
Review Article
Ann Nutr Disord & Ther. 2015; 2(3): 1026.
Amino Acids, Glucose Metabolism and Clinical Relevance for Phenylketonuria Management
Pena MJ1, Rocha JC1,2,3 and Borges N3,4*
1Medical Genetics Center Doctor Jacinto de Magalhães, Portugal
2Faculty of Health Sciences, University Fernando Pessoa, Portugal
3Center for Health Technology and Services Research, Portugal
4Faculty of Sciences Nutrition and Food, University of Porto, Portugal
*Corresponding author: Nuno Borges; Faculty of Science, Nutrition and Food Science, University of Porto, Rua Dr. Roberto Frias, 4200-465 Porto, Portugal
Received: February 20, 2015; Accepted: August 12, 2015; Published: September 21, 2015
Abstract
It is general knowledge that glycaemia is affected by digested nutrients. Amino acids intake appears to be an important regulator in this regard. Many questions need to be answered, such as the real mediators of this response and the mechanisms underlying this metabolic behavior. Studies have been undertaken in order to investigate the role of amino acids on metabolic parameters. Their main findings suggest that the ingestion of free amino acids have a pivotal role in avoiding glycaemia excursions, improving glucose tolerance. In parallel, several important molecules for glucose metabolism have been exploited. Insulin and glucagon-like peptide – 1 (GLP-1) release seem to be the main triggers of this response. This insulinogenic effect is attributed to some amino acids, particularly the branched-chain amino acids (leucine, isoleucine and valine). GLP-1 may exert its effects by activating its receptor in pancreas and enhancing insulin release by β-cells or through its extrapancreatic actions. The mechanisms that may justify the aforementioned effects remain to be answered, being the mTOR pathway activation a possible key. These metabolic effects may have a special interest within the nutritional management of Phenylketonuria (PKU), an inborn metabolic disease of phenylalanine (Phe) catabolism. Since a Phe restricted diet is the mainstay of PKU treatment, a chronic supplementation with a Phe-free amino acid mixture is used. Although scientific evidence is scarce, it is hypothesized whether this chronic ingestion may modulate glycaemia.
Keywords: Amino acids; Glycaemia; Insulin; Glucagon-like peptide-1; Phenylketonuria
Abbreviations
GIP: Glucose-dependent Insulinotropic Polypeptide; GLP-1: Glucagon-Like Peptide-1; mTOR: Mammalian Target of Rapamycin; DPP-IV: Dipeptidyl Peptidase IV; PKU: Phenylketonuria; Phe: Phenylalanine; BH4: Tetrahydrobiopterin
Introduction
Glucose homeostasis, in a holistic perspective, is the set of responses that maintains the blood glucose levels within the physiological range. However, glucose homeostasis goes beyond the simple concept of glucose dynamics and implies the activation of different organic systems and the recruitment of several hormones [1]. Glucose as the prime fuel for some cells and tissues, such as brain, is a core molecule for human metabolism [2]. Along with this, it is foreseeable that impairment in glycaemia can result in diabetes, the paradigm of glucose metabolism disturbances. The long-term complications associated with these metabolic imbalances contribute to multi-organ injury, highlighting the importance of blood glucose management [2].
The effect of amino acids on glucose metabolism has been documented for years [3] but the mechanisms underlying this interaction are quite complex and not completely understood [4]. Amino acid intake may have a pivotal role on glucose homeostasis, exhibiting glucose-lowering effects [5,6] suggesting that amino acids intake with meals may mitigate glycemic fluctuations [7]. The explanation brought forward to elucidate this response is a higher insulin secretion, as studied either in humans [8] or animals [5]. Out of all essential amino acids, branched-chain amino acids (leucine, isoleucine and valine) [9], appear to act as potent insulin secretagogues, with special emphasis to leucine [10]. Furthermore, we should also keep in mind that nutrients, including amino acids, may modulate the synthesis of hormones at the gut level. These are capable of modulating glycaemia by boosting insulin release or through a direct effect, not closely associated with pancreatic metabolism [11]. These substances are called in cretins. The most recognized in cretins are Glucose-dependent Insulinotropic Polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) [12]. The crosstalk between amino acids, glucose, insulin and GLP-1 is illustrated on Figure 1.
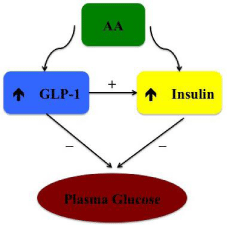
Figure 1: Interplay between amino acids, glucose, insulin and GLP-1. AA:
Amino Aids; GLP-1: Glucagon-Like Peptide-1.
Effect of amino acids on glucose metabolism
Several tissues regulate the glucose metabolism after nutrient intake. Liver and muscle tissue probably represent the best examples [13]. Blood glucose homeostasis results from the balance between the rate of plasma glucose appearance and its plasma clearance. The most important glucoregulatory hormones are insulin and glucagon produced in the pancreas, but also GLP-1 and GIP synthetized in the gut [14].
A growing body of evidence has demonstrated that a tight blood glucose control after a meal may benefit either type 2 diabetic patients [15] or healthy individuals. Particularly in this healthy group, it seems that a cardiovascular disease [16] and type 2 diabetes [17] risk reduction may occur.
Amino acids have been recognized to play a significant role in modulating blood glucose concentrations. Many studies have been performed in order to investigate in detail the effect of amino acids on glucose metabolism, both in humans and animals. Nilsson and colleagues (2007) carried out a study in healthy volunteers, where the ingestion of a drink containing five-amino acids (leucine, isoleucine, valine, lysine and threonine) displayed significantly lower blood glucose levels compared to a glucose drink, used as reference. Nevertheless, when the number of amino acids was reduced to two or three, the same trend was found, although without statistical significance [6]. In the same line, Gunnerud and colleagues (2012) argued that the ingestion of different protein sources by healthy subjects result in different postprandial blood glucose levels. During the study, fourteen healthy volunteers underwent a standard meal (ham sandwich) with either water or different pre-meal protein drinks. These pre-meal protein drinks were based on either whey or soy protein isolates, with or without addition of the five-amino acids (isoleucine, leucine, lysine, threonine and valine) or the five referred amino acids plus arginine. All protein drinks significantly reduced blood glucose concentrations compared to the reference meal. In addition, meals with whey protein appeared to be more effective in decreasing glycaemia than soy protein. Furthermore, this effect of reducing blood glucose levels was amplified by the co-ingestion of amino acids [18].
The study of Bernard and colleagues (2011), performed in Sprague-Dawley rats, was designed to investigate the effect of amino acid mixtures on the glucose response. The rats were gavaged with either glucose, glucose plus an amino acid mixture, glucose plus an amino acid mixture with increased leucine concentration or water. Blood glucose levels were significantly lower for the two experiments supplemented with amino acid mixtures compared to glucose. Likewise, the overall glucose area under the curve was lower after amino acid mixtures [5]. The same results were found by the study of Clemmensen and colleagues (2013), when an oral administration of L-arginine was performed to lean and obese mice [19].
Effect of amino acids on insulin secretion
Insulin is a key hormone for human metabolism. Absence of insulin results in severe metabolic disruptions, since its presence is essential for survival. This hormone is produced in and secreted from the β-cells of the Langerhans islets at the endocrine pancreas [20]. Insulin is secreted in a biphasic way, consisting in one transient phase, called the “first phase”, followed by the “second phase” that is more prolonged in time [20]. Beyond glucose itself, amino acids can also trigger insulin release [2,6,9] especially leucine, isoleucine, valine, lysine, threonine and arginine [6,19].
The reasons that may explain the insulin release by amino acids remain to be answered. Literature has pointed out some potential mechanisms, including generation of metabolic coupling factors, depolarization of the plasma membrane or enhancement of mitochondrial function. The activation of the mammalian target of rapamycin (mTOR) pathway in β-cell has been suggested to play an important role in this regard [21,22].
Effect of amino acids on GLP-1 secretion
Incretins are hormones synthesized in the gut after nutrient exposure, with paramount importance in glucose homeostasis [23]. The best-studied incretins regarding its influence on glucose metabolism are GLP-1 and GIP. GLP-1 is an incretin, produced in and secreted from L-cells, located in the gut, mainly in distal ileum and colon whereas GIP is produced in and released from gut K-cells [24]. They share many common actions directly at the pancreatic tissue, although their individual effects in other organs can be independent [25]. GLP-1 and GIP, both increase the magnitude of insulin release after food ingestion [23], even though GLP-1 appears to have a more pronounced incretin effect compared to GIP, mainly in type 2 diabetes [26,27]. The two biologically active forms of GLP- 1 are GLP-1 (7-37) amide and GLP-1 (7-36) amide and they are produced after post-translational processing of proglucagon. In vivo, GLP-1 (7-36) amide is the most abundant GLP-1, with a short halflife, less than two minutes due to rapid inactivation by the ubiquitous enzyme dipeptidyl peptidase IV (DPP-IV) [12].
As with insulin, GLP-1 is secreted in a biphasic manner, consisting in a short duration phase (10-15 minutes) followed by a more sustained phase (30-60 minutes) [28]. Outside of the pancreas, GLP-1 has receptors in multiple organs, such as brain, liver, muscle, adipose tissue, stomach and gut, exhibiting pleiotropic actions, which include regulation of food intake, gastric emptying and nutrient storage [12]. The existence of various target sites of GLP-1 biological actions illustrates the complexity of glucose regulation by GLP [29].
It is known that amino acids are capable of stimulating GLP-1 release. The study of Reimann and colleagues (2004) was carried out in order to investigate whether glutamine stimulates GLP-1 secretion from GLUTag cells in comparison with glucose and the results found by this research team showed that the amino acid glutamine acted as potent GLP-1 secretagogue [30]. Clemmensen and colleagues (2013) reported that an oral administration of L-arginine in lean and dietinduced obese mice increased plasma levels of insulin and GLP-1. They also studied the role of GLP-1 receptor signaling to understand its contribute for the expected results and found reduced effect of L-arginine to cause insulin secretion and improve glucose tolerance in GLP-1 receptor knockout mice [19].
The above-referred role of amino acids on GLP-1 secretion underpins the fact that some amino acids have a pronounced insulinogenic effect that may be partially mediated by incretin system activation.
Metabolic differences between free amino acids and whole protein
The molecular structure of proteins is critical for human metabolism and there is still debate regarding this matter. Nitrogen can be ingested in different molecular forms (free amino acids, peptides and proteins), which may affect the efficiency of its utilization [31]. The study carried out by Daenzer and colleagues (2001) sought to evaluate the effect of a diet containing casein or free amino acid mixture with a corresponding amino acid pattern to casein in some metabolic parameters. The assay revealed that the rats fed with free amino acid mixture had higher urinary nitrogen excretion and lower weight gain compared to those given the casein diet, showing that free amino acids are not preferentially targeted to protein synthesis. The diets were also enriched with labeled leucine and lysine and it was found that in rats fed with casein-bound leucine, leucine was used more efficiently than in rats fed free leucine, which resulted in a greater efficiency of incorporation into liver and plasma proteins. Kinetic differences were found regarding lysine, whose incorporation into liver proteins was higher in rats fed free amino acids. This study concluded that there are differences between intact protein and free amino acids regarding its metabolic use and the results cannot be extrapolated from one amino acid to another given the fact that different amino acids have different sites of catabolism [31]. A more recent study of Lacroix and colleagues (2006) was performed in healthy volunteers to compare the metabolic fate of different labeled milk products (casein, milk soluble protein isolate and total milk protein) regarding blood and urinary nitrogen pools. The main findings of this study were the higher but temporary hyperaminoacidemia observed in the group fed with milk soluble protein isolate in the postmeal period and the earlier transference of dietary nitrogen to urea and plasma amino acids in the same group as well, followed by casein and total milk protein groups. Taking into account these studies, it is important to mention the protein composition of milk products that consists of two major fractions, whey and casein. The amino acid profiles of soluble proteins and caseins are different, which is not the only difference between them, because during the digestive process, whey proteins are rapidly emptied from the stomach, whereas caseins precipitate, which delays amino acids supply to the gut. In addition, slowly digested proteins generate better utilization of nitrogen compared to rapidly digested proteins, because amino acids are released at different rates into blood stream [32].
This segmentation into rapid and slow proteins can be used by analogy for free amino acids versus whole protein.
Taking into consideration that protein and amino acids are wellknown insulin secretagogues, it will be expectable that the magnitude of the effect will be different too.
Metabolic impact of amino acids and its clinical relevance – The Phenylketonuria case
The metabolic effects of amino acids ingestion deserve a deep analysis considering its relevance for human nutrition and health. As described before, amino acid intake seems to be an important regulator of glucose metabolism, both in health and disease. Although their metabolic effects could be first directed mainly to diabetes management, other diseases can also benefit from a deeper knowledge. Within the scope of inherited metabolic diseases there is a sub-group which includes the metabolic disorders of amino acid catabolism [33]. The most common aminoacidopathy is Phenylketonuria (PKU) that is characterized by defects in the hepatic enzyme phenylalanine (Phe) hydroxylase or in its cofactor, tetrahydrobiopterin (BH4), causing the build-up of blood Phe [34]. The mainstay of PKU treatment is a low Phe diet, consisting in a natural protein restriction, supplemented with Phe-free amino acid mixtures and special low protein foods [35]. Adequate energy demands should be satisfied in these patients in order to promote amino acid anabolism, preventing the increased nitrogen excretion observed especially when amino acids are purely ingested [36,37]. Probably as a consequence of the chronic non-compliance of this and others nutritional recommendations, nutritional outcomes in diet treated patients are not always excellent [38]. Nevertheless, even assuming that synthetic diet characteristics may have an important impact on nutritional status, this approach has been classified as unequivocally successful in preventing the neurological disabilities [39]. According to Rocha and colleagues (2012), PKU patients showed a lower fasting plasma glucose levels compared to controls, despite an absence of differences on basal insulin concentrations or insulin resistance [40]. On the other hand, compared to intact milk protein, free amino acid mixtures intake, commonly used in PKU treatment, resulted in a significantly higher insulinemia peak at 30 minutes [37]. Altogether, this raises the question of whether regular ingestion of free amino acids in several meals throughout the day can determine at least in part some particular glucose behavior in patients with PKU.
Conclusion
Taken together, human and animal studies suggest that amino acids influence blood glucose responses. Deeper knowledge about this issue may bring insight into the management of PKU patients, besides the more immediate impact in the metabolism of individuals with diabetes.
References
- Grayson BE, Seeley RJ, Sandoval DA. Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci. 2013; 14: 24-37.
- Triplitt CL. Examining the mechanisms of glucose regulation. Am J Manag Care. 2012; 18: S4-10.
- Gannon MC, Nuttall FQ. Amino acid ingestion and glucose metabolism--a review. IUBMB Life. 2010; 62: 660-668.
- Iverson JF, Gannon MC, Nuttall FQ. Interaction of ingested leucine with glycine on insulin and glucose concentrations. J Amino Acids. 2014; 2014: 521941.
- Bernard JR, Liao YH, Hara D, Ding Z, Chen CY, Nelson JL, et al. An amino acid mixture improves glucose tolerance and insulin signaling in Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2011; 300: E752-760.
- Nilsson M, Holst JJ, Björck IM. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. The American journal of clinical nutrition. 2007; 85: 996-1004.
- Gunnerud U, Holst JJ, Östman E, Björck I. The glycemic, insulinemic and plasma amino acid responses to equi-carbohydrate milk meals, a pilot- study of bovine and human milk. Nutr J. 2012; 11: 83.
- Fajans SS, Floyd JC Jr, Knopf RF, Conn FW. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967; 23: 617-662.
- Floyd JC Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966; 45: 1487-1502.
- van Loon LJ. Leucine as a pharmaconutrient in health and disease. Curr Opin Clin Nutr Metab Care. 2012; 15: 71-77.
- Dardevet D, Moore MC, Neal D, DiCostanzo CA, Snead W, Cherrington AD. Insulin-independent effects of GLP-1 on canine liver glucose metabolism: duration of infusion and involvement of hepatoportal region. American Journal of Physiology-Endocrinology and Metabolism. 2004; 287: E75-E81.
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007; 132: 2131-2157.
- Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999; 48: 1198-1214.
- Aronoff SL, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectrum. 2004; 17: 183-190.
- Ceriello A, Colagiuri S. International Diabetes Federation guideline for management of postmeal glucose: a review of recommendations. Diabet Med. 2008; 25: 1151-1156.
- Ceriello A, Colagiuri S, Gerich J, Tuomilehto J. Guideline Development Group. Guideline for management of postmeal glucose. Nutr Metab Cardiovasc Dis. 2008; 18: S17-33.
- Goletzke J, Herder C, Joslowski G, Bolzenius K, Remer T, Wudy SA, et al. Habitually higher dietary glycemic index during puberty is prospectively related to increased risk markers of type 2 diabetes in younger adulthood. Diabetes care. 2013; 36: 1870-1876.
- Gunnerud UJ, Heinzle C, Holst JJ, Östman EM, Björck IM. Effects of pre-meal drinks with protein and amino acids on glycemic and metabolic responses at a subsequent composite meal. PLoS One. 2012; 7: e44731.
- Clemmensen C, Smajilovic S, Smith EP, Woods SC, Bräuner-Osborne H, Seeley RJ, et al. Oral L-arginine stimulates GLP-1 secretion to improve glucose tolerance in male mice. Endocrinology. 2013; 154: 3978-3983.
- Rorsman P, Eliasson L, Renström E, Gromada J, Barg S, Göpel S. The Cell Physiology of Biphasic Insulin Secretion. News Physiol Sci. 2000; 15: 72-77.
- Newsholme P, Brennan L, Bender K. Amino acid metabolism, ß-cell function, and diabetes. Diabetes. 2006; 55: S39-S47.
- Petzke KJ, Freudenberg A, Klaus S. Beyond the role of dietary protein and amino acids in the prevention of diet-induced obesity. Int J Mol Sci. 2014; 15: 1374-1391.
- Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007; 117: 24-32.
- Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013; 17: 819-837.
- Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008; 60: 470-512.
- Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987; 2: 1300-1304.
- Mentis N, Vardarli I, Köthe LD, Holst JJ, Deacon CF, Theodorakis M, Meier JJ. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes. 2011; 60: 1270-1276.
- Baggio LL, Drucker DJ. Clinical endocrinology and metabolism. Glucagon-like peptide-1 and glucagon-like peptide-2. Best Pract Res Clin Endocrinol Metab. 2004; 18: 531-554.
- Lamont BJ, Li Y, Kwan E, Brown TJ, Gaisano H, Drucker DJ. Pancreatic GLP-1 receptor activation is sufficient for incretin control of glucose metabolism in mice. J Clin Invest. 2012; 122: 388-402.
- Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004; 47: 1592-1601.
- Daenzer M, Petzke KJ, Bequette BJ, Metges CC. Whole-body nitrogen and splanchnic amino acid metabolism differ in rats fed mixed diets containing casein or its corresponding amino acid mixture. J Nutr. 2001; 131: 1965-1972.
- Lacroix M, Bos C, Léonil J, Airinei G, Luengo C, Daré S, et al. Compared with casein or total milk protein, digestion of milk soluble proteins is too rapid to sustain the anabolic postprandial amino acid requirement. Am J Clin Nutr. 2006; 84: 1070-1079.
- Saudubray JM, Berghe G, Walter JH. Inborn metabolic diseases. 5th edn. Springer. 2012.
- Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010; 376: 1417-1427.
- MacDonald A, Rocha JC, van Rijn M, Feillet F. Nutrition in phenylketonuria. Mol Genet Metab. 2011; 104 Suppl: S10-18.
- Mönch E, Herrmann ME, Brösicke H, Schöffer A, Keller M. Utilisation of amino acid mixtures in adolescents with phenylketonuria. Eur J Pediatr. 1996; 155 Suppl 1: S115-120.
- Weigel C, Rauh M, Kiener C, Rascher W, Knerr I. Effects of various dietary amino acid preparations for phenylketonuric patients on the metabolic profiles along with postprandial insulin and ghrelin responses. Annals of Nutrition and Metabolism. 2007; 51: 352-358.
- Enns GM, Koch R, Brumm V, Blakely E, Suter R, Jurecki E. Suboptimal outcomes in patients with PKU treated early with diet alone: revisiting the evidence. Mol Genet Metab. 2010; 101: 99-109.
- van Spronsen FJ. Phenylketonuria: a 21st century perspective. Nat Rev Endocrinol. 2010; 6: 509-514.
- Rocha JC, van Spronsen FJ, Almeida MF, Soares G, Quelhas D, Ramos E, et al. Dietary treatment in phenylketonuria does not lead to increased risk of obesity or metabolic syndrome. Mol Genet Metab. 2012; 107: 659-663.