
Perspective
Ann Nutr Disord & Ther. 2015; 2(3): 1028.
The Niemann-Pick C1 Gene Interacts with Dietary Lipids to Manifest Distinct Metabolic Phenotypes
Castillo JJ and Garver WS*
Department of Biochemistry and Molecular Biology, University of New Mexico Health Sciences Center, USA
*Corresponding author: Garver WS, Department of Biochemistry and Molecular Biology, School of Medicine, University of New Mexico Health Sciences Center, Albuquerque, New Mexico, USA
Received: September 29, 2015; Accepted: October 06, 2015; Published: October 08, 2015
Perspective
The worldwide prevalence of obesity and diabetes has increased dramatically prompting the World Health Organization to declare these metabolic diseases a global epidemic [1]. To investigate the molecular etiology of obesity and diabetes, a large number of Genome-Wide Association Studies (GWAS) have been performed resulting in the hypothesis-free identification of obesity and diabetes susceptibility genes. It is anticipated that understanding the function of these susceptibility genes and encoded proteins will provide mechanistic insight for future development of targeted medicinal and/or nutritional therapies. The objective of this perspective is to provide insight concerning the Niemann-Pick C1 (NPC1) gene and encoded protein as a model for future use of nutritional therapies to treat common metabolic diseases.
The human NPC1 gene represents one of the first susceptibility genes found to be associated with common obesity and/or diabetes [2-4]. These studies have been confirmed using our NPC1 mouse models with decreased gene dosage fed a high-fat diet that promotes metabolic disease phenotypes closely resembling human obesity and diabetes [5-8]. Without a doubt, the NPC1 protein represents a strong candidate for an obesity and diabetes susceptibility gene as a result of having a central role in maintaining cellular, tissue, and whole body lipid homeostasis. Moreover, studies have reported that the NPC1 gene is expressed in all tissues and that the encoded protein regulates the transport of Low Density Lipoprotein (LDL) derived lipids (cholesterol and fatty acids) from the lumen of lysosomes into the cytoplasm, where these lipids promote feedback inhibition of the Sterol Regulatory Element Binding Protein (SREBP) pathway and feed forward activation of the Liver X Receptor (LXR) pathways, thereby orchestrating the expression of many genes involved in maintaining energy balance [9-11]. Consistent with this important cellular function, our mouse model studies have revealed that the NPC1 gene is directly regulated by dietary lipids (cholesterol and fatty acids) to impart distinct metabolic disease phenotypes [12] (Figure 1). For example, the NPC1 gene is down regulated (at the transcriptional level) by dietary fatty acids, but not cholesterol, through feedback inhibition of the SREBP pathway and is associated with an increase in body weight and impaired glucose tolerance. In direct contrast, the NPC1 gene is also up regulated (at the posttranscriptional level) by dietary cholesterol, but not fatty acids, purportedly through inhibited ubiquitination of the NPC1 protein and targeting to proteasomes for degradation. In this latter case, the increased amount of NPC1 protein significantly improved glucose tolerance independent of body weight. Therefore, the increased consumption of specific dietary lipids (cholesterol or fatty acids) also known to serve as substrates for the NPC1 protein, has a profound effect on expression of the NPC1 gene and predisposition to distinct metabolic phenotypes.
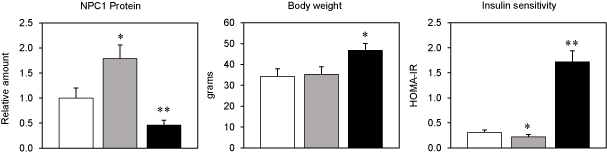
Figure 1: The effects of feeding wild-type C57BL/6J male mice one of three experimental diets until 30 weeks of age on the relative amounts of NPC1 protein,
body weight (grams), and glucose tolerance (HOMA-IR). Values are represented by the means ± SE; n = 9-10 mice. Asterisks represent a statistically significant
difference using Kruskal-Wallis 1-way ANOVA with P<0.05. White bars represent mice fed a low-fat diet with 0.00% cholesterol. Gray bars represent mice fed a lowfat
diet with 1.00% cholesterol. Black bars represent mice fed a high-fat diet (45% fat) with 0.02% cholesterol. HOMA-IR, homeostasis model assessment-insulin
resistance. This figure was adapted from our previous publication as indicated by reference [12].
In summary, the NPC1 gene represents one of at least a couple hundred susceptibility genes found to be associated with obesity and diabetes. For the vast majority of these genes neither the expression nor protein function has been characterized. We anticipate that further investigation into the expression of these genes and function of their encoded proteins will provide future development of targeted medicinal and/or nutritional therapies to more effectively treat obesity and diabetes, which together are known to represent major health care problems. As with the NPC1 gene, these studies will likely be performed using well defined mouse models followed by appropriate translational and clinical studies within ethnically diverse populations.
Acknowledgement
This article was supported by private donations through the University of New Mexico Foundation for the investigation of genetic and metabolic diseases.
References
- Caballero B. The global epidemic of obesity: An overview. Epidmiol Rev. 2007; 29: 1-5.
- Al-Daghri NM, Cagliani R, Forni D, Alokail MS, Pozzoli U, Alkharfy KM, et al. Mammalian NPC1 genes undergo positive selection and human polymorphisms associate with type 2 diabetes. BMC Med. 2012; 10: 140.
- Garver WS, de la Torre L, Brennan MC, Luo L, Jelinek D, Castillo JJ, et al. Differential association of Niemann-Pick C1 gene polymorphisms with maternal pre-pregnancy overweight and gestational diabetes. J Diabetes Obes. 2015; 2: 1-7.
- Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009; 41: 157-159.
- Jelinek D, Castillo JJ, Garver WS. The C57BL/6J Niemann-Pick C1 mouse model with decreased gene dosage has impaired glucose tolerance independent of body weight. Gene. 2013; 527: 65-70.
- Jelinek D, Castillo JJ, Heidenreich RA, Garver WS. The C57BL/6J Niemann-Pick C1 mouse model with decreased gene dosage is susceptible to increased weight gain when fed a high-fat diet: Confirmation of a gene-diet interaction. Gene. 2015; 568: 112-113.
- Jelinek D, Heidenreich RA, Erickson RP, Garver WS. Decreased NPC1 gene dosage in mice is associated with weight gain. Obesity. 2010; 18: 1457-1459.
- Jelinek D, Millward V, Birdi A, Trouard TP, Heidenreich RA, Garver WS. NPC1 haplo insufficiency promotes weight gain and metabolic features associated with insulin resistance. Hum Mol Genet. 2010; 20: 312-321.
- Garver WS, Jelinek D, Oyarzo JN, Flynn J, Zuckerman M, Krishnan K, et al. Characterization of liver disease and lipid metabolism in the Niemann-Pick C1 mouse. J Cell Biochem. 2007; 101: 498-516.
- Garver WS, Xie C, Repa JJ, Turley SD, Dietschy JM. Niemann-Pick C1 expression is not regulated by the amount of cholesterol flowing through cells in the mouse. J Lipid Res. 2005; 46: 1745-1754.
- Zhang J, Dudley-Rucker N, Crowley JR, Lopez-Perez E, Issandou M, Schaffer JE, et al. The steroidal analog GW707 activates the SREBP pathway through disruption of intracellular cholesterol trafficking. J Lipid Res. 2004; 45: 223-231.
- Jelinek D, Castillo JJ, Richardson LM, Luo L, Heidenreich RA, Garver WS. The Niemann-Pick C1 gene is down regulated in livers of C57BL/6J mice by dietary fatty acids, but not dietary cholesterol, through feedback inhibition of the SREBP pathway. J Nutr. 2012; 142: 1935-1942.