1996; 106: 291-297.
- Erkekoglu P, Zeybek ND, Giray B, Asan E, Arnaud J, Hincal F. Reproductive toxicity of di(2-ethylhexyl) phthalate in selenium-supplemented and selenium-deficient rats. Drug Chem Toxicol. 2011; 34: 379-389.
- Erkekoglu P, Giray B, Rachidi W, Hininger-Favier I, Roussel AM, Favier A, et al. Effects of di(2-ethylhexyl)phthalate on testicular oxidant/antioxidant status in selenium-deficient and selenium-supplemented rats. Environ Toxicol. 2014; 29: 98-107.
- Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and met α-analysis. J Clin Endocrinol Metab. 2010; 95: 2560-2575.
- Marks LS, Mazer NA, Mostaghel E, Hess DL, Dorey FJ, Epstein JI, et al. Effect of testosterone replacement therapy on prostate tissue in men with late-onset hypogonadism: a randomized controlled trial. JAMA. 2006; 296: 2351-2361.
- Eaton NE, Reeves GK, Appleby PN, Key TJ. Endogenous sex hormones and prostate cancer: a quantitative review of prospective studies. Br J Cancer. 1999; 80: 930-934.