
Special Article - Anemia
Ann Nutr Disord & Ther. 2017; 4(2): 1043.
New Experimental Anemic Model by Using a Nitrogen- Containing Bisphosphonate
Otsuka H¹, Soeta S², Yagi H³, Endo Y4 and Nakamura M1*
¹Department of Oral Anatomy and Developmental Biology, Showa University, Japan
²Department of Veterinary Anatomy, Nippon Veterinary and Animal Science University, Japan
³Department of Pharmaceutical, International University of Health and Welfare, Japan
4Division of Oral and Maxillofacial Surgery, Tohoku University, Japan
*Corresponding author: Masanaori Nakamura, Department of Oral Anatomy and Developmental Biology, Showa University, School of Dentistry, 1-5-8 Hatanodai, Shinagawa-ku, Tokyo 142-8555, Japan
Received: March 27, 2017; Accepted: May 03, 2017; Published: May 10, 2017
Abstract
Anemia is a pathological condition associated with various diseases including cancer, and is indicated by a reduction in erythrocyte numbers or the amount of hemoglobin in the peripheral blood. The analysis of changes in hematopoiesis during anemia is important to develop novel therapies and to understand hematopoietic ontogeny. We previously reported that injecting animals with Nitrogen-Containing Bisphosphonate (NBP), which is an inhibitor of osteoclastic bone resorption, decreased erythropoiesis in Bone Marrow (BM). Moreover, we induced severe anemia in a mouse model by injecting NBP in combination with Phenylhydrazine (PHZ), and embryonic type globin mRNA was detected in both the BM and the liver in this anemia model. In addition, wine-colored capsuled structures were unexpectedly observed in the abdominal cavity of this anemic mouse model, and active erythropoiesis was also observed in these structures. Here, we review recent insights into the pathogenic mechanisms underlying various forms of anemia that have been gained through our findings.
Keywords: Nitrogen-containing bisphosphonate; Extramedullary hematopoiesis; Embryonic hemoglobin; GCSF; SDF-1
Abbreviations
NBP: Nitrogen-Containing Bisphosphonate; GCSF: Granulocyte-Colony Stimulating Factor; PHZ: Phenylhydrazine; BM: Bone Marrow; SDF-1: Stromal Cell-Derived Factor 1; HSCs: Hematopoietic Stem Cells; HPCs: Hematopoietic Progenitor Cells; EPO: Erythropoietin
Introduction
Anemia is a pathological condition that involves a reduction in the number of erythrocytes or the amount of hemoglobin in the peripheral blood, and is caused by various diseases such as cancers and bone marrow disorders. The pathology of anemia is very complex, and studies that clarify the mechanism of recovery from anemia and its pathology are essential to establishing an understanding of the therapy, and the ontogeny of hematopoiesis and tissue engineering [1]. Many studies have reported experimental anemia models, including phlebotomy, schistosome parasite infection and draginduction, which are known to be different from the actual disease state (Table 1) [2]. Phenylhydrazine (PHZ) is used to experimentally induce hemolytic anemia in laboratory animals [3], by the mechanism of RBC lipid peroxidation [4,5].
Hb reduction
Reticulocytosis
Proerythroblast
Bled [2]
+
+
++
Parasite [2]
+
+
+
PHZ injection [2]
+
++
+
+: Significant increase compared with normal. ++: Significant increase compared other group.
Table 1: The comparing among the experimental anemic models.
Hematopoiesis occurs in the Bone Marrow (BM) of adult mammals under normal conditions. BM possesses a specialized microenvironment called the ‘niche’ that maintains Hematopoietic Stem Cells (HSCs) [6,7]. The stem cell niche, which is composed of cellular compartments, produces several cytokines, such as G-CSF, GM-CSF and SDF-1 [8,9].
Nitrogen-Containing Bisphosphonates (NBPs) have strong antibone resorption effects, and are used as therapeutic agents against bone resorption disorders such as osteoporosis and metastatic bone diseases [10-12]. We previously reported that mice injected with NBP developed decreased erythropoiesis in their BM (Figure 1) [13]. We induced severe anemia in a mouse model by injecting NBP in combination with Phenylhydrazine (PHZ), and we then analyzed the erythropoiesis and the levels of different types of hemoglobin in this model. Here, we review recent insights from the established severely anemic mouse model, and provide further understanding of the new proposed mechanism for recovery from anemia.
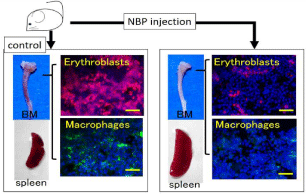
Figure 1: NBP injection impacts hematopoiesis.
The BM from NBP treated mice become white compared to that of control
mice, and splenomegaly is induced by NBP injection. NBP injection also
caused a reduction in the number of erythroblasts and resident macrophages
in the BM.
Bars = 50μm.
Effects of nitrogen-containing bisphosphonate on normal and splenectomized mice
The use of NBPs results in strong anti-bone resorption effects. In addition, NBPs have inflammatory side effects including fever, jaw osteomyelitis, osteonecrosis and extramedullary erythropoiesis [13- 15]. Our previous study reported that a single injection of a relatively large dose of NBP into mice induced Extramedullary erythropoiesis by depleting resident BM macrophages and increasing the number of granulocytes in the peripheral blood [13]. Moreover, the injection of NBP into splenectomized mice induced extramedullary erythropoiesis without anemia and caused changes in EPO concentrations in the liver [16].
A severely anemic mouse model
There are many experimental models of anemia that have been used to study this disease. We analyzed the changes in hematopoiesis after induction of anemia by inhibiting erythropoiesis in the BM. Splenectomized mice were treated with NBP to inhibit erythropoiesis in the BM, and with PHZ to induce hemolytic anemia. Treating animals with both NBP and PHZ induced more serious anemia than administering PHZ alone, and the concentration of EPO in the serum was also significantly increased (Figure 2A & 2B). Moreover, numerous nucleated erythrocytes and reticulocytes were observed in the peripheral blood of mice treated with both NBP and PHZ (Figure 2C).
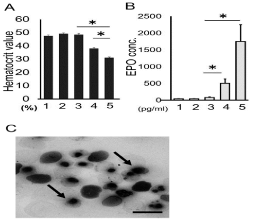
Figure 2: Blood analysis.
A .The hematocrit values of all groups at 5 days after treatment.
No change was observed following treatment with NBP alone, while a
significant reduction was observed following PHZ treatment. Severe anemia
was induced in the animals treated with both NBP and PHZ.
B. The serum EPO concentration. The PHZ and NBP group had significantly
increased EPO concentrations compared to the control.
C. Blood smears stained with May-GrÜnwaldGiemsa stain in the animals
treated with both NBP and PHZ. Nucleated erythroid cells were easily
detected in the both NBP- and PHZ-treated mice (arrows).
Bar = 10μm. *: P<0.05.
The number of erythroid lineage cells in the BM was significantl decreased following NBP injection. However, the proportion of these cells in the mice treated with NBP and PHZ was significantly increased despite the use of NBP [17]. This indicates that splenectomized mice treated with both NBP and PHZ become critically anemic and display a significant increase in EPO levels, which enhances erythropoiesis. The presence of nucleated erythrocytes in a blood smear suggested that abnormal erythropoiesis was induced, because nucleated erythrocytes are not normally observed in the blood in mammals, except during the early embryonic stages.
Expression of types of embryonic hemoglobin
Mammalian hematopoiesis occurs in two distinct waves, commonly referred to as the primitive and definitive waves, which originate in the yolk sac, and in the fetal liver and BM, respectively [18]. Yolk sac-derived primitive erythroid cells remain nucleated and enucleated terminally in circulation, whereas definitive erythroid cells produced in the fetal liver are released into circulation after complete maturation [5,6,19,20]. The components of the globin tetramer are encoded by the α- and β-globin gene loci. There are 3 functional α-globins (ζ-, α1- and α2) and 4 β -globins (Ey-, βh1-, β1- and β2-) in mice [6,21,22]. Primitive erythrocytes express the embryonic complement of globin chains, which initially consist of ζ- and βh1- globins, followed by α1- and α2-, and Ey-globins at the primitive proerythroblast stage [6,21-23]. Definitive erythroid cells complete their maturation and enucleation at erythropoietic sites, the fetal liver and BM, where the adult complement of globin chains consisting of α1-, α2-, β1-, and β2- are expressed.
Since we observed nucleated erythrocytes in the peripheral blood of severely anemic mice in our anemia model, we determined the expression of embryonic globins. The embryonic globins ζ-, βh1- and Ey- and the adult globins α- and β major were expressed in the BM and the liver in the severely anemic mice. We also identified the cell clusters that expressed the embryonic globins ζ-, βh1- and Ey- in the BM and liver of severely anemic mice [17]. These results suggest that abnormal erythropoiesis may occur in this critical anemia model, and that embryonic globins may be activated as a response to hypoxemia.
The factors relating to embryonic globin expression
The expression of globin genes is jointly regulated by elements in the promoter regions and an upstream enhancer region called the Locus Control Region (LCR) [6]. A variety of nuclear factors that are involved in transcriptional regulation are thought to be related to globin gene expression and switching between them [24,25]. Members of the KLF (Kruppel-like factor) family are essential transcription factors that bind GC-rich sequences to regulate the biological dynamic changes in globin expression during development [26]. KLF1 (EKLF: Erythroid Kruppel-like Factor) plays an essential role in erythropoiesis and is involved in the expression of β-like embryonic globin by binding to its promoter region [27,28]. KLF2 regulates biological activity in various tissues and enhances the expression of β-like embryonic globin [29,30].
The expression of several embryonic globin transcription factors was analyzed in severely anemic model mice. The expression of Klf1, Klf2 and Gata1was up-regulated. Moreover, the βh1- and Ey-globin promoters were bound to KLF1 and KLF2 in the BM and livers of mice treated with NBP and PHZ. These results indicate that KLF1 and/or KLF2 regulate the transcription of embryonic β-like globins in the BM and liver.
Induction of newly discovered hematopoietic structures
Erythropoiesis occurs in the bone marrow and the spleen even in healthy adult mice. Extramedullary erythropoiesis occurs in various organs, including the lungs, heart, thymus, and hemal nodes, under abnormal conditions such as acute anemia [31-38].
Unexpectedly, wine-colored structures appeared in the omentum or pancreas of our severely anemic model mice [39]. Histological examination identified lymphoid follicle-like cell clusters and welldeveloped sinusoids, similar to the spleen, and revealed that some hematopoietic cells, including mega karyocytes and erythroblasts, populated these structures [39]. The lymphoid follicle-like clusters were filled with erythroblasts, and most of these cells were proliferating. This suggests that these newly discovered structures in a severely anemic mouse model could be the site of extramedullary hematopoiesis.
HSC mobilization & G-CSF
G-CSF is a cytokine related to granulopoiesis and G-SCF production is enhanced in inflammation and stimulates granulopoiesis. G-CSF has other functions, namely the suppression of osteoblast lineage cells and inhibition of the expression of SDF-1, SCF and VCAM-1, which results in the mobilization of HSCs and HPCs from the BM to the peripheral tissues [40,41]. We examined the cell population in the peripheral blood and BM, and the GCSFlevels in our anemic mouse model.
We first determined the number of lineage-negative and c-kitpositive HPCs in the BM and peripheral blood in order to detect the mobilization of HPCs from the BM to the peripheral tissues. The population of HPCs was significantly decreased in the BM after NBP injection, whereas it was markedly increased in the peripheral blood during the same period following treatment. One day after NBP injection, the G-CSF levels increased precipitously and remained at a high concentration for up to 3 days after treatment, which correlated with the decrease in the number of HPCs in the BM. NBP is known to enhance granulopoiesis and inflammation [13], which might be mediated by G-CSF stimulation. Our results suggest that G-CSF production is stimulated by NBP treatment, which then provoked the mobilization of HPCs from the BM to the peripheral tissue (Figure 3).
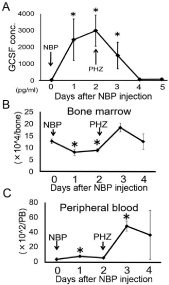
Figure 3: G-CSF concentration in serum and HPC numbers of severely
anemic mice.
Time-dependent change in serum G-CSF concentration. The serum G-CSF
concentration significantly increased 1-3 days after NBP injection compared
with 0 days after NBP injection. Number of lineage-negative, c-kit-positive
HPCs in the BM significantly decreased 1 and 2 days after NBP injection.
The number of HPCs in the peripheral blood significantly increased 1 day
after NBP injection.
*: P<0.05 (vs. control or 0 day).
Factors relating to extramedullary hematopoiesis
The recruitment of progenitors to the vascular niche involves signaling through SDF-1 [42]. The vascular niche seems to be associated with Extramedullary hematopoiesis in the liver and spleen through the expression of SDF-1, which plays an essential role in the homing of Hematopoietic Stem Cells (HSCs) and HPCs to the BM [43,44].
We detected some cytokines that are related to hematopoiesis, including SCF and SDF-1, in the omentum, even in normal conditions [39]. In particular, SDF-1 plays an essential role in the homing of HSCs and HPCs, and is associated with Extramedullary hematopoiesis [43,44].
The omentum seems to possess a suitable microenvironment for hematopoiesis because it expresses cytokines, including SDF1, even under normal conditions. Increased levels of these cytokines might support the observed mobilization of HPC homing, colonization and differentiation in the peripheral tissues in this study.
Conclusion
We established a severely anemic mouse model by sequential treatment with NBP and PHZ. This model demonstrated the emergence of nucleated erythrocytes in the peripheral blood, the expression of embryonic types of hemoglobin at hematopoietic sites, and the induction of newly discovered hematopoietic structures (Figure 4). Our results indicate the flexibility of hematopoiesis and suggest that that yolk sac-derived primitive erythroid cells may persist into adulthood in mice. Our insights from this severely anemic mouse model will contribute towards understanding the mechanism of anemia, and our ability to treat affected patients.
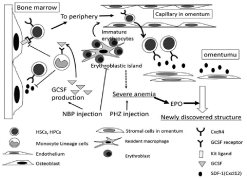
Figure 4: The pathological conditions and mechanisms of newly discovered
structure formation in the severely anemic mouse model.
The increased G-CSF production following NBP injection mediated HSC and/
or HPC mobilization, which colonized the omentum and provided the niche for
hematopoiesis by expressing some hematopoiesis-related factors, including
SDF-1. Moreover, the high EPO level stimulated erythropoiesis, resulting in
the expression of embryonic globins and the formation the new hematopoietic
structures.
Acknowledgement
Funding: This study was supported in part by JSPS KAKENHI Grants (Number 15K20367 and 15K11022) from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The authors declare no competing interests.
References
- Sankaran VG, Weiss MJ. Anemia: progress in molecular mechanisms and therapies. Nat Med. 2015; 21: 221-230.
- Moreau R, Malu TD, Dumais M, Dalko E, Gaudreault V, Rome H, et al. Alterations in bone and erythropoiesis in hemolytic anemia: comparative study in bled, phenylhydrazine treated and plasmodium -infected mice. PlosOne. 2012; 7: 46101.
- Perry JM, HarandiOF, Paulson RF. BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood. 2007; 109: 4494-4502.
- Berger J. Phenylhydrazinehaematotoxicity. J. Appl. Biomed. 2007; 5: 125-130.
- Zhu X, Liu J, Feng Y, Pang W, Qi Z, Jiang Y, et al. Phenylhydrazine administration accelerates the development of experimental cerebral malaria. Exp Parasitol. 2015; 156: 1-11.
- Palis J. Molecular biology of erythropoiesis. Wickrema A, Kee B, editors. In: Molecular Basis of Hematopoiesis. Springer. 2009; 73-93.
- McGrath KE, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol. 2005; 33: 1021-1028.
- Johns JL, Christopher MM. Extramedullaryhematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Pathol. 2012; 49: 508-523.
- Rankin EB, Wu C, Khatri R, Wilson TL, Andersen R, Araldi E, et al. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012; 149: 63-74.
- Body JJ. Rationale for the use of bisphosphonates in osteoblastic and osteolytic bone lesions. Breast. 2003; 12: 37-44.
- Cremers SC, Eekhoff ME, Den Hartigh J, Hamdy NA, Vermeij P, Papapoulos SE. Relationships between pharmacokinetics and rate of bone turnover after intravenous bisphosphonate (olpadronate) in patients with Paget's disease of bone. J Bone Miner Res. 2003; 18: 868-875.
- Oura S, Hirai I, Yoshimasu T, Kokawa Y, Sasaki R. Clinical efficacy of bisphosphonate therapy for bone metastasis from breast cancer. Breast Cancer. 2003; 10: 28-32.
- Nakamura M, Yagi H, Endo Y, Kosugi H, Ishi T, Itoh T. A time kinetic study of the effect of aminobisphosphonate on murinehaemopoiesis. Br J Haematol. 1999; 107: 779-790.
- Endo Y, Shibazaki M, Yamaguchi K, Nakamura M, Kosugi H. Inhibition of inflammatory actions of aminobisphosphonates by dichloromethylenebisphosphonate, a non-aminobisphosphonate. Br J Pharmacol. 1999; 126: 903-910.
- Yamaguchi K, Oizumi T, Funayama H, Kawamura H, Sugawara S, Endo Y. Osteonecrosis of the jawbones in 2 osteoporosis patients treated with nitrogen-containing bisphosphonates: osteonecrosis reduction replacing NBP with non-NBP (etidronate) and rationale. J Oral Maxillofac Surg. 2010; 68: 889-897.
- Otsuka H, Yagi H, Endo Y, Nonaka N, Nakamura M. Kupffer cells support extramedullaryerythropoiesis induced by nitrogen-containing bisphosphonate in splenectomized mice. Cell Immunol. 2011; 271: 197-204.
- Otsuka H, Takito J, Endo Y, Yagi H, Soeta S, Yanagisawa N, et al. The expression of embryonic globin mRNA in a severely anemic mouse model induced by treatment with nitrogen-containing bisphosphonate. BMC Hematol. 2016; 16: 4-16.
- Palis J, Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 2001; 29: 927-936.
- McGrath K, Palis J. Ontogeny of erythropoiesis in the mammalian embryo. Curr Top Dev Biol. 2008; 82: 1-22.
- Kingsley PD, Malik J, Fantauzzo KA, Palis J. Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood. 2004; 104: 19-25.
- Baron MH, Isern J, Fraser ST. The embryonic origins of erythropoiesis in mammals. Blood. 2012; 119: 4828-4837.
- Kingsley PD, Malik J, Emerson RL, Bushnell TP, McGrath KE, Bloedorn LA, et al. "Maturational" globin switching in primary primitive erythroid cells. Blood. 2006; 107: 1665-1672.
- McGrath KE, Frame JM, Fromm GJ, Koniski AD, Kingsley PD, Little J, et al. A transient definitive erythroid lineage with unique regulation of the beta-globin locus in the mammalian embryo. Blood. 2011; 117: 4600-4608.
- Sankaran VG, Xu J, Orkin SH. Advances in the understanding of haemoglobin switching. Br J Haematol. 2010; 149: 181-194.
- Tsiftsoglou AS, Vizirianakis IS, Strouboulis J. Erythropoiesis: model systems, molecular regulators, and developmental programs. IUBMB Life. 2009; 61: 800-830.
- McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010; 90: 1337-1381.
- Hodge D, Coghill E, Keys J, Maguire T, Hartmann B, McDowall A, et al. A global role for EKLF in definitive and primitive erythropoiesis. Blood. 2006; 107: 3359-3370.
- Drissen R, von Lindern M, Kolbus A, Driegen S, Steinlein P, Beug H, et al. The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability. Mol Cell Biol. 2005; 25: 5205-5214.
- Basu P, Lung TK, Lemsaddek W, Sargent TG, Williams DC, Jr, Basu M, et al. EKLF and KLF2 have compensatory roles in embryonic beta-globin gene expression and primitive erythropoiesis. Blood. 2007; 110: 3417-3425.
- Alhashem YN, Vinjamur DS, Basu M, Klingmuller U, Gaensler KM, Lloyd JA. Transcription factors KLF1 and KLF2 positively regulate embryonic and fetal beta-globin genes through direct promoter binding. J Biol Chem. 2011; 286: 24819-24827.
- Lenox LE, Perry JM, Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood. 2005; 105: 2741-2748.
- Bowling MR, Cauthen CG, Perry CD, Patel NP, Bergman S, Link KM, et al. Pulmonary extramedullary hematopoiesis. J Thorac Imaging. 2008; 23: 138-141.
- Meo A, Cassinerio E, Castelli R, Bignamini D, Perego,L, Cappellini MD. Effect of hydroxyurea on extramedullaryhaematopoiesis in thalassaemiaintermedia: case reports and literature review. Int J Lab Hematol. 2008; 30: 425-431.
- Halder RC, Abe T, Mannoor MK, Morshed SR, Ariyasinghe A, Watanabe H, et al. Onset of hepatic erythropoiesis after malarial infection in mice. Parasitol Int. 2003; 52: 259-268.
- Turner DR. The vascular tree of the haemal node in the rat. J Anat. 1969; 104: 481-493.
- Zidan M, Pabst R. Histology of hemal nodes of the water buffalo (Bosbubalus). Cell Tissue Res. 2010; 340: 491-496.
- Zhang W, Nasu T, Yasuda M. A mechanism for selective lymphocyte homing in bovine hemal nodes. Vet ImmunolImmunopathol. 2003; 156: 211-214.
- Casteleyn CR, Breugelmans S, Simoens P, Van den Broeck W. Morphological and immunological characteristics of the bovine temporal lymph node and hemal node. Vet ImmunolImmunopathol. 2008; 126: 339-350.
- Otsuka H, Yagi H, Endo Y, Soeta S, Nonaka N, Nakamura M. Nitrogen-containing bisphosphonate induced a newly discovered hematopoietic structure in the omentum of an anemic mouse model by stimulating G-CSF production. Cell Tissue Res. 2017; 367: 297-309.
- Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005; 106: 3020-3027.
- GreenbaumAM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011; 25: 211-217.
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004; 10: 64-71.
- Inra CN, Zhou BO, Acar M, Murphy MM, Richardson J, Zhao Z, et al. A perisinusoidal niche for extramedullaryhaematopoiesis in the spleen. Nature. 2015; 527: 466-471.
- Mendt M, Cardier JE. Role of SDF-1 (CXCL12) in regulating hematopoietic stem and progenitor cells traffic into the liver during extramedullaryhematopoiesis induced by G-CSF, AMD3100 and PHZ. Cytokine. 2015; 76: 214-221.