
Special Article - Vitamin D Deficiency
Ann Nutr Disord & Ther. 2017; 4(1): 1041.
Vitamin D Levels in Lebanese Patients with Schizophrenia: A Case-Control Study
Haddad C¹, Zoghbi M1,2, Hallit S1,3,4,5, Bou Assi T¹, Medlej-Hashim M6, Nabbout R6,#, Azar J1,7,#,*
¹Psychiatric Hospital of the Cross, Lebanon
²Department of Family Medicine, Saint-Joseph University, Lebanon
³School of Pharmacy, University of Saint Joseph, Lebanon
4University of Saint-Esprit Kaslik, Lebanon
5School of Pharmacy, Lebanese University, Lebanon
6Faculty of Science, Lebanese University, Lebanon
7Department of Medicine, Lebanese American University, Lebanon
#Last co-authors
*Corresponding author: Jocelyne Azar, Department of Medicine, Lebanese American University, Psychiatric Hospital of the Cross, Jall-Eddib, Lebanon
This work has been done in the frame of the “Behavioral and Cognitive Neuroscience Master 2 Program”, Faculty of Sciences, Lebanese University
Received: February 13, 2017; Accepted: March 07, 2017; Published: March 14, 2017
Abstract
Objectives: To compare the vitamin D status in schizophrenics to that of healthy controls; secondary objectives were to assess the correlation between severe vitamin D deficiency and the severity of schizophrenia symptoms and to evaluate the potential factors influencing this status.
Methods: A case-control study, conducted between March and July 2016, recruited 100 patients with schizophrenia and 100 controls with no known psychiatric disorders and matched for age and sex.
Results: Schizophrenic patients had a more severe vitamin D deficiency compared to healthy controls (9% vs. 3%). Vitamin D insufficiency was found in 28% of schizophrenic patients compared to 37% in healthy controls (p=0.053). BCRS and MRSS scales were significantly associated with a more severe vitamin D deficiency (p=0.006). We couldn’t detect any association between the total PANSS score and its three subscales with vitamin D deficiency (p>0.05). Sun exposure, intermediate and high socioeconomic levels would significantly increase the odds of having an increased level of vitamin D as compared to low socioeconomic level (OR=1.046, OR=141.919 and OR=132.110) respectively. Higher MRSS scores would significantly increase the odds of having a more severe vitamin D deficiency by 15.2% (OR=0.848, CI 0.731 - 0.984, p=0.029).
Conclusion: Vitamin D insufficiency is prevalent in patients with schizophrenia. Further work is needed to answer the question of causality. Is vitamin D deficiency the result or the cause of schizophrenia? Future cohort studies may investigate the association with longer follow-up. Spreading awareness among health care professionals for routine serum vitamin D testing, along with patient education, are necessary to avoid this problem.
Keywords: Vitamin D; Schizophrenia; BCRS; MRSS; Schizophrenia symptoms
Introduction
Vitamin D deficiency has been extensively linked to a wide range of neuropsychiatric disorders, including schizophrenia [1-4]. In fact, vitamin D is involved in various brain processes including neurodevelopment, neurotransmitter expression, neurotrophic and growth factor regulation and is thought to be neuroprotective [3]. In addition to its well-known role in calcium and bone metabolism, vitamin D has been shown to be a potent inducer of nerve growth factor synthesis [5-7]. McGrath suggested that a prenatal deficiency of vitamin D might be a risk factor for schizophrenia [5].
A pathophysiological effect of vitamin D levels on schizophrenia development, with a reversible effect after correction has been postulated [8]. Studies have shown that schizophrenics tend to be born in winter/spring seasons during which the Ultraviolet rays (UVB) required to synthesize vitamin D are reduced [9,10]. In addition, schizophrenic patients are more likely to stay indoors and have reduced exposure to sunlight, hence the increased risk of low vitamin D level [3,7]. Furthermore, most schizophrenic patients do not consume enough dietary vitamin D [11,12] with the latter shown to reduce the risk of psychosis or schizophrenia [6,13]. These factors may not be causal but may arise due to confusion with independent risk factors [8], such as ethnicity, dietary factors, sedentary lifestyle, physical activity, body mass index, urban living and socioeconomic status [7,8,14,15].
Vitamin D has also been linked to mental illnesses including Alzheimer’s [16], premenstrual mood disorder [17], major depression [18] and psychosis [19]. However, the relationship between vitamin D status and brain functions is still not elucidated [19], despite some evidence of negative, cognitive [20,21] and mood [22] effects in people with insufficient vitamin D levels. A recent study showed that in schizophrenic patients, a significantly greater severity of negative symptoms was correlated with lower vitamin D status, while overall symptom severity and positive symptom severity approached a significant relationship with vitamin D status. These findings lead researchers to hypothesize that inadequate vitamin D status may account for some portion of the symptom burden experienced by schizophrenic persons [1].
Lebanon has a high prevalence of 25(OH)-D deficiency in adults and older adults [23,24] however, data regarding vitamin D levels in psychiatric patients is still lacking. Therefore, the primary objective of this study was to compare the vitamin D status in schizophrenics to that of healthy controls; secondary objectives were to assess the correlation between severe vitamin D deficiency and the severity of schizophrenia symptoms and to evaluate the potential factors influencing this status.
Materials and Methods
Study design and participants
A case-control study was conducted at the Psychiatric Hospital of the Cross (PHC) - Lebanon, between March and July 2016. A hundred patients with schizophrenia and 100 controls with no known psychiatric disorders were recruited and matched for age and sex. The study received approval by the research and ethics committee of the hospital and participants or their legally representative provided a written informed consent before entering the study.
Patients newly admitted to the hospital, suffering from schizophrenia for no longer than ten years, diagnosed by the treating psychiatrist according to medical file diagnosis [25].
Individuals were excluded if they had any other mental illness, had a diagnosis of alcohol or substance dependence, were treated with vitamin D supplement, or had a metabolic disease that may affect serum vitamin D concentrations.
Clinical and psychiatric evaluations
Demographic (age, gender, geographic region, marital status, occupation, educational level, monthly income per house) and clinical information of the participants (diagnosis, duration of illness, medications intake at the time of the study, blood pressure, history of medical illness and family history of mental disorders) were collected from medical files. The socioeconomic status divided into three levels (low (<1000USD), intermediate (1000-2000USD), high (>2.000USD), was collected from medical files based on the income, education and occupation of each patient. The number of weekly hours of sun exposure, physical activities (indoor and outdoor) and consumption of certain foods (milk, fish, eggs, meat, fruits and vegetables) were also obtained from all participants via a face-to-face interview by 3 investigators. The Body Mass Index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters) and classified according to WHO (World Health Organization) classification [26]. Underweight (<18.5), Normal (18.5-24.9), Overweight (25.0-29.9) and Obese (= 30.0) [26].
Two psychologists performed all the diagnostic assessments of symptoms and functioning using the Positive and Negative Syndrome Scale for schizophrenia (PANSS) [27], the Brief Cognitive Rating Scale (BCRS) [28] and the Morningside Rehabilitation Status Scale (MRSS) [29]. Briefly, the PANSS is used for measuring symptom severity in patients with schizophrenia and evaluating positive and negative symptoms of psychotic disorders [27]. The BCRS describes the severity of cognitive impairment providing five main axes (concentration, short term memory, long term memory, orientation, and self-care ability) and five co-axes (language, psychomotor, mood and behavior, drawing skills, calculating skills) each rated on a 7-step scale (1=no impairment; 7=most severe impairment) [28]. The MRSS scale is used to evaluate the level of general functioning in psychiatric patients. This scale allows the clinician to rate social adaptation of the subject on four axes: dependence/Independence, activity/inactivity level, the level of social integration/ isolation in addition to the effects of present symptoms. Each axis is rated on a 7-step scale, with 0 meaning no disorder and 7 meaning an extreme degree of disability, with higher scores indicating greater dysfunction [29].
Vitamin D measurement blood samples and laboratory analysis
The laboratory analysis was performed at the Laboratory department of the PHC. 6ml of blood were drawn from the antecubital vein of each participant for the measurement of vitamin D. Vitamin D levels (serum 25-hydroxyvitamin D (25 OHD) were determined by chemiluminescence immunoassay (Architect I, Abbott Laboratories).
We divided the Vitamin D level in four groups to take into consideration participants with severe deficiency. These cut-offs were defined almost exclusively based on data from older populations and from western countries [30].
Statistical analysis
Statistical Package for the Social Sciences (SPSS) 22 was used for the data analysis. Student’s t-test was used to compare continuous variables in two groups. Pearson correlation was used for linear correlation between continuous variables. For categorical variables, the χ2 and Fisher exact tests were used. Multivariate analysis logistic regressions were carried out using variables that showed a p-value <0.2 in the bivariate analysis.
A backward logistic regression test was performed to identify the independent variables that affect 25(OH) D levels. Vitamin D variable categorized into two groups (Vitamin D<25nmol/l, Vitamin D>25nmol/l) [31,32] were used as dependent variables. Significance will be defined as a p-value less than 0.05 levels.
Results
Sociodemographic characteristics of the study sample
Overall 200 participants were enrolled. The mean age of schizophrenic patients was 37.00 ± 11.65 years compared to 38.77±10.61 years for the controls (age ranging between 18 and 65 years for the whole sample). Most of the participants were male in each group (72% in control group, 70% in cases group). The majority of cases were single (76%), having an intermediate and below level of education (60%), and a low socioeconomic level (54%). On the other hand, 44% of the control group was single, 60% had secondary and high levels of education and the majority of them had an intermediate socio-economic level (76%). More than half of the patients were smokers (60%) versus 48% of the controls.
Most of the patients didn’t have a history of medical illness (71%) and 42% of them had a family history of mental disorders, while all controls didn’t have a family history of mental disorders 100% and almost all of them 88% didn’t have a history of medical illness.
Almost half of the patients had normal BMI (48%), with 52% being overweight or obese, while most of the controls were overweight and obese (62%) and 38% having normal BMI.
A significant difference between the two groups was found for the geographic region, marital status, education level, socioeconomic level, history of medical illness, family history of psychiatric illness (p<0.005).
Comparison of vitamin D category between schizophrenic patients and healthy control
Figure 1 shows the percentage of vitamin D level categorized according to IOM guideline between schizophrenic patients and healthy control. The results showed that 9% of schizophrenic patients had a severe vitamin D deficiency as compared to 3% in the control group. Almost half of the schizophrenic patients (49%) had vitamin D deficiency compared to 54% in healthy controls. Vitamin D insufficiency was found in 28% of schizophrenic patients compared to 37% in healthy controls, while vitamin D sufficiency was found in 14% of schizophrenic patients compared to 6% in healthy controls. The difference in vitamin D categories between controls and schizophrenics tended to significance (p=0.053).
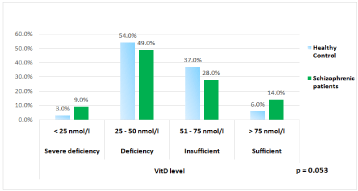
Figure 1: Vitamin D category between schizophrenic patients and healthy
control.
Correlation between the severity of vitamin D deficiency in schizophrenic patients and schizophrenia symptoms, severity of cognitive impairment and the level of general functioning
Significantly higher BCRS and MRSS mean scores were found in schizophrenic patients with a more severe vitamin D deficiency (p=0.006 for both variables). However, we couldn’t detect any significant difference between the total PANSS score and its three subscales with vitamin D level (p>0.05) (Table 1).
Vitamin D < 25 nmol/l
Vitamin D > 25 nmol/l
p-value
Mean ± SD
Mean ± SD
BCRS
19.06 ± 6.69
14.79 ± 5.24
0.006
MRSS
16.44 ± 5.24
12.97 ± 4.33
0.006
P-PANSS*
15.50 ± 6.55
17.75 ± 7.31
0.55
N-PANSS*
16.50 ± 4.79
17.78 ± 7.81
0.748
G-PANSS*
26.75 ± 2.06
34.16 ± 11.01
0.422
Total-PANSS
58.75 ± 9.10
69.69 ± 21.63
0.32
*P-PANSS: Positive PANSS; N-PANSS: Negative PANSS; G-PANSS: Cognitive PANSS.
Table 1: Vitamin D level in schizophrenic patients and psychological scales.
Bivariate analysis
Table 2 displays the bivariate analysis of the factors that might be correlated with severe vitamin D levels. Females had significantly more severe vitamin D levels than males (p<0.001). Having a family history of mental disorders and taking anticholinergic drugs were significantly associated with a more severe vitamin D deficiency (p=0.043 and p=0.029) respectively. Higher BCRS and MRSS mean scores were significantly associated with more severe vitamin D deficiency (p=0.006 for both). An increased mean time of outdoor activities and sun exposure were significantly associated with a higher level of vitamin D (p=0.033 and p=0.026) respectively. No significant difference was found between typical antipsychotics, atypical antipsychotics, antidepressants, mood stabilizers, benzodiazepines, and socioeconomic level, BMI and the two categories of vitamin D (p>0.05).
Vitamin D < 25 nmol/l
Vitamin D > 25 nmol/l
p-value
Frequency (%)
Frequency (%)
Gender
Male
14 (43.8%)
128 (76.2%)
Female
18 (56.3%)
40 (23.8%)
<0.001
Socioeconomic level
Low
14 (43.8%)
60 (35.7%)
0.124
Intermediate
15 (46.9%)
103 (61.3%)
High
3 (9.4%)
5 (3.0%)
Family History of Mental disorders
Yes
11 (34.4%)
31 (18.5%)
0.043
No
21 (65.6%)
137 (81.5%)
Anti-cholinergic drug
Yes
10 (43.5%)
16 (20.8%)
0.029
No
13 (56.5%)
61 (79.2%)
Typical antipsychotics drug
Yes
6 (66.7%)
55 (60.4%)
0.715
No
3 (33.3%)
36 (39.6%)
Atypical antipsychotics drug
Yes
18 (78.3%)
53 (68.8%)
0.382
No
5 (21.7%)
24 (31.2%)
Anti-depressant drug
Yes
4 (17.4%)
10 (13.0%)
0.593
No
19 (82.6%)
67 (87.0%)
Mood stabilizers drug
Yes
11 (47.8%)
32 (41.6%)
0.594
No
12 (52.2%)
45 (58.4%)
Benzodiazepine drugs
Yes
5 (21.7%)
21 (27.3%)
0.595
No
18 (78.3%)
56 (72.7%)
Case-control group
Healthy Control
9 (28.1%)
91 (54.2%)
0.074
Schizophrenic Patients
23 (71.9%)
77 (45.8%)
Mean ± SD
Mean ± SD
BCRS
19.06 ± 6.69
14.79 ± 5.24
0.006
MRSS
16.44 ± 5.24
12.97 ± 4.33
0.006
BMI
28.23 ± 7.48
26.22 ± 4.89
0.153
Indoor activities
7.83 ± 15.57
15.38 ± 37.06
0.067
Outdoors activities
12.00 ± 25.34
26.19 ± 53.59
0.026
Sun exposure (in minutes)
37.67 ± 74.96
67.41 ± 68.74
0.033
Table 2: Bivariate analysis for vitamin D divided into two categories.
Multivariable analysis
A backward logistic regression, using the two-category vitamin D level as dependent variable, showed that intermediate and high socioeconomic level would significantly increase the odds of having an increased level of vitamin D as compared to low socioeconomic level by 54.5% and 34.1% respectively (OR= 141.919, CI 1.545 - 13036.060, p = 0.032 and OR= 132.110, CI 1.341 - 13016.132, p = 0.037 respectively). Sun exposure would significantly increase the odds of having an increased vitamin D level by 4.6% (OR= 1.046, CI 1.007- 1.086, p = 0.022). Furthermore, higher MRSS scores would significantly increase the odds of having a more severe vitamin D deficiency by 15.2% (OR= 0.848, CI 0.731 – 0.984, p =0.029) (Table 3).
Logistic regression taking vitamin D divided into two categories (<25 nmol/l and >25 nmol/l) as dependent variable
OR
95% C.I
p-value
Sun exposure
1.046
1.007
1.086
0.022
MRSS
0.848
0.731
0.984
0.029
Socioeconomic level
0.099
Intermediate socioeconomic level
141.919
1.545
13036.06
0.032
High socioeconomic level
132.11
1.341
13016.132
0.037
Table 3: Multivariable analysis.
Discussion
To our knowledge, this is the first study that investigates the level of vitamin D in Lebanese schizophrenic patients. Our results showed that patients with schizophrenia, compared to healthy controls, had similar vitamin D levels, although severe deficiency in vitamin D levels were demonstrated three times more in the patients. This could be due to insufficient sample size or the existence of vitamin D deficiency in controls.
The desirable vitamin D levels, and thus hypovitaminosis D, have been set using international guideline based cut-offs and lately have been a matter of great debate. The Endocrine Society guidelines in 2011 [33] had set a cut-off point at 30ng/mL (75nmol/L) while the Institute of Medicine Report (IOM) had set a cut off at 20ng/mL (50nmol/L) in 2011 [34]. According to the IOM guidelines vitamin D levels are categorized into deficient (below 50nmol/L), insufficient (50-75 nmol/L) and sufficient (more than 75nmol/L) [34,35]. A level below 25nmol/l (10ng/ml) was considered as severe deficiency [31,32]. A meta-analysis, including 19 studies (2804 participants), showed a strong association between vitamin D deficiency and schizophrenia [2], with 65% of them being vitamin D deficient [2]. Other studies [6,36,37] showed that adults with schizophrenia have significantly lower serum concentrations of vitamin D as compared with healthy controls. It was expected that vitamin D levels in the Lebanese population would be higher due to the sunny climate. In fact, Lebanon is located at appropriate latitudes (33° 35’N) [38] for the synthesis of vitamin D [39] and thus, higher levels of total vitamin D would be expected [39]. Furthermore, our results indicated that patients with schizophrenia, as well as the healthy ones, had a vitamin D deficiency, similar to previous studies [2,4,36,37,40,41]. Other studies conducted in Lebanon had shown a hypovitaminosis D in the healthy population [35,42-45].
Schizophrenic patients are on a wide variety of medications, including anticonvulsants and antipsychotic drugs [46,47]. Our findings showed no significant association between those taking antipsychotics and vitamin D level, in line with other studies that showed no effect of antipsychotics on vitamin D level [1,48,49]. It is hypothesized that anti psychotics would cause hypogonadism and might predispose the patient to a higher risk of osteoporosis [50] however, it is not known whether there are direct effects of this class of medications on vitamin D status [1]. Some anticonvulsant medications might increase the rate of vitamin D removal from the body via the liver [51] by enhancing the catabolism of vitamin D [47], thus, putting the patient at risk of low vitamin D levels.
Vitamin D deficiency may be due to cultural requirements that limit sun exposure, as well as to low vitamin D intake [35]. In addition, big cities have a higher degree of air pollution that contains ozone, thus leading to an efficient atmospheric absorption of UVB photons, thereby reducing the skin photosynthesis of vitamin D52. Unfortunately, Beirut is actually a much polluted city, with no continuous measurement of its pollution level [43].
We recorded an inverse association between vitamin D level MRSS and BCRS scales respectively. No study has evaluated the association between MRSS, BCRS and hypovitaminosis D in schizophrenic patients. Increasing the behavioral and cognitive function would increase outdoor activities and exposure to sunlight [1,48]. Different studies had reported that insufficiency or deficiency of vitamin D is associated with cognitive impairment [1,23,53-56]. Vitamin D deficiency can be associated with muscle weakness that might lead to cognitive impairment [57]. Furthermore, our schizophrenic patients had a sedentary lifestyle, including social isolation and lack of motivation, leading to poorer nutrition and less time spent outdoors that would contribute to lower vitamin D levels [58].
Our findings did not show a significant association between vitamin D levels and schizophrenia symptoms, in opposite to the study of Graham et al. [1] which was the first to report an association of vitamin D insufficiency with more severe negative symptoms and poorer neurocognitive function in patients with schizophrenia. This can be due to the fact that our patients were still in early adulthood, confirming the fact that low vitamin D status in late adolescence or early adulthood is unlikely to be causally related to schizophrenia risk in these subjects 1. These results need to be confirmed by future studies.
Our results showed that sun exposure and the socioeconomic level was significantly associated with increased vitamin D level. We observed that the number of weekly reported hours of sun exposure was positively correlated with an increased level of vitamin D. Eyles et al. [3] suggested that lower exposure to sunlight, which is required for the production of vitamin D, is associated with higher schizophrenia risk. Another study done by Itzhaky et al [36] did not find a significant difference between daily sun exposure and schizophrenia. Our finding reinforces the importance of sun exposure as a source of vitamin D. This study was done during spring and summer season where sun exposure is appropriate for the synthesis of vitamin D. Season affect vitamin D synthesis in the skin [35]. Lower serum 25-hydroxyvitamin D levels are reported in winter and spring compared to summer and fall in studies from temperate regions in the developing world [35].
Our results showed that intermediate and high Socioeconomic Status (SES) was associated with increased level of vitamin D, similar to a study done by Arabi et al [35] which reported that individuals with hypovitaminosis D were mostly of low socioeconomic status. However, previous studies [9,59] did not show a relation between vitamin D and SES. Families with low SES had restricted access to the nutritional variety and food quality (food cost is an important factor affecting food choice) and have low activities lifestyles (low income could discourage outdoor activities) [60,61].
Limitations
There are several limitations of this study. It’s a single center study, thus does not reflect vitamin D statuses among all hospitalized schizophrenic patients. The sample size for this study remains small, with additional larger studies needed to confirm our findings. The season during which this study was conducted might have influenced our results. Further prospective studies should take this limitation into account. An information bias is possible since the evaluation of lifestyle activities, including diet, outdoor activity, and daily sun exposure was done through a direct interview and is subjective. The information collected directly from patients could have been affected by the low level of cognition due to the negative effect of antipsychotics treatment. In addition, we did not screen specifically for malabsorption problems in both schizophrenics and control groups, which might confer a higher risk of vitamin D deficiency.
Conclusion
Vitamin D insufficiency is prevalent in patients with schizophrenia. Further work is needed to answer the question of causality. Does vitamin D deficiency play a vital role in the pathogenesis of schizophrenia? The theory of a genetic interaction between schizophrenia and vitamin D is possible. Future cohort studies may investigate the association with longer follow-up.
In the meantime, it is important that health care professionals be aware of the high rates of vitamin D deficiency among schizophrenics. Spreading awareness among health care professionals for routine serum vitamin D testing, along with patient education, are necessary to avoid this problem.
Acknowledgments
The authors would like to thank Maha Abdel Razzak and Afaf Greij (Masters Students), Dr Sahar Obeid and Miriam Slikhanian for their help in the accomplishment of this work.
Funding
Special thanks to Pharmaline pharmaceutical company for funding the laboratory tests.
References
- Graham KA, Keefe R, Lieberman J, Calikoglu AS, Lansing K, Perkins DO. Relationship of low vitamin D status with positive, negative and cognitive symptom domains in people with first-episode schizophrenia. Early intervention in psychiatry. 2015; 9: 397-405.
- Valipour G, Saneei P, Esmaillzadeh A. Serum vitamin D levels in relation to schizophrenia: a systematic review and meta-analysis of observational studies. The Journal of Clinical Endocrinology & Metabolism. 2014; 99: 3863-3872.
- Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Frontiers in neuroendocrinology. 2013; 34: 47-64.
- Murri MB, Respino M, Masotti M, Innamorati M, Mondelli V, Pariante C, et al. Vitamin D and psychosis: mini meta-analysis. Schizophrenia research. 2013; 150: 235-239.
- McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophrenia research. 1999; 40: 173-177.
- McGrath J, Saari K, Hakko H, Jokelainen J, Jones P, Järvelin MR, et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophrenia research. 2004; 67: 237-245.
- McGrath JJ, Burne TH, Féron F, Mackay-Sim A, Eyles DW. Developmental vitamin D deficiency and risk of schizophrenia: a 10-year update. Schizophrenia bulletin. 2010; 36: 1073-1078.
- Taylor AE, Burgess S, Ware JJ, Gage SH, Richards JB, Smith GD, et al. Investigating causality in the association between 25 (OH) D and schizophrenia. Scientific reports. 2016; 6.
- Davies G, Welham J, Chant D, Torrey EF, McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. National Institute of Mental Health. 2003; 29: 587-593.
- Kinney DK, Teixeira P, Hsu D, Nepoleon SC, Crowley DJ, Miller A, et al. Relation of schizophrenia prevalence to latitude, climate, fish consumption, infant mortality, and skin color: a role for prenatal vitamin d deficiency and infections? Schizophrenia bulletin. 2009: 35: 582-595.
- Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermato-endocrinology. 2013; 5: 51-108.
- Ferder M, Inserra F, Manucha W, Ferder L. The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. American Journal of Physiology-Cell Physiology. 2013; 304: 1027-1039.
- Hedelin M, Löf M, Olsson M, Lewander T, Nilsson B, Hultman CM, et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33 000 women from the general population. BMC psychiatry. 2010; 10: 38.
- McNamee L, Mead G, MacGillivray S, Lawrie SM. Schizophrenia, poor physical health and physical activity: evidence-based interventions are required to reduce major health inequalities. 203: 239-241.
- McGrath JJ, Lawlor DA. The search for modifiable risk factors for schizophrenia. Am Psychiatric Assoc. 2011.
- Balion C, Griffith LE, Strifler L, Henderson M, Patterson C, Heckman G, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012; 79: 1397-1405.
- Eskandari F, Martinez PE, Torvik S, Phillips TM, Sternberg EM, Mistry S, et al. Low bone mass in premenopausal women with depression. Arch Intern Med. 2007; 167: 2329-2336.
- Gracious BL, Finucane TL, Friedman-Campbell M, Messing S, Parkhurst MN. Vitamin D deficiency and psychotic features in mentally ill adolescents: a cross-sectional study. BMC Psychiatry. 2012; 12: 38.
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002; 13: 100-105.
- Annweiler C, Schott AM, Allali G, et al. Association of vitamin D deficiency with cognitive impairment in older women: cross-sectional study. Neurology. 2010; 74: 27-32.
- Annweiler C, Schott AM, Rolland Y, Blain H, Herrmann FR, Beauchet O. Dietary intake of vitamin D and cognition in older women: a large population-based study. Neurology. 2010; 75: 1810-1816.
- Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008; 264: 599-609.
- Darwish H, Zeinoun P, Ghusn H, Khoury B, Tamim H, Khoury SJ. Serum 25-hydroxyvitamin D predicts cognitive performance in adults. Neuropsychiatric disease and treatment. 2015; 11: 2217.
- Medlej-Hashim M, Jounblat R, Hamade A, et al. Hypovitaminosis D in a Young Lebanese Population: Effect of GC Gene Polymorphisms on Vitamin D and Vitamin D Binding Protein Levels. Ann Hum Genet. 2015; 79: 394-401.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA. American Psychiatric Association. 2013.
- World Health Organization, BMI classification. 2006.
- Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry research. 1988; 23: 99-110.
- Reisberg B, Schneck M, Ferris SH, Schwartz G, DeLeon M. The brief cognitive rating-scale (BCRS)-findings in Primary Degenerative Dementia (PDD). Psychopharmacology Bulletin. 1983; 19: 47-50.
- Affleck JW, McGuire RJ. The measurement of psychiatric rehabilitation status. A review of the needs and a new scale. The British Journal of Psychiatry. 1984; 145: 517-525.
- Fuleihan GEH, Rahme M, Bassil D. Do desirable vitamin D levels vary globally? Nutritional influences on bone health: Springer. 2013: 273-299.
- Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, et al. IOM committee members respond to Endocrine Society vitamin D guideline. The Journal of Clinical Endocrinology & Metabolism. 2012; 97: 1146-1152.
- Spiro A, Buttriss J. Vitamin D: An overview of vitamin D status and intake in Europe. Nutrition Bulletin. 2014; 39: 322-350.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism. 2011; 96: 1911-1930.
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know? The Journal of Clinical Endocrinology & Metabolism. 2011; 96: 53-58.
- Arabi A, El Rassi R, Fuleihan GEH. Hypovitaminosis D in developing countries—prevalence, risk factors and outcomes. Nature Reviews Endocrinology. 2010; 6: 550-561.
- Itzhaky D, Amital D, Gorden K, Bogomolni A, Arnson Y, Amital H. Low serum vitamin D concentrations in patients with schizophrenia. IMAJ-Israel Medical Association Journal. 2012; 14: 88.
- Jamilian H, Bagherzadeh K, Nazeri Z, Hassanijirdehi M. Vitamin D, parathyroid hormone, serum calcium and phosphorus in patients with schizophrenia and major depression. International journal of psychiatry in clinical practice. 2013; 17: 30-34.
- Beirut, Lebanon Lat Long Coordinates.
- Amato R, Pinelli M, Monticelli A, Miele G, Cocozza S. Schizophrenia and vitamin D related genes could have been subject to latitude-driven adaptation. BMC evolutionary biology. 2010; 10: 351.
- Humble MB, Gustafsson S, Bejerot S. Low serum levels of 25-hydroxyvitamin D (25-OHD) among psychiatric out-patients in Sweden: relations with season, age, ethnic origin and psychiatric diagnosis. The Journal of steroid biochemistry and molecular biology. 2010; 121: 467-470.
- Yüksel RN, Altunsoy N, Tikir B, Kuluk MC, Unal K, Goka S, et al. Correlation between total vitamin D levels and psychotic psychopathology in patients with schizophrenia: therapeutic implications for add-on vitamin D augmentation. Therapeutic advances in psychopharmacology. 2014; 4: 268-275.
- Fuleihan GEH, Nabulsi M, Choucair M, Salamoun M, Hajj Shahine C, Kizirian A, et al. Hypovitaminosis D in healthy schoolchildren. Pediatrics. 2001; 107: E53.
- Gannagé-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. Journal of bone and mineral research. 2000; 15: 1856-1862.
- Gannagé-Yared MH, Helou E, Zaraket V. Serum 25 hydroxyvitamin D in employees of a Middle Eastern university hospital. Journal of endocrinological investigation. 2014; 37: 541-546.
- Medlej-Hashim M, Jounblat R, Hamade A, Ibrahim JN, Rizk F, Azzi G, et al. Hypovitaminosis D in a young Lebanese population: effect of GC gene polymorphisms on vitamin D and vitamin D binding protein levels. Annals of human genetics. 2015; 79: 394-401.
- Zhou C, Assem M, Tay JC, Watkins PB, Blumberg B, Schuetz EG, et al. Steroid and xenobiotic receptor and vitamin D receptor crosstalk mediates CYP24 expression and drug-induced osteomalacia. The Journal of clinical investigation. 2006; 116: 1703-1712.
- Lauth M, Rohnalter V, Bergström Å, Kooshesh M, Svenningsson P, Toftgård R. Antipsychotic drugs regulate hedgehog signaling by modulation of 7-dehydrocholesterol reductase levels. Molecular pharmacology. 2010; 78: 486-496.
- Crews M, Lally J, Gardner-Sood P, Howes O, Bonaccorso S, Smith S, et al. Vitamin D deficiency in first episode psychosis: a case–control study. Schizophrenia research. 2013; 150: 533-537.
- Gracious BL, Finucane TL, Friedman-Campbell M, Messing S, Parkhurst MN. Vitamin D deficiency and psychotic features in mentally ill adolescents: a cross-sectional study. BMC psychiatry. 2012; 12: 38.
- Meaney AM, Smith S, Howes O, O’brien M, Murray RM, O’keane V. Effects of long-term prolactin-raising antipsychotic medication on bone mineral density in patients with schizophrenia. The British Journal of Psychiatry. 2004; 184: 503-508.
- Verrotti A, Coppola G, Parisi P, Mohn A, Chiarelli F. Bone and calcium metabolism and antiepileptic drugs. Clinical neurology and neurosurgery. 2010; 112: 1-10.
- Holick MF. Environmental factors that influence the cutaneous production of vitamin D. The American journal of clinical nutrition. 1995; 61: 638-645.
- Granic A, Hill T, Kirkwood T, Davies K, Collerton J, Martin-Ruiz C, et al. Serum 25-hydroxyvitamin D and cognitive decline in the very old: the Newcastle 85+ Study. European journal of neurology. 2015; 22: 106-107.
- Llewellyn DJ, Lang IA, Langa KM, Graciela MT, caroline Pl, Antonio C, et al. Vitamin D and Risk of Cognitive Decline in Elderly Persons. Obstetrical & Gynecological Survey. 2011; 66: 354-355.
- der Schaft JV, Koek H, Dijkstra E, Verhaar H, der Schouw YV, Emmelot-Vonk M. The association between vitamin D and cognition: a systematic review. Ageing research reviews. 2013; 12: 1013-1023.
- Wilson VK, Houston DK, Kilpatrick L, Yaffe K, Cauley JA, Lovato J, et al. Relationship between 25-Hydroxyvitamin D and Cognitive Function in Older Adults: The Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2014; 62: 636-641.
- Muir SW, Montero-Odasso M. Effect of vitamin D supplementation on muscle strength, gait and balance in older adults: a systematic review and meta-analysis. Journal of the American Geriatrics Society. 2011; 59: 2291-2300.
- Berg AO, Melle I, Torjesen PA, Lien L, Hauff E, Andreassen OA. A cross-sectional study of vitamin D deficiency among immigrants and Norwegians with psychosis compared to the general population. The Journal of clinical psychiatry. 2010; 71: 1598-1604.
- Shirazi L, Almquist M, Malm J, Wirfält E, Manjer J. Determinants of serum levels of vitamin D: a study of life-style, menopausal status, dietary intake, serum calcium, and PTH. BMC women's health. 2013; 13: 33.
- Dubowitz T, Heron M, Bird CE, Lurie N, Finch BK, Basurto-Dávila R, et al. Neighborhood socioeconomic status and fruit and vegetable intake among whites, blacks, and Mexican Americans in the United States. The American journal of clinical nutrition. 2008; 87: 1883-1891.
- Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the Black Women's Health Study. American Journal of Epidemiology. 2010; 171: 564-570.