
Special Article - Iron Deficiency
Ann Nutr Disord & Ther. 2016; 3(2): 1036.
Prevalence of Iron Deficiency in Healthy Adolescents
Urrechaga E¹*, Izquierdo-Álvarez S², Llorente MT³ and Escanero JF4
¹Core Laboratory, Hospital Galdakao-Usansolo, Spain
²Department of Clinical Biochemistry, University Hospital Miguel Servet, Spain
³Institute of Toxicology of Defence, Central Hospital of Defence Gómez Ulla, Spain
4Department of Pharmacology and Physiology, University of Zaragoza, Spain
*Corresponding author: Urrechaga E, Core Laboratory, Hospital Galdakao-Usansolo, Hematology, Galdakao, Vizcaya, Spain
Received: November 17, 2016; Accepted: December 28, 2016; Published: December 30, 2016
Abstract
Objective: Investigate iron status in a well-defined, healthy population of adolescents in our region in the northern coast of Spain.
Material and Methods: We conducted an observational study in healthy adolescents of our area during October 2015. Criteria of inclusion: adolescents undergoing the official health control on their 15-16 years. Pregnant, thalassemia carriers, C-reactive protein (>5 mg/L) or underlying causes for anemia and subjects registered of Hospital admission in the previous 3 months were excluded. 1407 females and 852 males were enrolled. Hemograms were analyzed on XN analyzers (Sysmex, Kobe, Japan). Serum ferritin, serum iron and transferrin were measured with a chemical analyzer Cobas c711 (Roche Diagnostics). The adolescents were classified according to their Iron status: Normal Hb >120 g/L (females) >130 g/L (males); s-Ferritin > 50 μg/L; Latent Iron Deficiency (LID) Hb >120 g/L (females) >130 g/L (males) s-Ferritin 50-16 μg/L; Depletion of Iron stores (DS) Hb >120 g/L (females) >130 g/L (males) s-Ferritin <16 μg/L; Iron Deficiency Anemia (IDA) Hb <120 g/L (females) <130 g/L (males).
Results: Females 112 (7.9%) IDA; 1295 non-anemic divided in 180 (12.8%) DS, 705 (50.2%) LID, 409 (29.1%) normal. Males 68 (8.6%) IDA; 784 nonanemic divided in 30 (3.8%) DS, 295 (37.5%) LID and 456 (58.0%) normal.
Conclusion: Our data show higher prevalence of iron deficiency than reported in other western countries. Adolescent girls constitute a group at risk and specific attention should be given to them during adolescence.
Keywords: Iron deficiency; Iron deficiency anemia; Iron status; Adolescents
Introduction
Iron is an essential micronutrient for humans, functioning as a component of a number of proteins which play important roles in physiological functions. Iron Deficiency (ID) is in the top 20 risk factors for the global distribution of burden of disease [1] and the most common nutritional disorder and leading cause of anemia in the world [2]. Iron deficiency anemia still represents a formidable health challenge [3]. Adolescence is characterized by an accelerated growth and rate of development. During this period adolescents acquire 15-25% of adult size, 40-50% of adult weight and gain around 10 Kg body weight.
Iron is required to satisfy the increased Hemoglobin (Hb) demand for the expansion of blood volume, myoglobin for the higher muscular mass and enzymes necessary for growth [4]. In this period increase Hb concentrations by 50-100 g/L/year to reach adult levels, in addition it is characterized by gonadal sex steroids output. Iron requirements in girls begin to increase after menarche, with 30-40 ml of blood loss in each menstruation, leading to a loss of 15-30 mg of iron. In boys the testosterone secretion and the increased muscular mass development involves additional Iron requirements [5].
Not only physical development can be impaired due to iron deficiency, iron plays an important role in the nervous system function: brain and central nervous system are high demanding organs at this point of life, due to rapid grow and development and cognitive function can be hampered [6,7].
Iron Deficiency (ID) is a reduced content of total body iron. Hb within the reference interval does not exclude ID, because individuals with normal body iron stores must lose a large amount of body iron during a long period before the Hb falls below the concentration that defines anemia.
ID progresses through stages: Latent Iron Deficiency (LID), Depletion of Iron Stores (DIS) and Iron Deficiency Anemia (IDA).
Non-anemic iron deficiency is sometimes termed ‘latent iron deficiency’ or subclinical Iron Deficiency (LID). Iron depots are slowly used up in the process, while red blood cells are continuously produced in the bone marrow; standard indices Mean Cell Volume (MCV), Mean Cell Hemoglobin (MCH) and Red Blood Cell Count (RBC) tend to decline, but in the initial phase the values can still remain in the lower limit of reference intervals and only minor changes can be detected [8].
During the depletion of stores phase there is a progressive decrease in serum ferritin: normal Hb level with MCH in the lower limit of reference range can be detected, but the main laboratory finding is low ferritin. Anemia is established when the storage depletion is sufficient to restrict the synthesis of Hb, and thus its level decrease [9].
We conducted an observational study in apparently healthy adolescents to assess the iron status in this group of our population. The aim of the study was to investigate iron status in a well-defined, healthy population of adolescents in our region, Vizcaya, in the northern coast of Spain.
Material and Methods
The study was conducted in accordance with the guidelines established by the Institutional Review Board at Galdakao-Usansolo Hospital.
We performed an observational study in apparently healthy adolescents, undergoing the official health control on their 15-16 years, during October 2015.
Pregnant, thalassemia carriers, C-Reactive Protein (CRP) >5 mg/L or underlying causes for anemia and subjects registered of Hospital admission in the previous 3 months were excluded.
Fourteen subjects had high levels of CRP (9 boys and 5 girls) and were excluded, 1 boy was recognized as beta thalassemia carrier and 1 girl received a transfusion on previous months. Therefore, the present study of iron status was performed on a total of 2259 adolescents, 1407 girls and 852 boys, with no indication of the presence of neither inflammation nor underlying disease.
Analytical methods
Venous blood samples were drawn into evacuated tubes containing K2-EDTA (Vacutainer™ Becton-Dickinson, Rutherford, NJ, USA), kept at ambient temperature and processed within 6 hours from the time of blood collection. Hemograms were measured on XN analyzers (Sysmex, Kobe, Japan). Biochemical assays of iron status (serum iron, transferrin, and ferritin) were performed using standard methods on a Cobas c711 analyzer (Roche Diagnostics, Mannheim, Germany).
Statistical evaluation of analytical results
Statistical software package SPSS (SPSS; Chicago, IL, USA) version 23.0 for windows was applied for statistical analysis of the results. Kolmogorov-Smirnoff was applied to verify the distribution of the values of the different tests under study. Independent samples t test was applied in order to detect statistical deviations between the groups; P < 0.05 was considered statistically significant.
Results
The adolescents were classified according to their iron status:
-Normal iron status, Hb and ferritin within reference ranges: Hb >120 g/L (females) >130 g/L (males); s-Ferritin > 50 μg/L.
-Subclinical iron deficiency, ferritin below reference range and Hb >120 g/L (females) >130 g/L (males), which can be divided in two stages:
a. Latent Iron Deficiency (LID) ferritin below reference range and no anemia; Hb >120 g/L (females) >130 g/L (males) s-Ferritin < 50 μg/L and
b. Depletion Of Iron Stores (DS) ferritin below the threshold of depletion and no anemia Hb >120 g/L (females) >130 g/L (males) s-Ferritin <16 μg/L and finally anemia when Hb drops below the threshold value.
-Iron Deficiency Anemia (IDA), Hb <120 g/L (females) <130 g/L (males).
Figure 1 and 2 shows the following groups:
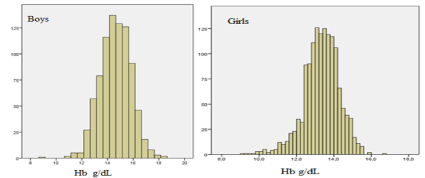
Figure 1: Histograms of Hb in 852 boys and 1407 girls, undergoing the official health control on their 15-16 years.
8.6% of the boys had IDA and 784 had Hb >130g/L.
7.9% of the girls had IDA and 1295had Hb >120g/L.
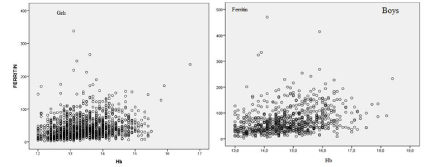
Figure 2: Relative distribution of Ferritin (μg/dL) and Hb (g/dL) in 852 non-anemic boys and 1407 non-anemic girls.
1) Females: 112 (7.9%) IDA; 1295 non-anemic divided in 180 (12.8%) DS, 705 (50.2%) LID, 409 (29.1%) normal iron status.
2) Males 68 (8.6%) IDA; 784 non-anemic divided in 30 (3.8%) DS, 295 (37.5%) LID and 456 (58.0%) normal iron status.
Table 1 and Table 2 summarize the analytical data of the nonanemic adolescent males and females, respectively. Prevalence of IDA was low in both groups, but iron status is different in girls and boys; less than 1/3 of the girls studied had normal iron status with Hb and ferritin in reference ranges and 50% presented LID, while 2/3 of boys had normal iron status.
S Ferritin interval µg/L
Statistical comparisons, P
Group 1
<16
Group 2
16-49
Group 3
=50
Group1
vs. group 2
Group 1
vs. group 3
Group 2
vs. group 3
Hb g/L
140 (8)
145 (9)
150 (10)
.002
.0001
.001
MCV fL
85.0 (69)
87.9 (4)
89.6 (4)
.005
.0001
.001
MCH pg
27.8 (2)
29.0 (1.5)
29.8 (1.5)
.001
.0001
.06
MCHC g/L
327 (61)
330 (65)
332 (59)
.001
.0002
.04
RDW %
13.3 (0.8)
13.0 (0.9)
12.9 (1)
.004
.002
.06
ST %
28 (10)
32 (9)
33 (10)
.005
.001
.001
S Ferritin µg/dL
11 (3)
34 (9)
98 (49)
.0001
.0001
.0001
Hb: Hemoglobin; MCV: Mean Cell Volume; MCH: Mean Cell Hemoglobin; MCHC: Mean Cell Hemoglobin Concentration; RDW: Red cell Distribution Width; ST: Transferrin Saturation.
68 (8.6 %) had IDA
784 non-anemic males divided in groups:
1 depletion of iron stores, n= 30 (3.8 %)
2 latent iron deficiency, n=295 (37.5 %)
3 normal iron status, n= 456 (58.0 %)
Table 1: Analytical data of 852 boys, with no indication of the presence of neither inflammation nor underlying disease.
S Ferritin interval µg/L
Statistical comparisons, P
Group 1
<16
Group 2
16-49
Group 3
=50
Group1
vs. group 2
Group 1
vs. group 3
Group 2
vs. group 3
Hb g/L
130 (7)
134 (8)
136 (7)
.002
.0001
.001
MCV fL
85.0 (4)
89.8 (4)
90.4 (4)
.005
.0001
.001
MCH pg
28.4 (2)
29.6(1.5)
29.9(1.6)
.0001
.06
.001
MCHC g/L
327 (71)
329 (63)
330 (64)
.001
.0002
.07
RDW %
13.0 (0.8)
12.7(0.6)
13.0 (1)
.001
.07
.001
ST %
28 (12)
30 (9)
31 (10)
.005
.001
.04
S Ferritin µg/dL
11 (3)
63 (9)
81 (34)
.0001
.0001
.0001
Hb: Hemoglobin; MCV: Mean Cell Volume; MCH: Mean Cell Hemoglobin; MCHC: Mean Cell Hemoglobin Concentration; RDW: Red Cell Distribution Width; ST: Transferrin Saturation.112 (7.9 %) had IDA
1295 non-anemic females divided in groups:
1 depletion of iron stores, n= 180 (12.8 %)
2 latent iron deficiency, n=705 (50.2 %)
3 normal iron status, n= 409 (29.1 %)
Table 2: Analytical data of 1407 girls, with no indication of the presence of neither inflammation nor underlying disease.
Discussion
Iron Deficiency (ID) is produced by an imbalance between requirements and the quantity ingested and absorbed. This deficiency proceeds is sequential stages: first a decrease in iron stores with serum ferritin bellow reference range, then the depletion of depots (ferritin <15 μg/L), but normal Hb; after the exhaustion of iron the imbalance between supply and requirements makes the erythropoiesis iron restricted, with low Hb synthesis and anemia.
ID is the most common and widespread nutritional disorder, has been recognized as a problem mainly in developing countries, it is also an enormous public health problem in the industrialized world. Most causes in the developing countries were sanitary and nutritional; in western countries increased demands in fertile age women, children and adolescents, due to higher physiological demands, are leading causes [10]. ID, even without anemia, is also associated with a variety of physical symptoms, including fatigue and decreased tolerance for exercise, as well as neuropsychological sequelae such as irritability, apathy, depressive symptoms, and decreased cognitive function and quality of life [11].
The high prevalence of ID and IDA found in our adolescents was unexpected, 40% of the boys and 63% of girls had s-ferritin below reference values. Our area, in the North coast of Spain, is a high income region; our 3,50,000 inhabitants live in villages of 30,000- 40,000 habitants where industry, agriculture and services are the main activities. In our opinion different causes related to lifestyle in our youths are contributing to this situation. A great number of factors predispose to ID and their diversity reflects the socioeconomic status and general development of society.
In low income countries micronutrient deficiencies due to an inadequate diet, leading to an insufficient intake of nutrients such as iron, folic acid, vitamin A, vitamin B12 and vitamin D, are still common; poor bioavailability is also present, with excessive phytate, phosphate, and oxalate and tannin intake. In addition to an inadequate diet and malnutrition, there are other possible associated conditions such as malabsorption due to parasitosis of the gastrointestinal tract [3,10].
In the develop world and western societies the etiology of ID is radically different, linked to lifestyle and habits. In industrialized countries dietary iron intake of adolescents may be poor as the result of inadequate level of iron in the diet with sufficient bioavailability to satisfy the body’s demands during this particular period of nutritional vulnerability. Another characteristic that is common among adolescents refers to a change in dietary habits resulting from peer influence, a need for self-affirmation within the family or as the result of the behavioural or social changes that teenagers face during this phase [3,12].
Adolescents’ dietary habits are often inadequate and more food is consumed outside the home. The consequences of current lifestyles, with increasing dependence on harmful fast food, since there are often important nutritional limitations with this type of food, including its high energy, fat and sodium content in conjunction with its poor fiber, vitamin, calcium and iron content [13]. The availability, ease and speed of food preparation and the influence of peers and of the media makes this food a favorite and most of these factors contribute to an iron-poor diet [14].
All articles revised and mentioned are concordant in the marked differences in iron status between boys and girls, due primarily from menstrual bleeding. Menarche and menstrual abnormalities in adolescents, in combination with an inadequate diet is the cause of the higher prevalence of ID in girls. Heavy menstrual bleeding is a common cause of ID and IDA in women of reproductive age. In these cases, menstrual bleeding is chronically heavier than normal, causing a negative iron balance [15,16].
Menarche in girls imposes additional requirements to balance menstrual iron losses. Biological differences, but also gender social roles could also contribute to the differences in iron status between boys and girls. Young people, but especially girls, at these ages tend to pay more attention on their appearances. Many girls feel pressure to be thin from peer pressure from friends and famous women on social media, leading to improper nutrition. Not controlled diets, low calorie consumption, excessive weight control a desire for a slim figure, lead to poor nutrition and improper dietary behavior [14].
Screening for ID is routinely tested in the primary care setting with a Complete Blood Count (CBC). However, these tests are unable to identify ID without anemia: normal Hb level does not exclude ID, because individuals must lose a large amount of body iron during a long period before anemia is established [17]. Studies on the prevalence of ID were previously based mainly on Hb determinations and focused on the prevalence of anemia caused by low iron. The distributions of the classic hematologic parameters of iron status, Hb red cell indices (MCH and MCV), are wide in normal subjects and show a marked overlap with the corresponding distributions in irondeficient subjects. These methods can therefore only validly be used when the severity of ID is marked. In highly industrialized countries, where this severity is usually mild. These methods are therefore too insensitive, both in the single patient and in epidemiologic studies. This may lead to underestimation of the prevalence of ID if a reasonably high specificity is ascertained [18].
Serum ferritin concentration is the accepted gold standard used for identification of storage iron depletion while no significant changes in the hemogram, due to long lifespan of erythrocytes, are seen in this stage [19].
Several thresholds were proposed for the diagnosis of ID with the use of ferritin concentrations across specialties and indications. The 12-15 μg/L thresholds for ferritin were proposed in guidelines, including those in the general population, women, children, and patients with kidney or digestive diseases. The 25-30 μg/L is the threshold for functional compartment impairment and iron deficient erythropoiesis. The 45-50 μg/L threshold is the limit of reference range for ferritin was proposed and involved the general population with a diagnosis of ID that was estimated as “probably” [20,21]. In our study the lower limit of the reference range defines ID and 16 μg/L the depletion of stores.
Several bias factors may influence the validity of the comparisons of the prevalence of ID in different groups examined; infection rates not considered and/or subjects with Hb genetic disorders with anemia but no ID not excluded; seasonal variations could affect the results obtained and are not usually even referred by authors. Our study was conducted in autumn and confounding factors (infection, inflammation, Hemoglobinopathies) excluded. Comparisons can be difficult because the glossary of terms can also differ, mostly when different Laboratory tests not generally available (soluble Transferrin Receptor, Zn protoporphyrin) are included, or different cut offs for s-ferritin define the groups [22,23].
In the last decade, the prevalence of ID in Spain was reported to be 8 .6% and 12.6% in boys and girls respectively. Iron stores were considered to be depleted when S-Ferritin <12 μg/L, and affected 2.2% in boys 6.3% in girls. The conclusions were that ID was relatively common and Health care programs for children and adolescents should aim specifically to prevent, detect and control ID [24].
This data are surpassed by the rates among adolescents in other European countries such as Denmark (16%), UK (28%), or Ireland; (43%); Finland and France presented lower prevalence (4.7% and 3.1% respectively), in the same decade [25]. In this study, the overall proportion of Iron depletion was 17.6%; it was significantly higher in girls (21.0%) than in boys (13.8%). The prevalence of iron depletion by geographical location was: 23% in Eastern Europe, 19% in Northern Europe, 18% in Western Europe, 17% in Central Europe and 15% in Southern Europe.
More recently, an evaluation of Iron status of the adolescents in Europe using biochemical indicators was conducted [26]. The proportion of anemia was 4.4%, consistent with the estimates of the worldwide prevalence of anemia during adolescence: 27% in developing countries and 6% in developed countries [27].
The proportion in our area more or less doubles this prediction: 8.6% and 7.9% in boys and girls respectively, while the proportion of subjects with iron depletion (ferritin <16 μg/L irrespectively from Hb level) is 20.7% in girls and 12.4% in boys, is in perfect concordance with HELENA study [26], reporting 21% and 13.8% in females and males respectively. The percentages also correspond to those reported in two studies in Sweden in the nineties. Using the same cutoff value for serum ferritin a 14-15% incidence in non-anemic adolescents was found [28,29].
All these studies suggest that ID with and without anemia remains a common yet treatable condition in adolescents in Europe. The concern is that the volunteers were not aware of their iron status, which can cause other health problems. Iron depletion may hamper cognitive development and lead to poor school performances in areas such as cognitive function, mathematics score, memory test, attention and verbal learning and Intelligent Quotient tests during a critical period of their lives where grades and future aspirations could be determined [30].
Conclusion
It may seem paradoxical that a nutrient deficiency disorder would be common among adolescents in highly industrialized countries with excess of foods.
The correlation of s-Ferritin with Hb and the higher percentage of iron depletion in girls with respect to boys suggest that the risk of developing IDA is higher in girls. If latent ID is not corrected, it may indeed progress to ID and finally anemia.
In view of the magnitude of this problem and the number of risk factors involved, urgent and systematic actions need to be taken to prevent and treat ID. Health care programs usually focus in children, while no special programs are conducted for adolescents. Those programs should be implemented, and aim specifically to prevent, detect and control ID in adolescents or at least girls.
Given the crucial role of general practitioners in the diagnosis and management of this condition, our findings may contribute to increase the awareness of ID among physicians as well as to reduce its occurrence among at-risk patients.
References
- Murray CJL, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, et al. Institute for Health Metrics and Evaluation. GBD 2010 change in leading causes and risks between 1990 and 2010. The Lancet. 2012; 380: 2063-2066.
- WHO. Serum ferritin concentrations for the assessment of Iron status and Iron deficiency in populations. Vitamin and Mineral Nutrition Information System. 2011.
- Pasricha SR, Drakesmith H, Black BJ, Hipgrave D, Biggs BA. Control of iron deficiency anaemia in low and middle income countries, Blood. 2013; 121: 2607-2612.
- Mesias M, Seiquer I, Navarro P. Iron Nutrition in Adolescence. Crit Rev Food Sci Nutr. 2013; 53: 1226-1237.
- Dallman PR. Changing Iron needs from birth through adolescence. S. J. Fomon, S. Zlotkin, editors. In: Nutritional Anemias: Raven Press. New York. 1992; 29-38.
- Mc Cann JC, Ames BN. An overview of evidence for a causal relation between Iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007; 85: 931-945.
- Akramipour R, Rezaei M, Rahimi Z. Prevalence of Iron deficiency among adolescent school girls from Kermanshah, Western Iran. Hematology. 2008; 13: 352-355.
- Muñoz M, Villar I, García-Erce JA. An update on iron physiology. World J Gastroenterol. 2009; 15: 4617-4626.
- Urrechaga E, Borque L, Escanero JF. Clinical value of markers of hypochromia in the detection of latent iron deficiency in non-anemic premenopausal women. J Clin Lab Analysis. 2016; 30: 623-627.
- Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014; 123: 615-624.
- Izquierdo Álvarez S, Urrechaga Igartua E, Llorente Ballesteros MT, Escanero Marcén JF. The Role of Iron and Other Trace Elements on Mental Development and Cognitive Function. Gargiulo, Pascual Ángel Arroyo, Humberto Luis Mesones, editors. In: Psychiatry and Neuroscience Update: Bridging the Divide. 2015.
- Nathan GD, Orkin SH. Appendices - Reference values in infancy and childhood. Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux SE, editors. In: Nathan and Oski’s hematology of infancy and childhood, 5th ed. Philadelphia: WB Saunders. 1998.
- Orkin SH, Nathan DG, Ginsburg D, Look AT, Fisher DE, Lux SE. Nathan and Oski’s hematology of infancy and childhood. 7th ed. Philadelphia. PA: Saunders. 2009. 911-1015.
- De Andrade Cairo RC, Rodrigues Silva L, Carneiro Bustani N, Ferreira Marques CD. Iron deficiency anemia in adolescents; a literature review. Nutr Hosp. 2014; 29: 1240-1249.
- Lang AT, Johnson S, Sturm M, O' Brien SH. Iron Deficiency without Anemia: A Common Yet Under-Recognized Diagnosis in Young Women with Heavy Menstrual Bleeding. J Pediatr Adolesc Gynecol. 2016.
- Joffe A. High Prevalence of Iron Deficiency without Anemia in Girls with Heavy Menses, NEJM Journal Watch.
- Asberg AE, Mikkelsen G, Aune MW, Asberg A. Empty Iron stores in children and young adults—the diagnostic accuracy of MCV, MCH, and MCHC. Int J Lab Hematol. 2014; 36: 98-104.
- Hallberg L, Hulten L, Lindstedt G, Lundberg PA, Mark A, Purens J, et al. Prevalence of Irondeficiency in swedish adolescents. Pediatric. 1993; 34: 680-687.
- Ferraro S, Mozzi R, Panteghini M. Revaluating serum ferritin as a marker of body Iron stores in the traceability era. Clin Chem Lab Med. 2012; 50: 1911-1916.
- Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of Iron deficiency across indications: a systematic review. 2015; 102: 1585-1594.
- BC Guidelines. Iron deficiency–Investigation and management. 2010.
- Coad J, Pedley K. Iron deficiency and Iron deficiency anemia in women. Scand J Clin Lab Invest. 2014; 74: 82-89.
- Mousa SMO, Saleh SM, Higazi AMM, Ali HAA. Iron deficiency and Iron deficiency anemia in adolescent girls in rural upper Egypt. International Blood Research & Reviews. 2016; 5: 1-6.
- Durá Travé T, Aguirre Abad P, Mauleón Rosquil C, Oteiza Flores MS, Díaz Velaz L. Iron deficiency in adolescents 10-14 years of age. Aten Primaria. 2002; 29: 72-78.
- Hercberg S, Preziosi P, Galan P. Iron deficiency in Europe. Public Health Nutr. 2001; 4:537-545.
- Ferrari M, MisturaL, Patterson E, Sjöström M, Díaz LE, Stehle P, et al. Helena study group. Evaluation of iron status in european adolescents through biochemical iron indicators: the HELENA study. Eur J Clin Nutr. 2011; 65: 340-349.
- Kara B, Cal S, Aydodan A, Sarper N. The prevalence of anaemia in adolescents: a study from Turkey. J Pediatr Hematol Oncol. 2006; 28: 316-321.
- Bergström E, Hernell O, Lönnerdal B, Persson LA. Sex differences in Iron stores of adolescents: what is normal?, J Pediatr Gastroenterol Nutr. 1995; 20: 215-224.
- Samuelson G, Bratteby LE, Berggren K, Elverby JE, Kempe B. Dietary Iron intake and Iron status in adolescents. Acta Paediatr. 1996; 85: 1033-1038.
- Halterman JS, Kaczorowski JM, Aligne CA, Auinger P, Szilagyi PG. Iron Deficiency and Cognitive Achievement among School-Aged Children and Adolescents in the United States. Pediatrics. 2001; 107: 1381-1386.