
Special Article - Vitamin D Deficiency
Ann Nutr Disord & Ther. 2016; 3(2): 1035.
Vitamin-D Deficiency and Male Reproduction
Taglianetti S, De Rocco Ponce M, Ghezzi M and Foresta C*
Department of Medicine, University of Padova, Italy
*Corresponding author: Foresta C, Department of Medicine, University of Padova, Unit of Andrology and Reproductive Medicine, Via Giustiniani, 2, 35128 Padova, Italy
Received: November 15, 2016; Accepted: December 22, 2016; Published: December 26, 2016
Abstract
Purpose: Vitamin D (VD) can be considered a functional steroid hormone with a well-established effect on musculoskeletal health. The purpose of this systematic review was to investigate the role of vitamin D in male reproduction, presenting current evidence from experimental animal and human studies. The basis of this interplay lays on the presence of Vitamin D-Receptor (VDR) and Vitamin D (VD) metabolizing enzymes in testis and male reproductive tract.
Methods: Using PubMed, we searched for publications during the last 30 years that investigated the role of vitamin D in male reproduction.
Results: Evidence from animal and human studies suggests a possible role of vitamin D in male reproduction. Epidemiological studies suggest a positive association between 25-hydroxy-vitamin D [25(OH)D] and semen parameters and androgen levels. On the other hand, several studies reported that high vitamin D levels may have a negative effect on gonadal function.
Conclusions: Further large prospective studies are warranted to prove a casual relationship between vitamin D and male reproduction and the impact of vitamin D supplementation on gonadal function.
Keywords: Vitamin D; Androgens; Fertility; Male reproduction; Sperm; Supplementation
Abbreviations
1a,25(OH)2D3: 1alpha,25-Dihydroxyvitamin D3; BMD: Bone Mineral Density; BMI: Body Mass Index; CYP24A1: 24R-Hydroxylase; CYP27B1: 1a-Hydroxylase; CYP2R1: 25-Hydroxylase; FSH: Follicle Stimulating Hormone; GPRC6A: G-Protein Coupled Receptor Family C Group 6 Member A; HCG: Human Chorionic Gonadotropin; INSL3: Insulin-Like 3; LH: Luteinizing Hormone; OC: Osteocalcin; 25(OH)D: 25-Hydroxyvitamin D; PKA: Protein Kinase A; PKC: Protein Kinase C; RXR: Retinoid X Receptor; SHBG: Sex Hormone- Bindng Globuline; SOCE: Store-Operated Calcium Entry; TRPV6: Transient Receptor Potential Cation Channel Subfamily V Member 6; ucOC: uncarboxylated-OC; VD: Vitamin D; VDR: Vitamin D Receptor; VDREs: Vitamin D-Responsive Elements.
Introduction
Vitamin D (VD) is a versatile signaling molecule that could be properly considered a functional steroid hormone. Currently, there is great interest in VD for its possible “non-classical” effects in addition to the well-known role on bone metabolism, especially on male gonadal function [1,2].
Vitamin D synthesis and role
In humans the VD status is mainly determined by ultraviolet-B radiation of the skin, while VD intake by nutrition and supplements plays only a minor role [3].
Thereafter, VD is hydroxylated at the C25 position of the side chain. This hydroxylation takes place in the liver and other tissues such as testes by 25-hydroxylase (identified as CYP2R1 or CYP27A1). The product of this reaction is 25-hydroxyvitamin D [25(OH) D], which is commonly used to evaluate VD levels. 1alpha,25- dihydroxyvitamin D3 [1a,25(OH)2D3], is the active metabolite obtained by 1a-hydroxylase (CYP27B1) from 25(OH)D in the kidneys, as well as in other tissues including human testis [4].
The broad biological actions of VD which involve the regulation of about 3% of the human genome are mediated through the Vitamin D Receptor (VDR) [1].
The VDR acts as a transcription factor binding to Vitamin D Responsive Elements (VDREs) in the promoter region of target genes after forming a VDR-RXR heterocomplex with the Retinoid X Receptor (RXR) [5]. The VDR is almost ubiquitously expressed in human cells, suggesting an endocrine role of the VD [3,6,7]. In the kidney, 25(OH)D and 1a,25(OH)2D3 are catabolized by 24R-hydroxylase (identified as CYP24A1) to 24R,25(OH)2D3 and 1-a 24R,25(OH)3D3, respectively [8] (Figure 1).
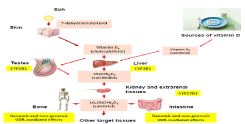
Figure 1: Vitamin D metabolism.
- Most studies both in animals and humans showed an association between low vitamin D levels and impaired gonadal function. Infertile subjects with low vitamin D levels may then beneficiate from a vitamin D supplementation.
- Some studies showed a possible association between high levels of vitamin D and impaired gonadal function, so indiscriminate vitamin D supplementation is not suggested.
- Keeping in mind the hydroxylation function of Leydig cells, subjects with low vitamin D levels and impaired testicular function should be better treated with activated vitamin D formulation (i.e. 25(OH) vitamin D).
Materials and Methods
We performed a systematic review of the literature by searching in Pubmed for relevant English language papers published until November 2016. We used the following search terms: ‘vitamin D’ and ‘fertility’, ‘vitamin D’ and ‘male reproduction’, ‘vitamin D’ and ‘infertility’. In addition, we also used the search terms ‘25-hydroxyvitamin D (25(OH)D)’, ‘1,25-dihydroxyvitamin D’, and ‘calcitriol’ instead of vitamin D. We also used listed references from selected articles to expand the search. We excluded editorials, case reports, and letters to editors, duplicate publications and studies pertaining to female subjects. The results of original articles up to 2016 have been summarized and discussed in a critical manner.
Vitamin D hypovitaminosis and male gonadal function
Hypovitaminosis D (<30 ng/ml) in the general population is very prevalent. It has been estimated that 20-100% of U.S., Canadian, and European elderly men and women still living in the community are VD deficient (<30 ng/ml) [3,9-12], while 32% of healthy students and physicians at a Boston hospital showed 25(OH)D below 20 ng/ml [13, 14]. VD deficiency has been linked to various health disorders including bone, cardiovascular, infectious, oncologic, musculoskeletal, neuropsychologic and reproductive disorders, as well as to overall mortality [15-23]. While the role of VD deficiency in reduced bone mass is evident, its relation to other health disorders is subject of debate.
Accumulating evidence from animal and human studies suggests that VD is involved in reproductive function in both genders. It has been shown that the VDR and the VD metabolizing enzymes are expressed in reproductive organs [2,24]. They are concomitantly expressed, in males, in Sertoli cells, germ cells, Leydig cells, mature spermatozoa and in the epithelial cells lining the male reproductive tract [2,4,25]. There is increasing literature supporting the existence of a complex relation between VD and androgen metabolism. For example, data demonstrate that androgens can increase 1-alphahydroxylase [26]. Moreover, the regulation of gene expression by VD metabolites can be modified depending on androgen levels [27]. VD has probably a role in the developing gonad, considering that VDR is early expressed in human gonocytes, Leydig and immature Sertoli cells from gestational week 16 [28].
VD has widespread biological functions, including an essential role for systemic calcium homeostasis [1]. Optimal sperm function may thus depend on a direct effect of VD or be indirectly influenced through calcium homeostasis, which is known to play a role in male reproductive function [29,30,31].
On the other hand, the role for calcium in the maturation of human spermatozoa is well documented and highlighted by the 2–3- fold higher calcium concentration in human epididymal and prostate fluid compared with serum [4,32].
Similarly to what happens in the kidney, which shares with the male reproductive tract a common development origin, VD in the epididymis and efferent ducts may regulate transcellular calcium transport through action of TRPV6. This membrane calcium channel is expressed in the epididymis and its ablation may compromise calcium absorption, resulting in impaired sperm motility and infertility in mice [33].
Moreover, Blomberg Jensen and colleagues have shown in vitro that in ejaculated mature sperm, activated VD (1a,25(OH)2D3) increased the intracellular calcium concentration through Inositol Trisphosphate (IP3)-mediated calcium-release from an intracellular IP3-receptor-gated calcium store in the neck of human spermatozoa, then increasing sperm motility and inducing the acrosome reaction [34]. One possible mechanism that links the extracellular calcium with intracellular calcium was proposed by Blomberg Jensen [4]. Blomberg Jensen speculates the existence of a “Store-Operated Calcium Entry” (SOCE) in the neck of human spermatozoa. SOCE is a mechanism in which the depletion of calcium from the intracellular stores induces calcium entry from the extracellular space. Based on Blomberg Jensen’s hypothesis it is possible that hypovitaminosis D impairing calcium release from intracellular stores can affect SOCE and the whole mechanism of calcium regulation. The final consequence may be a reduced motility and impaired acrosome reaction (Figure 2).
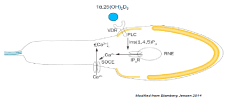
Figure 2: 1a, 25-dihydroxyvitamin D3 activates VDR in the neck region of
spermatozoa and determines inositol trisphosphate (IP3)-mediated calciumrelease
from an intracellular IP3- receptor-gated calcium store resulting in
increase of intracellular calcium-release from RNE activates SOCE resulting
in calcium entry from the extracellular space.
Abbreviations: [Ca2+]: Intracellular concentration of Calcium Ions; PLC:
Phospholipase C; RNE: Redundant Nuclear Envelope; SOCE: Store-
Operated Calcium Entry; VDR: Vitamin D Receptor.
Another possible indirect effect of VD on male fertility may depend on its central regulation of male reproductive function. In fact, VDR and 1a-hydroxylase expression have been showed in neurons, glial cells, substantia nigra, human pituitary samples and hypothalamus. Several data have shown a relevant interaction between VD and Pituitary Transcription Factor-1 (Pit-1), which is involved in the development of the anterior pituitary gland and influences growth hormone (GH) and prolactin gene expression [35-38].
Influence of Hypovitaminosis D in Male Gonadal Function: Animal Experimental Data
Experimental data support a dual way of action of VD in testis. Actually, VD achieves its effects either through a genomic pathway or through a no-genomic pathway involving PKA and PKC or calcium/ potassium channels in the plasma membrane [39-40]. The cyclic AMP/PKA complex acts as a mediator of 1a,25(OH)2D3 in both genomic and no-genomic effects [41]. It has been shown that VD deficiency in rodents leads to reduced sperm counts, impaired sperm motility and lower fertility rates in females inseminated with semen from VD deficient males [42].
Moreover, VDR knockout mice present with gonadal insufficiency, with decreased sperm count and motility and histological abnormalities of the testis as well as high LH and FSH levels that may indicate the presence of a testicular damage. The explanation remains unclear but data show a possible pathophysiological mechanism in these mice which involves a reduced gonadal aromatase activity and gene expression in the testis and epididymis compared with the wild-type ones [6]. This reduced aromatase activity may in part explain the gonadal abnormalities in mice lacking the VDR if we consider the importance of estrogens for testicular function including steroidogenesis.
VD might also influence the male reproductive functions by stimulating Sertoli cell secretory functions. In fact, Menegaz et al have suggested a PKA/PKC-dependent 1a,25(OH)2D3-VDR nongenotropic pathway leading to Cl- -channel and exocytosis activation in immature Sertoli cells thus promoting its release of ions, proteins and growth factors relevant to germ cell maturation [43] (Figure 3).
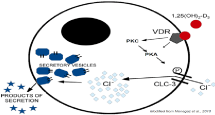
Figure 3: Chloride Channel-3 (ClC-3) opening in the membrane of secretory
vesicles is required for exocytosis. 1,25(OH)2-D3 may enhance ClC-3
phosphorylation and opening via a non-genomic pathway involving protein
kinase C (PKC)/protein kinase A (PKA) activation.
The influence of VD on male reproduction might be mediated by calcium levels, since the impaired fertility in some animal models was partly restored by normalization of serum calcium levels [31,44]. Furthermore Sun and colleagues, employing a 1a-hydroxylase-/- mouse model, have recently showed that sperm count, motility, histological structure of testis, and spermatogenesis can depend on calcium and phosphorus levels rather than VD. In fact, these hypocalcemic and hypophosphatemic male mice exhibited fertility abnormalities characterized by hypergonadotropic hypogonadism, with downregulation of testicular calcium channels, lower intracellular calcium levels, decreased proliferation of spermatogenic cells with down regulation of cyclin E and CDK2 and up regulation of p53 and p21 expression; these abnormalities were all reversed with diet modification without VD supplementation, suggesting that the regulation of 1a,25(OH)2D3 in the male reproductive system can be mediated through extracellular calcium and phosphate [45].
It has also been suggested a protective role of vitamin D from oxidative stress and cellular toxicity in diabetic rat testes and maintenance of the number and motility of sperm in these animals [46,47].
Finally, Sood et al have shown in mice with impairment of Sertoli and Leydig cells function and fed with VD deficient diet, that VD supplementation was able to reverse these alterations. However the authors observed a worsening of function (testicular sperm count, total testicular GTP activity and Leydig cell count) at higher dose of VD supplementation, suggesting the existence of an optimal dose for male fertility [48].
Influence of Hypovitaminosis D in Male Gonadal Function: Human Data
Vitamin D and seminal parameters
Epidemiological studies support a positive association between serum 25(OH)D levels and sperm motility in both fertile and infertile men [4]. Interestingly, the expression of VDR and CYP24A1 is higher in spermatozoa from fertile than infertile men [49]. CYP24A1 is expressed at the annulus in 1% of spermatozoa from subfertile men, whereas fertile men express CYP24A1 in 25% of their spermatozoa [49]. Moreover, several studies have suggested that the presence of VDR and VD metabolizing enzymes may be useful as positive predictive marker for semen quality [29,49-51].
Our group has shown in a study in which participated 98 patients with a cytological diagnosis of hypospermatogenesis or Sertoli cellonly syndrome a higher prevalence of hypovitaminosis D, higher parathyroid hormone levels and lower gene and protein expression of CYP2R1 compared with healthy controls [52]. Another study performed in 300 men with severe hypovitaminosis D (25(OH)D < 25 nmol/L) has shown the presence of alterations in semen parameters (forward motility and normal morphology) compared with those with sufficient VD levels [25(OH)D > 75 nmol/L] [34]. Yang et al, moreover, have found that lower 25(OH)D concentrations were associated with impaired sperm motility and morphology only in infertile men [53]. Based on these data, a supplementation of 10-20 µg per day for men with infertility in order to obtain serum VD levels of 10-50 ng/ml, has been suggested [54].
Nevertheless, while association studies documented a clear negative effect of hypovitaminosis D on sperm parameters, we lack interventional studies to prove a positive effect of vitamin D supplementation on these parameters.
In fact there is only one prospective study on infertile men who presented idiopathic oligoasthenozoospermia, in which a threemonth course of supplementation with VD (200 IU/day) and calcium (600 mg/day) determined a significant improvement in sperm quality compared with a supplementation with vitamin E (100 mg/day) [55].
Lastly, there are only few studies regarding a positive relationship between VD status and successful conception [55,56], whereas other authors did not observe any correlation between VD status and fertility parameters or pregnancy outcomes among men undergoing subfertility treatment [51,57,58]. Taken together, these data suggest a positive correlation between semen parameters and VD status but further prospective studies are warranted to clarify whether VD supplementation can be beneficial for infertile men regarding reproductive outcomes.
Vitamin D and androgen levels
There are conflicting data regarding the possible influence of VD on androgen levels.
Several epidemiological studies have shown a positive linear relationship between androgen and 25(OH)D levels, especially in elderly men. This might be particularly important because of very high prevalence of VD and androgen deficiency in elderly men that are associated at high risk for all-cause and cardiovascular mortality suggesting that a parallel deficiency is a powerful marker of poor health [59,60].
Large multi-center, cross-sectional studies [61,62] have shown positive linear association between 25(OH)D and androgen levels.
Insufficient and deficient 25(OH)D concentrations have been associated with lower total testosterone levels and free androgen index compared with sufficient 25(OH)D concentrations in an Australian study considering men at cardiovascular risk (2229 men, mean age 62 ± 11 years referred for coronary angiography). In the same study, the two groups showed a similar seasonal variations of androgens with a peak of 25(OH)D levels at the end of summer [63]. This association has been also documented in a large Chinese study with 2854 men (mean age 53 ± 13.5 years) in which a higher risk of hypogonadism (OR 1.5, 95% confidence interval 1.14-1.97) was found in men within the lowest 25(OH)D quartile (= 35.4 nmol/L) compared with those in the highest one (= 48.8 nmol/L) [64]. Moreover, Bellastella and colleagues have recently reported, in a case-control study involving 122 men with type 2 diabetes, a significant difference in the VD levels (20.1 ± 6.58 vs 24.0 ± 5.6 ng/ml, p <0.01) in hypogonadal men (n=51) compared with eugonadal ones (n=71) [65]. These epidemiological studies did not, however, explain the physiopathological link between VD and androgen levels. The 25-hydroxylation activity performed by the Leydig cell could justify these findings.
In fact the hydroxylating enzyme CYP2R1 (microsomal VD 25-hydrosylase), the major enzyme involved in VD 25-hydroxylation, is highly expressed in Leydig cells [52,66,67]. This enzyme participates in the activation of the VD precursor cholecalciferol and it is expressed by Leydig cells of the testis under the influence of hCG/LH [52,66,67]. Low expression of CYP2R1 in patients with Leydig cell dysfunction leads to low serum levels of 25(OH)D and low bone mineral density (BMD) [52,66,67]. Impaired CYP2R1 expression (and perhaps INSL3 production), which leads to low levels of 25(OH)D, is found not only in cases of overt hypogonadism (primary and secondary) but also in cases of subclinical hypogonadism [66]. Furthermore, men with low 25(OH)D levels are at risk of clinical sequelae (such as low BMD and osteoporosis) even if they have normal testosterone levels [52,66,67]. 25(OH)D (and LH) levels are more sensitive markers of Leydig cell impairment than testosterone, so that it may be useful in the diagnosis of male hypogonadism (Figure 4).
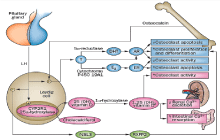
Figure 4: Schematic representation of the crosstalk between testis and bone.
Leydig cells contribute to bone metabolism by producing testosterone and
INSL3 and expressing the CYP2R1 enzyme that hydroxylates cholecalciferol
to 25-hydroxyvitamin D. Testosterone, directly or after conversion to E2 and
DHT, acts on osteoblasts and osteoclasts through AR and ER, whereas INSL3
acts only on osteoblasts through its receptor RXFP2. 1,25-dihydroxyvitamin D
regulates calcium homeostasis and bone metabolism by acting on the kidney,
intestine and osteoblasts. The osteoblast protein, osteocalcin, promotes
testosterone production in Leydig cells by activating steroidogenesis
enzymes.
Abbreviations: 25 (OH) vitamin D: 25-Hydroxyvitamin D; 1,25 (OH)2
vitamin D: 1,25-Dihydroxyvitamin D; AR: Androgen Receptor; DHT:
Dihydrotestosterone; E2: 17β-Estradiol; ER: Estrogen Receptor; INSL3:
Insulin-Like 3; LH: Luteinizing Hormone; T: Testosterone.
Only one interventional prospective study investigated the effect of VD supplementation on testosterone levels reporting that VD therapy (average dose 3332 IU/day for 1 year) might increase testosterone levels in non diabetic obese men with VD deficiency undergoing weight reduction [68]. The mechanism involved has not been clarified, but possibilities are a vitamin-D-receptor-mediated effect on Leydig cells or an effect on the pituitary gland, but current literature does not clarify these hypotheses.
In fact, Hofer et al. have reported that 1a,25(OH)2D3 increases testosterone production and mRNA expression of enzymes involved in androgen production and their precursors (CYP11A1, HSD3B2, CYP19A1, CYP3A4, and SRD5A1) in human primary testicular cells [69]. In this study, this effect seems to be VDR-depended and can be directly or synergistically related to LH. It is also possible that 1a,25(OH)2D3 exerts an influence on steroidogenesis by modulating the calcium-dependent LH response [4].
Nevertheless, a multilevel interaction between testosterone and VD that involves 25-hydroxylation of VD by Leydig cells, and stimulation of testosterone production by VD, is plausible and deserves further investigation.
Actually, many others studies among young and health men failed to find an association between VD and androgen levels in men [22,70-73]. Furthermore, no difference in VD levels was found in men with congenital hypogonadotropic hypogonadism compared with healthy controls [74].
The possible reasons for these controversial findings may be differences in age, healthy and fertility status, seasonal variations and BMI. In particular, striking differences between young and older men (>60 years of age) have been suggested and attributed to indirect effects of VD in older men [4]. It is likely that some indirect VD effects on testosterone levels in older men could be mediated by calcium and phosphate homeostasis, SHBG or osteocalcin production [4,51]. This hypothesis is supported by some data from the European Male ageing study, including 3369 community-dwelling men aged 40-79 years. In this study, a positive association of 25(OH)D levels with total testosterone and free testosterone lost significance after adjusting for age and lifestyle factors [61].
The possible role of osteocalcin
Osteocalcin (OC) is a small protein secreted by bone-forming osteoblast that showed ability to modulate Leydig cell function, such as the production of testosterone. In fact the experiments of Oury and colleagues, conducted ex vivo and in vivo in loss- and gain-offunction models, showed that OC is able to regulate the expression of enzymes required for testosterone synthesis in Leydig cells in a CREB-dependent manner, suggesting an endocrine regulation of male reproduction by the skeleton [75]. A posttranslational gamma-carboxylation of glutamate residues of OC is thought to strongly influence its biological activity. In fact, uncarboxylated- OC (ucOC), which lacks of gamma- carboxylation of glutamate residues at position 17, 21, and 23, is the only form able to influence Leydig cell function [75,76]. The receptor mediating the activity of OC is probably the G protein-coupled receptor GPRC6A, which is expressed at high levels in Leydig cells as well [75,77]. Animal data by Pi et al showed that GPRC6A-deficient mice presented a number of bone/metabolic impairment which were probably related to the altered steroidogenesis [78]. Actually, GPRC6A -/- mice had reduced testosterone levels along with defective mineralization of bone and impaired osteoblast function, glucose intolerance, and metabolic syndrome [78]. Moreover, our group has recently showed a positive correlation between serum ucOC and 25(OH)D levels in 40 overweight male patients and 21 controls, suggesting that OC may contribute with LH to 25(OH)D production by Leydig cells [79]. Taken together, these data underline the crucial role of Leydig cells in the crosstalk between testis and bone function (Figure 4).
Vitamin D, Seminal Parameters and Androgen Levels: U-Shaped Association?
The majority of studies supports a positive linear association between 25(OH)D concentrations and gonadal function, while some researchers have highlighted the possible negative effects of high 25(OH)D levels as summarized in Table 1. These apparent conflicting data may be because of differences in study design, baseline 25(OH) D concentrations, different proportion of men with VD sufficiency, dietary VD intake, age, ethnicity among the participants and assay methodology. Moreover, it is important to underline that some effects mediated by VD in humans are exclusively paracrine effects and that there are a lot of other systemic and autocrine factors involved in the VD metabolism [4,8,80-82]. In fact, a positive linear association between 25(OH)D and gonadal function has not been found in several studies, that instead have reported a possible inverse U-shaped association. The first study to report possible negative effects of high VD levels (94 - 227 nmol/l) on semen volume, sperm count and morphology was a cross-sectional study by Ramlau-Hansen [22].
Animal studies
Human studies
Positive linear association
Sperm count
Kwiecinski et al., 1989 [42]
Foresta et al., 2011 [52]
Kinuta et al., 2000 [6]
Blomberg Jensen, 2014 [4]
Ding et al., 2016 [46]
Sperm motility
Kwiecinski et al., 1989 [42]
Blomberg Jensen et al., 2011 [34]
Kinuta et al., 2000 [6]
Yang et al., 2012 [53]
Ding et al., 2016 [46]
Blomberg Jensen, 2014 [4]
Sperm morphology
Blomberg Jensen et al., 2011[34]
Yang et al., 2012 [53]
Steroidogenesis
Kinuta et al., 2000 [6]
Wehr et al., 2010 [63]
Lerchbaum &Obermayer-Pietsch, 2012 [59]
Lerchbaum et al., 2012 [60]
Lee et al., 2012 [61]
Nimptsch et al., 2012 [62]
Ferlin et al., 2013 [66]
Bellastella et al., 2014 [65]
Wang et al., 2015 [64]
U-shaped association
Sperm count, motility, morphology and steroidogenesis
Ramlau-Hansen et al., 2011 [22]
Hammoud et al., 2012 [73]
Lerchbaum et al., 2014 [83]
Table 1: Vitamin D association with gonadal function in animal and human studies.
In 2012, Hammoud et al. reported similar findings regarding semen parameters. They showed a U-shaped association between 25(OH)D concentrations and semen quality in a cross-sectional study of 152 healthy men. Men with higher (= 50 ng/ml) and lower (= 20 ng/ml) 25(OH)D levels presented worse sperm concentrations and motility compared with men with 20-50 ng/ml [73].
Another more recent cross-sectional study on 225 men (median age 35 years), has suggested a U-shaped association between VD and hypogonadism; men in lowest (= 43.9 nmol/L) and highest (> 101.8 nmol/L) 25(OH)D quintiles presented an increased risk of hypogonadism, even after adjustment for possible confounders factors [83]. In this study there was a relatively large proportion of men with 25(OH)D levels = 75 nmol/L that allowed a balanced evaluation of high 25(OH)D levels with the possibility to evaluate non-linear associations.
As regards this particular U-shaped association between 25(OH) D and gonadal function, the increased risk of hypogonadism in men with higher VD concentrations is difficult to interpret. It has been hypothesized that high VD levels may affect VD metabolism within the target tissues, leading to increased 24-hydroxylation [84]. Thus, in presence of high circulating 25(OH)D levels, the concentration of the biologically active 1a,25 (OH)2D3 might be reduced in target tissues such as testis and the pituitary gland.
Furthermore, the negative relation between high levels of VD and semen parameters can be explained by the experimental finding of Aquila and colleagues that, increasing doses of VD resulted in a negative effect at higher concentrations of VD on intracellular calcium, sperm motility and acrosin reaction, leading to hypothesize that high levels of VD might induce alterations in the systemic or local calcium and zinc levels, both known to play a role in spermatogenesis [29,30,85].
Other studies have also suggested a possible U-shaped or nonlinear associations in other medical fields: for example, between VD and cancer mortality [86], breast cancer [87], prostate cancer [88], overall and cancer mortality [89] and cardiovascular disease [90]. Interestingly, a meta-analysis including 14 prospective studies involving 5562 deaths reports a reverse J-shaped association between serum 25(OH)D and all-cause mortality [91]. This type of association was also suggested by data from the Third National Health and Nutrition Examination Survey (NHANES III) cohort [92]. The suggested optimal 25(OH)D concentrations for all-cause mortality were 75-87.5 nmol/L [91] and 70-90 nmol/L [92], respectively.
Conclusions
Taken together, these data clearly show that hypovitaminosis D is associated with impaired gonadal function. Further interventional studies are needed to prove a causal relationship and a positive effect of VD supplementation and whether VD exerts its effects on reproductive male function directly or indirectly through other VD regulated endocrine factors, such as calcium or estrogen levels, that may play an important role in reproductive outcomes. Furthermore, we still lack universally accepted therapeutically target for VD levels. However it is now clear that, in clinical practice, clinicians can no longer treat deficiency states of VD in the same way, but must differentiate between the various forms of hypovitaminosis D distinguishing them according to pathophysiological causes. In particular, in man, hypogonadism can be associated with a deficiency of VD that can strongly influence the type of VD metabolite used to treat these patients. It should be taken into account the form of the microsomal deficiency of 25-hydroxylase (CYP2R1) and, consequently, administer to these subjects active forms of VD (calcifediol) in order to reach adequate blood VD levels.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstujf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008; 29: 726-776.
- Blomberg Jensen M, Nielsen JE, Jorgensen A, Skakkebaek NE, Juul A, Leffers H, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010; 25: 1303-1311.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357: 266-281.
- Blomberg Jensen M. Vitamin D and male reproduction. Nat Rev Endocrinol. 2014; 10: 175-186.
- Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1 a,25(OH)2 vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011; 25: 543-559.
- Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000; 141: 1317-1324.
- Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013; 12: 976-989.
- Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab. 2011; 25: 531-541.
- Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005; 90: 3215-3224.
- Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006; 260: 245-254.
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006; 81: 353-373.
- Greene-Finestone LS, Berger C, de Groh M, Hanley DA, Hidiroglou N, Sarafin K, et al. 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int. 2011; 22: 1389-1399.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011; 96: 1911-1930.
- Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002; 112: 659-662.
- Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metabol. 2010; 95: 471-478.
- Baker AM, Haeri S, Camargo CA Jr, Espinola JA, Stuebe AM. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010; 95: 5105-5109.
- Faserl K, Golderer G, Kremser L, Lindner H, Sarg B, Wildt L, et al. Polymorphism in vitamin D binding protein as a genetic risk factor in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2011; 96: 233-241.
- Gallagher JC, Sai AJ. Vitamin D insufficiency, deficiency, and bone health. J Clin Endocrinol Metab 2010; 95: 2630-2633.
- Osei K. 25-OH vitamin D: is it the universal panacea for metabolic syndrome and type 2 diabetes? J Clin Endocrinol Metab. 2010; 95: 4220-4222.
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011; 96: 53-58.
- Ward KA, Das G, Roberts SA, Berry JL, Adams JE, Rawer R, et al. A randomized, controlled trial of vitamin D supplementation upon musculoskeletal health in postmenarchal females. J Clin Endocrinol Metab. 2010; 95: 4643-4651.
- Ramlau-Hansen CH, Moeller UK, Bonde JP, Olsen J & Thulstrup AM. Are serum levels of vitamin D associated with semen quality? Results from a cross-sectional study in young healthy men. Fertil Steril. 2011; 95: 1000-1004.
- Ciccone MM, Zito A, Dentamaro I, Vestito D, Scicchitano P, Iacoviello M. Vitamin D deficiency and cardiovascular diseases. G Ital Cardiol (Rome). 2015; 16: 16-20.
- Corbett ST, Hill O, Nangia AK. Vitamin D receptor found in human sperm. Urology. 2006; 68:1345-1349.
- Nangia AK, Hill O, Waterman MD, Schwender CE, Memoli V. Testicular maturation arrest to testis cancer: spectrum of expression of the vitamin D receptor and vitamin D treatment in vitro. J Urol. 2007; 178: 1092-1096.
- Somjen D, Katzburg S, Stern N, Kohen F, Sharon O, Limor R, et al. 25 hydroxy-vitamin D(3) - 1alpha hydroxylase expression and activity in cultured human osteoblasts and their modulation by parathyroid hormone, estrogenic compounds and dihydrotestosterone. J Steroid Biochem Mol Biol. 2007; 107: 238-244.
- Mordan-McCombs S, Brown T, Wang WL, Gaupel AC, Welsh J, Tenniswood M. Tumor progression in the LPB-Tag transgenic model of prostate cancer is altered by vitamin D receptor and serum testosterone status. J Steroid Biochem Mol Biol. 2010; 121: 368-371.
- Blomberg Jensen M, Jørgensen A, Nielsen JE, Steinmeyer A, Leffers H, Juul A, et al. Vitamin D metabolism and effects on pluripotency genes and cell differentiation in testicular germ cell tumors in vitro and in vivo. Neoplasia. 2012; 14: 952-963.
- Aquila S, Guido C, Middea E, Perrotta I, Bruno R, Pellegrino M, et al. Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reprod Biol Endocrinol. 2009; 7: 140.
- Mendoza FJ, Perez-Marin CC, Garcia-Marin L, Madueno JA, Henley C, Aguilera-Tejero E, et al. Localization, distribution and function of the calcium-sensing receptor in sperm. J Androl. 2012; 33: 96-104.
- Dabrowski FA, Grzechocinska B, Wielgos M. The role of vitamin D in reproductive health - a Trojan Horse or the Golden Fleece? Nutrients. 2015; 7: 4139-4153.
- Clulow J, Jones RC, Hansen LA. Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: comparisons with the homologous metanephric proximal tubule. Exp Physiol. 1994; 79: 915-928.
- Weissberger P, Kriebs U, Tsvilovskyy V, Olausson J, Kretz O, Stoerger C, et al. Male fertility depends on Ca2+ absorption by TRPV6 in epididymal epithelia. Sci Signal. 2011; 4: 27.
- Blomberg Jensen M, Bjerrum PJ, Jessen TE, Nielsen JE, Joensen UN, Olesen IA, et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011; 26: 1307-1317.
- Perez-Fernandez R, Alonso M, Sequra C, Munoz I, Garcia-Caballero T, Diguez C. Vitamin D receptor gene expression in human pituitary gland. Life Sci. 1997; 60: 35-42.
- Seoane S, Ben I, Centeno V, Perez-Fernandez R. Cellular expression levels of the vitamin D receptor are critical to its transcriptional regulation by the pituitary transcription factor Pit-1. Mol Endocrinol. 2007; 21: 1513-1525.
- Seoane S, Perez-Fernandez R. The vitamin D receptor represses transcription of the pituitary transcription factor Pit-1 gene without involvement of retinoid X receptor. Mol Endocrinol. 2006; 20: 735-748.
- Nandi A, Sinha N, Ong E, Sonmez H, Poretsky L. Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Invest. 2016; 25: 15-28.
- Avila E, Diaz L, Barrera D, Halhali A, Méndez I, González L, et al. Regulation of vitamin D hidroxylases gene expression by 1,25-dihydroxyvitamin D3 and cyclic AMP in cultured human syncytiotrophoblasts. J Steroid Biochem Mol Biol. 2007; 103: 90-96.
- Zanatta L, Zamoner A, Gonçalves R, Zanatta AP, Bouraïma-Lelong H, Bois C, et al. Effect of 1 a,25-dihydroxyvitamin D3 in plasma membrane targets in immature rat testis: ionic channels and gamma-glutamyl transpeptidase activity. Arch Biochem Biophys. 2011; 515: 46-53.
- Akerstrom VL, Walters MR. Physiological effects of 1,25-dihydroxyvitamin D3 in TM4 Sertoli cell line. Am J Physiol Endocrinol Metab. 1992; 262: 884-890.
- Kwiecinski GG, Petrie GI, DeLuca HF. Vitamin D is necessary for reproductive functions of the male rat. J Nutr. 1989; 119: 741-744.
- Menegaz D, Barrientos-Duran A, Kline A, Silva FR, Norman AW, Mizwicki MT, et al. 1,alpha,25(OH)2-Vitamin D3 stimulation of secretion via chloride channel activation in Sertoli cells. J Steroid Biochem Mol Biol. 2010; 119: 127-134.
- Uhland AM, Kwiecinski GG, DeLuca HF. Normalization of serum calcium restores fertility in vitamin D-deficient male rats. J Nutr. 1992; 122: 1338-1344.
- Sun W, Chen L, Zhang W, Wang R, Goltzman D, Miao D. Active vitamin D deficiency mediated by extracellular calcium and phosphorus results in male infertility in young mice. Am J Physiol Endocrinol Metab. 2015; 308: 51-62.
- Ding C, Wang Q, Hao Y, Ma X, Wu L, du M, et al. Vitamin D supplement improved testicular function in diabetic rats. Biochemical and Biophysical Research Communications. 2016; 473: 161-167.
- Kägi U, Chafouleas JG, Norman AW, Heizmann CW. Developmental appearance of the Ca2+-binding proteins parvalbumin, calbindin D-28K, S-100 proteins and calmodulin during testicular development in the rat. Cell Tissue Res. 1988; 252: 359-365.
- Sood S, Reghunandanan R, Reghunandanan V, Marya RK, Singh PI. Effect of vitamin D repletion on testicular function in vitamin D-deficient rats. Ann Nutr Metab. 1995; 39: 95-98.
- Blomberg Jensen M, Jørgensen A, Nielsen JE, Bjerrum PJ, Skalkam M, Petersen JH, et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. Int J Androl. 2012; 35: 499-510.
- Aquila S, Guido C, Perrotta I, Tripepi S, Nastro A, Andò S. Human sperm anatomy: ultrastructural localization of 1alpha,25-dihydroxyvitamin D receptor and its possible role in the human male gamete. J Anat. 2008; 213: 555-564.
- Boisen IM, Bøllehuus Hansen L, Mortensen LJ, Lanske B, Juul A, Blomberg Jensen M. Possible influence of vitamin D on male reproduction. J Steroid Biochem Mol Biol. 2016.
- Foresta C, Strapazzon G, De Toni L, Perilli L, Di Mambro A, Muciaccia B, et al. Bone mineral density and testicular failure: evidence for a role of vitamin D 25-hydroxylase in human testis. J Clin Endocrinol Metab. 2011; 96: 646-652.
- Yang B, Sun H, Wan Y, Wang H, Qin W, Yang L, et al. Associations between testosterone, bone mineral density, vitamin D and semen quality in fertile and infertile Chinese men. Int J Androl. 2012; 35: 783-792.
- Buhling KJ, Laakmann E. The effect of micronutrient supplements on male fertility. Curr Opin Obstet Gynecol. 2014; 26: 199-209.
- Deng XL, Li YM, Yang XY, Huang JR, Guo SL, Song LM. Efficacy and safety of vitamin D in the treatment of idiopathic oligoasthenozoospermia. Zhonghua Nan Ke Xue. 2014; 20: 1082-1085.
- Tartagni M, Matteo M, Baldini D, Tartagni MV, Alrasheed H, De Salvia MA. Males with low serum levels of vitamin D have lower pregnancy rates when ovulation induction and timed intercourse are used as a treatment for infertile couples: results from a pilot study. Reprod Biol Endocrinol. 2015; 13: 127.
- Neville G, Martyn F, Kilbane M, O’Riordan M, Wingfield M, McKenna M, et al. Vitamin D status and fertility outcomes during winter among couples undergoing in vitro fertilization/intracytoplasmic sperm injection. Int J Gynaecol Obstet. 2016; 135: 172-176.
- Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril. 2014; 102: 460-468.
- Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility-a systematic review. Eur J Endocrinol. 2012; 166: 765-778.
- Lerchbaum E, Pilz S, Boehm BO, Grammer TB, Obermayer-Pietsch B, Marz W. Combination of low free testosterone and low vitamin D predicts mortality in older men referred for coronary angiography. Clin Endocrinol (Oxf). 2012; 77: 475-483.
- Lee DM, Tajar A, Pye SR, Boonen S, Vanderschueren D, Bouillon R, et al. Association of hypogonadism with vitamin D status: the European Male Ageing Study. Eur J Endocrinol. 2012; 166: 77-85.
- Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin Endocrinol (Oxf). 2012; 77: 106-112.
- Wehr E, Pilz S, Boehm BO, Marz W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol. 2010; 73: 243-248.
- Wang N, Han B, Li Q, Chen Y, Chen Y, Xia F, et al. Vitamin D is associated with testosterone and hypogonadism in Chinese men: results from a cross-sectional SPECT-China study. Reprod Biol Endocrinol. 2015; 13: 74.
- Bellastella G, Maiorino MI, Olita L, Capuano A, Rafaniello C, Giugliano D, et al. Vitamin D deficiency in type 2 diabetic patients with hypogonadism. J Sex Med. 2014; 11: 536-542.
- Ferlin A, Selice R, Carraro U, Foresta C. Testicular function and bone metabolism - beyond testosterone. Nat Rev Endocrinol. 2013; 9: 548-554.
- Foresta C, Selice R, De Toni L, Di Mambro A, Carraro U, Plebani M, et al. Altered bone status in unilateral testicular cancer survivors: role of CYP2R1 and its luteinizing hormone-dependency. J Endocrinol Invest. 2013; 36: 379-384.
- Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011; 43: 223-225.
- Hofer D, Munzker J, Schwetz V, Ulbing M, Hutz K, Stiegler Ph, et al. Testicular synthesis and vitamin D action. J Clin Endocrinol Metab. 2014; 99: 3766-3773.
- Chen RY, Nordin BE, Need AG, Scopacasa F, Wishart J, Morris HA, et al. Relationship between calcium absorption and plasma dehydroepiandrosterone sulphate (DHEAS) in healthy males. Clin Endocrinol (Oxf). 2008; 69: 864-869.
- Ceglia L, Chiu GR, Harris SS & Araujo AB. Serum 25-hydroxyvitamin D concentration and physical function in adult men. Clin Endocrinol (Oxf). 2011; 74: 370-376.
- Jorde R, Grimnes G, Hutchinson MS, Kjaergaard M, Kamycheva E, Svartberg J. Supplementation with vitamin d does not increase serum testosterone levels in healthy males. Horm Metab Res. 2013; 45: 675-681.
- Hammoud AO, Meikle AW, Peterson CM, Stanford J, Gibson M, Carrell DT. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl. 2012; 14: 855-859.
- Meric C, Sonmez A, Aydogdu A, Tapan S, Haymana C, Basaran Y, et al. Osteoprotegerin, fibroblast growth factor 23, and vitamin D3 levels in male patients with hypogonadism. Horm Metab Res. 2014; 46: 955-958.
- Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011; 144: 796-809.
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130: 456-469.
- Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem. 2005; 280: 40201-40209.
- Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008; 3: e3858.
- De Toni L, De Filippis V, Tescari S, Ferigo M, Ferlin A, Scattolini V, et al. Uncarboxylated Osteocalcin Stim-ulates 25-Hydroxy Vitamin D Production in Leydig Cell Line Through a GPRC6a-Dependent Pathway. En-docrinology. 2014; 155: 4266-4274.
- Nyomba BL, Bouillon R & De Moor P. Evidence for an interaction of insulin and sex steroids in the regulation of vitamin D metabolism in the rat. J Endocrinol. 1987; 115: 295-301.
- Li SA, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs in mice. Cell Struct Funct. 2004; 29: 91-99.
- Imai M, Ishikawa K, Matsukawa N, Kida I, Ohta J, Ikushima M, et al. Klotho protein activates the PKC pathway in the kidney and testis and suppresses 25-hydroxyvitamin D3 1 a-hydroxylase gene expression. Endocrine. 2004; 25: 229-234.
- Lerchbaum E, Pilz S, Trummer C, Rabe T, Schenk M, Heijboer AC, et al. Serum vitamin D levels and hypogonadism in men. Andrology. 2014; 2: 748-754.
- Miller GJ, Stapleton GE, Hedlund TE & Moffat KA. Vitamin D receptor expression, 24-hydroxylase activity, and inhibition of growth by 1alpha,25-diihydroxyvitamin D3 in seven human prostatic carcinoma cell lines. Clin Cancer Res. 1995; 1: 997-1003.
- Ebisch IM, Pierik FH, DE Jong FH, Thomas CM, Steegers-Theunissen RP. Does folic acid and zinc sulphate intervention affect endocrine parameters and sperm characteristics in men? Int J Androl. 2006; 29: 339-345.
- Pilz S, Kienreich K, Tomaschitz A, Ritz E, Lerchbaum E, Obermayer-Pietsch B, et al. Vitamin D and cancer mortality: systematic review of prospective epidemiological studies. Anticancer Agents Med Chem. 2013; 13: 107-117.
- Abbas S, Chang-Claude J, Linseisen J. Plasma 25-hydroxyvitamin D and premenopausal breast cancer risk in a German case-control study. Int J Cancer. 2009; 124: 250-255.
- Tuohimaa P, Tenkanen L, Ahonen M, Lumme S, Jellum E, Hallmans G, et al. Both high and low levels of blood vitamin D are associated with a higher prostate cancer risk: a longitudinal, nested case-control study in the Nordic countries. Int J Cancer. 2004; 108: 104-108.
- Michaelsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundstrom J, et al. Am J Clin Nutr. 2010; 92: 841-848.
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008; 117: 503-511.
- Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr. 2012; 95: 91-100.
- Sempos CT, Durazo-Arvizu RA, Dawson-Hughes B, Yetley EA, Looker AC, Schleicher RL, et al. Is there a reverse J-shaped association between 25-hydroxyvitamin D and all-cause mortality? Results from the U.S. nationally representative NHANES. J Clin Endocrinol Metab. 2013; 98: 3001-3009.