
Special Article - Malnutrition
Ann Nutr Disord & Ther. 2016; 3(1): 1030.
Detecting Severe Acute Malnutrition in Children under Five at Scale: The Challenges of Anthropometry to Reach the Missed Millions
Hammond W¹, Badawi AE² and Deconinck H³*
¹Public Health and Nutrition, USA
²Public Health and Nutrition, Sudan
³Health Research Institute and Society, Catholic University of Louvain, Belgium
*Corresponding author: Hedwig Deconinck, Health Research Institute and Society, Catholic University of Louvain, Clos Chapelle-aux-Champs, B3016 30, 1200 Brussels, Belgium
Received: August 30, 2016; Accepted: October 28, 2016; Published: October 31, 2016
Abstract
Objective: Severe Acute Malnutrition (SAM) interventions aim to detect and treat children at highest risk of death who benefit most from treatment. SAM services reach less than 20% of affected children worldwide, and innovative policy changes are needed to scale up services. This paper discusses anthropometry to diagnose SAM as one pathway to improve the effectiveness coverage of SAM services.
Results: WHO defines SAM by either MUAC <115 mm or WHZ <-3 or the presence of nutritional oedema. Both MUAC and WHZ are proxy indicators of a clinical condition, and neither is a gold standard. Because they measure different characteristics of the same illness, MUAC and WHZ identify different SAM populations that overlap differently in different contexts across and within countries. MUAC is a better predictor of mortality and has the practical advantages of simplicity, reliability and accuracy. Using both indicators independently identifies more children and loses sensitivity to risk of death.
Discussion and Conclusion: Based on current evidence and operational and policy considerations, using MUAC only for diagnosing SAM with a countryadapted cut-off could feasibly scale up SAM services and improve coverage to reach the millions of missed children. Meanwhile, continued research on the biomedical consequences and policy implications of this approach, as well as innovations such as system dynamics modeling, may contribute to the evidence.
Keywords: Anthropometry; Mid-upper arm circumference; Severe acute malnutrition; Weight-for-height z-score; Child malnutrition; Nutrition disorders; Child
Abbreviations
MUAC: Mid-Upper Arm Circumference; SAM: Severe Acute Malnutrition; UNICEF: United Nations Children’s Fund; WHZ: Weight-For-Height Z-Score; WHO: World Health Organization.
Introduction
Worldwide, Severe Acute Malnutrition (SAM) affects about 16 million children under 5 at any time [1] and kills over half a million annually [2]. SAM is a serious illness caused by inadequate food intake or absorption or by infection. SAM alters metabolism, weakening the immune system and making children more susceptible to illness and nine times more likely to die than well-nourished children [3]. Until 2000, few children with SAM were treated, in routine hospital care or temporary emergency centers often far from their homes. The advent of ready-to-use therapeutic food in the 1990s allowed treatment of uncomplicated SAM in decentralized primary care and made scaleup possible [4]. The global annual SAM treatment caseload grew from a few thousand in 2000 to over 3 million in 2014 in about 80 countries [5], but less than 20% of SAM children received care [5]. Scale-up has slowed, and accelerated change is required. One way to reach the millions of missed children with SAM more efficiently is to improve early detection of children at highest risk of death. This paper discusses the challenges of anthropometry in diagnosing SAM as one pathway to improve the effectiveness coverage of SAM services. The authors examined published literature, policies and practices on the use of Mid-Upper Arm Circumference (MUAC) and/or Weight-for- Height Z-Score (WHZ) to detect SAM for treatment.
Detecting sAM for treatment
The World Health Organization (WHO) defines SAM as MUAC less than (<) 115 mm or WHZ < minus 3 standard deviations of the median value of the 2006 WHO Child Growth Standards or the presence of bilateral pitting (nutritional) oedema [6]. Both MUAC and WHZ are proxy indicators of the clinical condition of SAM and are more practical in resource-poor environments than biomarkers such as hormonal and metabolic changes, but neither is a gold standard. Late detection of SAM or presentation for treatment increases the risk of medical complications requiring intensive inpatient care [7]. Early detection prevents development of complications and allows early start of treatment in primary healthcare, making treatment cheaper [7] and easier to scale up and integrate [8]. This improves health outcomes and maximizes effectiveness coverage (the proportion of children with SAM who recovered after treatment, the key outcome indicator of effective care) [9].
MUAC detects loss of subcutaneous fat and muscle mass in wasted children [10,11]. MUAC tapes are inexpensive, portable and easy to use with training and supervision [6]. Measurement requires placing the tape correctly and reading and recording measurements accurately. MUAC is influenced by age, sex and body composition of lean mass. MUAC <115mm in children 6-59 months detects younger children (with smaller arms than older children) and more girls (with smaller arms than boys) [11]. Child populations with more central fat, e.g., thin-fat phenotypes in South Asia, have lower MUAC readings than those with more peripheral fat [12] indicating MUAC’s sensitivity to fat distribution. Though using a single MUAC cut-off for all children 6-59 months has been questioned, as MUAC is age and gender dependent [13,14], using MUAC-for-age or MUAC-forheight did not improve predictive value of mortality [11,15].
WHZ is a composite indicator of weight relative to length (for children under 2) or height (for children over 2). Expressed in standard deviations, it describes how far and in what direction a child’s weight deviates from the median weight of a child of the same length or height in the WHO Child Growth Standards. WHZ requires good training and regular supervision and involves weighing and measuring length or height using scales and length/height boards that are not easily available and must be functional and calibrated for accuracy. In practice, children are rarely weighed naked, and measurements are often imprecise because the moving piece of the measuring board is incorrectly placed. WHZ also involves finding the intersection of height and weight on separate reference tables for boys and girls with either upper limits or ranges of cutoffs [16] and classifying nutritional status by z-scores. Because WHZ is more likely to identify boys than girls with SAM (the WHO SAM cutoff for girls at any given height is at a lower weight than for boys [17], so girls must have lower weight to be eligible for treatment) some researchers and policy makers recommended a unisex table based on the tables for boys to avoid discriminating against girls [18]. As WHZ compares ponderal growth with linear growth, child populations with larger heads, chests and abdomens and greater central body fat distribution [19], stunted growth or shorter legs generally have higher WHZ (or lower prevalence of wasting) [20,21]. Longer legs typically translate into lower WHZ (or higher prevalence of wasting), though linear growth reflects good health [22].
Since the first publications on MUAC [23] and WHZ [24] to diagnose malnutrition in pre-school children, their use has expanded and their popularity fluctuated. A first comparative study (1975) of anthropometric methods to diagnose ‘protein calorie malnutrition’ showed close agreement between the two indicators in the same population [24]. A major review of untreated SAM in the community found MUAC better at identifying children at risk of mortality [25]. Further comparative studies found MUAC less prone to error than WHZ [26], better able to detect children with nutritional oedema and as effective as WHZ in identifying SAM children with medical complications [26]. No difference was found in clinical and laboratory characteristics and treatment outcomes in children with SAM identified by either indicator [27]. A 2011 Kenya study recommended routine MUAC measurement as part of child hospital admissions in sub-Saharan Africa [28]. A 2013 Bangladesh study found that community health workers achieved 90% error-free case management of children identified with uncomplicated SAM by MUAC [29]. A 2015 Niger study found that mothers detected SAM in children using MUAC with good sensitivity and specificity [30]. A 2014 Kenya study found weight, length and height measurements reliable under controlled conditions but less so when combined into WHZ, risking failure to detect SAM [31]. A 2015 Bangladesh study found that WHZ misdiagnosed 12-14% of children with SAM, compared with only 1%-2% misdiagnosed by MUAC [32]. MUAC’s validity has been challenged [33] by comparing its sensitivity and positive predictive value to that of WHZ instead of mortality, mistakenly assuming that WHZ is the definitive standard for identifying SAM.
Different body shapes (which influences WHZ), stunting level and fat distribution (which influences MUAC) mean that MUAC and WHZ identify different children with SAM [11,19,26,34]. A 2016 study of anthropometric surveys from 47 countries found that on average, 52.7% of children were classified as SAM based on MUAC and 63.7% based on WHZ, with an overlap of 16.5% [35]. SAM prevalence based on either MUAC or WHZ and overlap of SAM child populations varied within and across countries, showing that the relationship between MUAC and WHZ was more complex than previously thought.
Improving effectiveness coverage of SAM services
Policy changes and innovative strategies are needed to break the deadlock of sustained low coverage and improve health outcomes when they are less severe and less costly to treat. Self-referral, active and routine detection of SAM by informed communities and health workers and better access to care are key to expanding coverage. Persistent barriers to treatment access, initiation, adherence and retention are understanding of the illness and treatment pathway, availability of services, transport costs, out-of-pocket expenditures and opportunity costs for carers [36], [37]. Improving SAM treatment access and uptake will increase coverage and reduce health costs and financial risk for carers. A 2015 Sierra Leone study found that using MUAC only for admission of SAM children significantly increased coverage (71%) over using WHZ (55%) and resulted in similar recovery rates (83% for MUAC and 71% for WHZ) [38].
Using MUAC only to detect SAM may improve coverage because of its ease of use, reliability and ability to identify children at risk of mortality. Its effectiveness has been studied in several contexts [39], and some countries have adopted it fully (Ghana, Somalia) or partially (Sierra Leone, South Sudan, Sudan). SAM detection and care based on MUAC enabled adding SAM to Integrated Community Case Management (iCCM) of diarrhoea, malaria and pneumonia in Bangladesh [29], Mali, Pakistan and South Sudan [40]. Moreover, MUAC only may reduce workload and improve planning and resource allocation [41], thus health outcomes. While MUAC only may detect SAM children at highest risk of death, arguments against such a policy underlined the risk of missing SAM children identified by WHZ who also have increased risk of death, although lower than by MUAC [28]. A counter-argument suggested increasing the MUAC cut-off to include more children with SAM identified by WHZ [42] who might otherwise be left out and would benefit from treatment. If SAM treatment capacity increases so that all cases of SAM identified by MUAC access treatment, then cases of SAM identified by WHZ that were not yet identified by MUAC should be targeted.
The challenge of scaling up SAM services, the case of sudan
The complexity of decision-making is illustrated by Sudan, which has a high burden of SAM and a weak health system. The government received support for SAM services in war-affected zones (about 20% of the country) [43] in 2015 expanded services to another 35% of health facilities with its own resources [44] but struggled to scale up further or integrate SAM case management into routine primary health care. The 2015-2017 national health plan proposed using MUAC only for SAM diagnosis and treatment [45] to accelerate scale-up [46] but faced resistance because of the lack of international guidance or clear evidence.
With 5.2 million children under 5 in 2010 and a SAM prevalence defined by WHZ of 5.3%, Sudan had an average of 275,600 children with SAM [47] and an annual caseload of 716,560 children (applying the global 1.6 incidence conversion factor) [48]. The real caseload was probably higher because the prevalence did not include SAM cases defined by MUAC or oedema and the incidence conversion facto was probably higher [49]. When applying the SAM treatment capacity in 2010 of 52,064 children [50], only one out of 14 children were admitted for treatment (coverage was probably lower because the caseload did not count children with SAM identified by either MUAC or oedema).
Sudan has diverse ethnic groups with different body shapes (e.g., pastoralist populations tend to have longer legs and hence higher sitting to standing height ration than agrarian populations). A 2016 study suggested that on average, the SAM population identified by either MUAC or WHZ overlaps by 20.4% and twice as many SAM children are identified by WHZ as by MUAC [35]. However, in 2013, North Darfur State had a SAM prevalence of 5.8% based on MUAC and 7.8% based on WHZ, while Kassala State had a SAM prevalence of 4.3% based on MUAC and 2.6% based on WHZ [51] (Figure 1), suggesting a different overlap of the two populations.
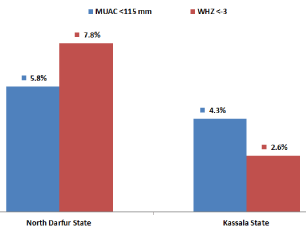
Figure 1: Diagnosis of SAM in children under 5 by MUAC and WHZ in two
states in Sudan, 2013.
Source: Federal Ministry of Health, 2013 [51].
The estimated SAM caseload based on population estimates in 2014 [52] and the prevalence rate from a 2013 survey, was 394,234 children identified by MUAC and 531,362 identified by WHZ [51]. The survey results did not indicate how much the SAM populations overlapped, but assuming 20.4% [35], 768,767 children would have been identified by either MUAC or WHZ (Figure 2). Thus, 51.3% of SAM children (394,234 with low MUAC/768,7677) were at higher risk of death. In 2014, Sudan’s SAM treatment capacity was 140,000 children. With the current policy and treatment capacity, one child in five (18.2% or 140,000/768,767) identified by either MUAC or WHZ (or oedema) accessed treatment. There was no guarantee that the one child who accessed treatment was identified with SAM by MUAC and thus was at higher risk of death. With, a MUAC only policy and the 2014 treatment capacity, one child in three (35.5% or 140,000/394/234) with a high risk of death would have accessed treatment.
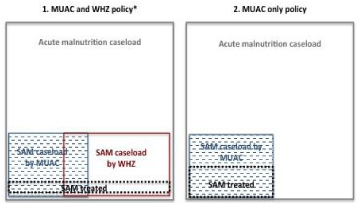
Figure 2: Annual caseloads of treated and untreated children with SAM in
Sudan, by diagnosis policy and treatment capacity, 2014.
The 2013 national survey identified 394,234 children with SAM by MUAC
<115 mm and 531, 362 by WHZ <–3. If we assume a 20.4% overlap of the
two populations, then 768,767 children were identified with SAM by either
MUAC or WHZ. SAM treatment capacity in 2014 was 140,000 children.
Calculation: (394, 234 + 531, 362) – (20.4/100 *X) = X, or 768,767 = X
Source: UNICEF, 2014 [47]; Federal Ministry of Health, 2013 [52].
Increasing SAM service coverage would require major increase in capacities and resources. A MUAC only policy would double coverage and save more lives by targeting children at highest risk of death. This example raises the question whether saving the lives of more serious SAM cases and treating less serious cases with less costly interventions (75% to 90% of SAM children may recover spontaneously [53]) may be more effective for overall health outcome.
Conclusion
This paper discussed the challenges of anthropometry in diagnosing SAM and ways to improve the effectiveness coverage of SAM. Innovative practices have been piloted to increase early detection and treatment of SAM children, but the clinical unknowns of the missed children resisted policy change. Simple and reliable anthropometry for early diagnosis could increase the number of children accessing treatment. But because of incomplete biomedical evidence, researchers are unlikely to agree soon on using MUAC only. This uncertainty is a serious barrier to decision-making for policy change to accelerate coverage of more effective, feasible and sustainable quality services at scale.
Based on current evidence and policy considerations, using MUAC only for diagnosis shows promise for closing the capacity gap to scale up services to reach missed children with SAM. Meanwhile, with so many children’s lives at risk, more and innovative research is needed on the biomedical consequences and policy implications of this approach. Effectiveness studies of SAM-specific health outcomes from implementation of various policies will fill in part of the picture.
Systems thinking can help explain why and how SAM policies work in complex and rapidly changing settings [54]. For example, mathematical modeling [55] and dynamic systems modeling over time [56] could compare the (cost-) effectiveness of different SAM policies in specific contexts. This paper calls for better understanding of the impacts of policy changes and for real-world decisions without making SAM children wait for treatment.
References
- United Nations Children's Fund, World Health Organization, World Bank Group. Levels and trends in child malnutrition. Joint child malnutrition estimates. Key findings of the 2015 edition. Geneva: WHO. 2015.
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013; 382: 427-451.
- United Nations Children's Fund, World Health Organization. WHO child growth standards and the identification of severe acute malnutrition in infants and children. Joint statement. Geneva: WHO. 2009.
- United Nations Children's Fund, UNSCN, World Food Programme, World Health Organization. Joint statement on community-based management of severe acute malnutrition. Geneva: WHO; 2007.
- United Nations Children's Fund. Nutri Dash 2014 global report. New York. UNICEF. 2014.
- World Health Organization. Guideline: updates on the management of severe acute malnutrition in infants and children. Geneva. WHO. 2013.
- Collins S, Dent N, Binns P, Bahwere P, Sadler K, Hallam A. Management of severe acute malnutrition in children. Lancet. 2006; 368: 1992-2000.
- Deconinck H, Hallarou ME, Pesonen A, Gerard JC, Criel B, Donnen P, et al. Understanding factors that influence the integration of acute malnutrition interventions into the national health system in Niger. Health Policy and Planning. 2016.
- Tanahashi T. Health service coverage and its evaluation. Bulletin of the World Health Organization. 1978; 56: 295-303.
- Briend A, Garenne M, Maire B, Fontaine O, Dieng K. Nutritional status, age and survival: the muscle mass hypothesis. European Journal of Clinical Nutrition. 1989; 43: 715-726.
- Myatt M, Khara T, Collins S. A review of methods to detect cases of severely malnourished children in the community for their admission into community-based therapeutic care programs. Food and Nutrition Bulletin. 2006; 27: S7-23.
- D'Angelo S, Yajnik CS, Kumaran K, Joglekar C, Lubree H, Crozier SR, et al. Body size and body composition: a comparison of children in India and the UK through infancy and early childhood. Journal of Epidemiology and Community Health. 2015; 69: 1147-1153.
- de Onis M, Yip R, Mei Z. The development of MUAC-for-age reference data recommended by a WHO Expert Committee. Bulletin of the World Health Organization. 1997; 75: 11-18.
- Fiorentino M, Sophonneary P, Laillou A, Whitney S, de Groot R, Perignon M, et al. Current MUAC cut-offs to screen for acute malnutrition need to be adapted to gender and age: The axample of Cambodia. PloS One. 2016; 11: e0146442.
- Garenne M, Maire B, Fontaine O, Briend A. Distributions of mortality risk attributable to low nutritional status in Niakhar, Senegal. The Journal of Nutrition. 2006; 136: 2893-2900.
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Methods and Development. Geneva. WHO. 2006.
- World Health Organization. Child growth standards: Arm circumference for age. WHO.
- Golden M, Grellety Y, Schwartz H, Tchibindat F. Report of a regional meeting to harmonise the criteria for monitoring and evaluation of the treatment of acute malnutrition in West and Central Africa. Dakar. UNICEF. 2010.
- Post CL, Victora CG. The low prevalence of weight-for-height deficits in Brazilian children is related to body proportions. The Journal of Nutrition. 2001; 131: 1290-1296.
- Myatt M, Duffield A, Seal A, Pasteur F. The effect of body shape on weight-for-height and mid-upper arm circumference based case definitions of acute malnutrition in Ethiopian children. Annals of Human Biology. 2009; 36: 5-20.
- Briend A, Khara T, Dolan C. Wasting and stunting--similarities and differences: policy and programmatic implications. Food and Nutrition Bulletin. 2015; 36: S15-823.
- Bogin B, Varela-Silva MI. Leg length, body proportion, and health: a review with a note on beauty. International Journal of Environmental Research and Public Health. 2010; 7: 1047-1075.
- Jelliffe DB. Arm circumference in children. Lancet. 1970; 1: 305-306.
- Osifo BO, Jegede IO, Omololu A. Evaluation of various methods in the detection of protein calorie malnutrition (PCM) of early childhood. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1975; 69: 221-225.
- Pelletier DL. The relationship between child anthropometry and mortality in developing countries: implications for policy, programs and future research. The Journal of Nutrition. 1994; 124: 2047s-2081s.
- Aguayo VM, Aneja S, Badgaiyan N, Singh K. Mid upper-arm circumference is an effective tool to identify infants and young children with severe acute malnutrition in India. Public Health Nnutrition. 2015; 18: 3244-3248.
- Isanaka S, Guesdon B, Labar AS, Hanson K, Langendorf C, Grais RF. Comparison of Clinical Characteristics and treatment outcomes of children selected for treatment of severe acute malnutrition using mid upper arm circumference and/or weight-for-height z-Score. PloS One. 2015; 10: e0137606.
- Mogeni P, Twahir H, Bandika V, Mwalekwa L, Thitiri J, Ngari M, et al. Diagnostic performance of visible severe wasting for identifying severe acute malnutrition in children admitted to hospital in Kenya. Bulletin of the World Health Organization. 2011; 89: 900-906.
- Puett C, Coates J, Alderman H, Sadler K. Quality of care for severe acute malnutrition delivered by community health workers in southern Bangladesh. Maternal and Child Nutrition. 2013; 9: 130-142.
- Blackwell N, Myatt M, Allafort-Duverger T, Balogoun A, Ibrahim A, Briend A. Mothers understand and can do it (MUAC): A comparison of mothers and community health workers determining mid-upper arm circumference in 103 children aged from 6 months to 5 years. Archives of Public Health = Archives belges de sante publique. 2015; 73: 26.
- Mwangome MK, Berkley JA. The reliability of weight-for-length/height z scores in children. Maternal & Child Nutrition. 2014; 10: 474-480.
- Modi P, Nasrin S, Hawes M, Glavis-Bloom J, Alam NH, Hossain MI, et al. Midupper arm circumference outperforms weight-based measures of nutritional status in children with diarrhea. The Journal of Nutrition. 2015; 145: 1582-1587.
- Tripathy J, Sharma A, Prinja S. Is mid-upper arm circumference alone sufficient to identify severe acute malnutrition correctly? Indian Pediatrics. 2016; 53: 166-167.
- Hop lT, Gross R, Sastroamidjojo S, Giay T, Schultink W. Mid-upper-arm circumference development and its validity in assessment of undernutrition. Asia Pacific Journal of Clinical Nutrition. 1998; 7: 65-69.
- Grellety E, Golden MH. Weight-for-height and mid-upper-arm circumference should be used independently to diagnose acute malnutrition: policy implications. BMC Nutrition. 2016; 2: 1.
- Jacobs B, Ir P, Bigdeli M, Annear PL, Van Damme W. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in low-income Asian countries. Health Policy and Planning. 2012; 27: 288-300.
- Rogers E, Myatt M, Woodhead S, Guerrero S, Alvarez JL. Coverage of community-based management of severe acute malnutrition programmes in twenty-one countries, 2012-2013. PloS One. 2015; 10: e0128666.
- Maust A, Koroma AS, Abla C, Molokwu N, Ryan KN, Singh L, et al. Severe and Moderate Acute Malnutrition Can Be Successfully Managed with an Integrated Protocol in Sierra Leone. The Journal of Nutrition. 2015; 145: 2604-2609.
- Emergency Nutrition Network, London School of Hygiene and Tropical Medicine, Save the Children, Irish Aid. Mid upper arm circumference and weight-for-height z-score as indicators of severe acute malnutrition: a consultation of operational agencies and academic specialists to understand the evidence, identify knowledge gaps and to inform operational guidance. Oxford: ENN; 2013.
- Friedman L, Wolfheim C. Linking nutrition and integrated community case management. A review of operational experiences. London: Children's Investment Fund Foundation, Save the Children. ACF. 2014.
- Deconinck H, Pesonen A, Hallarou M, Gerard JC, Briend A, Donnen P, et al. Challenges of estimating the annual caseload of severe acute malnutrition: The case of Niger. PloS One. 2016; 11: e0162534.
- Laillou A, Prak S, de Groot R, Whitney S, Conkle J, Horton L, et al. Optimal screening of children with acute malnutrition requires a change in current WHO guidelines as MUAC and WHZ identify different patient groups. PloS One. 2014; 9: e101159.
- United Nations Children's Fund. NutriDash global report New York: UNICEF. 2014.
- United Nations Children's Fund. UNICEF annual report 2014: Sudan. Khartoum. UNICEF. 2015.
- Government of Sudan Federal Ministry of Health. National plan for scaling up of CMAM in Sudan 2015- 2017 Khartoum. FMOH. 2014.
- Government of Sudan Federal Ministry of Health, Valid International, United Nation Children's Fund. Review of community management of acute malnutrition (CMAM). Khartoum. FMOH. 2013.
- Government of Sudan Federal Ministry of Health, Central Bureau of Statistics. Sudan household health survey second round 2010. Khartoum. Central Bureau of Statistics. 2011.
- United Nations Children's Fund. Management of severe acute malnutrition in children: Working towards results at scale. UNICEF programme guidance document. New York. UNICEF. 2015.
- Isanaka S, Grais RF, Briend A, Checchi F. Estimates of the duration of untreated acute malnutrition in children from Niger. American Journal of Epidemiology. 2011; 173: 932-940.
- United Nations Children's Fund. UNICEF annual report 2010: Sudan. Khartoum. UNICEF. 2011.
- Government of Sudan Federal Ministry of Health. Simple spatial surveying method (S3M) survey in Sudan. Khartoum. FMOH. 2013.
- Government of Sudan Central Bureau of Statistics. The total projected population of states for the period 2009 to 2018. Khartoum. Central Bureau of Statistics. 2014.
- Garenne M, Willie D, Maire B, Fontaine O, Eeckels R, Briend A, et al. Incidence and duration of severe wasting in two African populations. Public Health Nutrition. 2009; 12: 1974-1982.
- Adam T. Advancing the application of systems thinking in health. Health Research Policy and Systems / BioMed Central. 2014; 12: 50.
- Carrera C, Azrack A, Begkoyian G, Pfaffmann J, Ribaira E, O'Connell T, et al. The comparative cost-effectiveness of an equity-focused approach to child survival, health, and nutrition: A modelling approach. Lancet. 2012; 380: 1341-1351.
- Peters DH. The application of systems thinking in health: why use systems thinking? Health Research Policy and Systems / BioMed Central. 2014; 12: 51.