
Special Article - Eating Disorders
Ann Nutr Disord & Ther. 2017; 4(2): 1045.
Therapeutic Impact of Dysfunction in Reward Processing in Anorexia Nervosa - A Mini Review
Kulvinder Kochar Kaur¹*, Allahbadia G² and Singh M³
¹Centre for Human Reproduction, India
²Rotunda - A Centre for Human Reproduction, India
³Swami Satyanand Hospital, India
*Corresponding author: Kulvinder Kochar Kaur, Centre for Human Reproduction, Scientific Director, 721, G.T.B. Nagar, Jalandhar-144001, Punjab, India
Received: May 05, 2017; Accepted: May 25, 2017; Published: June 01, 2017
Abstract
Anorexia Nervosa (AN) is a disorder having a chronic course which is refractory to treatment in many patients and is one psychiatric disorder where greatest mortality rate exists. Hence understanding the basic pathophysiology is essential. Recently the dysfunction of reward processing has been highlighted in many reviews along with aberrant appetite motivators like leptin, ghrelin, BDNF and endocannabonoids signal is believed to affect not only homeostatic food systems but also hedonic circuits, just like importance of dopamine, acetylcholine, serotonin in reward processing is emphasized. This minireview tries to emphasize on the altered reward processing in such ED’s and in formulating newer therapies in a disorder not having any specific treatment till date.
Introduction
Cultural influences, psychological, biologic, genetic and social factors likely contribute to the development of eating disorders. Several have been associated with their development including a history of dieting, preoccupation with weight, athletic and artistic pursuits that favour leanness or involve subjective judging and possibly sexual abuse. Young women having a first degree relative with an eating or affective disorder or alcoholism are at an increased risk for developing an eating disorder. Linkage analysis has identified possible susceptibility loci for Anorexia Nervosa (AN) on chromosome1 and for Bulimia Nervosa (BN) on chromosome 10. Affective, anxiety And Obsessive Compulsive Disorders (OCD), personality disorders substance abuse are common in women with eating disorders. Family stresses referred to high perceived parental expectations (for success, achievement) poor communication and marital tension also may play a role.
The clinical spectrum of eating disorders varies on a limited period of amenorrhea associated with a crash diet in otherwise normal women to the grossly underweight anorexic having a distorted body image and the bulimic who cycle regularly between binge eating and purging behavior. The specific diagnostic criteria for AN and BN are defined in the Diagnostic &Statistical Manual of Mental Disorders (DSM IV) and are briefly summarized here. DSMIV categorizes those with clearly abnormal eating patterns and weight control habits which do not meet the specific criteria for AN or BN as having an eating disorder not otherwise specified.
Anorexia Nervosa (AN)
- Refuse to maintain weight within a normal range for height and age (<85%) of ideal body weight.
- Fear of gaining weigh tor becoming fat even though underweight.
- Disturbed body image with undue importance on weight or shape.
- In post menarcheal women functional amenorrhea.
- Episodic binge eating, consuming abnormally large amount of food with a sense of lack of control.
- Recurrent compensatory behavior including self induced vomiting or misuse of laxatives, diuretics or other medications, fasting or excessive exercise.
- Bingeing and purging behavior occurring at least twice/wk on average for an interval of 3months or more.
- Dissociation with body shape from image.
- The behavior does not occur exclusively during episodes of AN.
- In purging behavior includes regular self induced vomit or misuse of laxatives or diuretics.
- In 2nd other compensatory behaviors predominate like exercise.
Two subtypes of AN have been defined restricting and binge/ purging. In the 1st restriction of food intake is the first method of achieving weight control. In the second binging and purging via self induced vomiting or the use of laxatives or diuretics is the first method for controlling weight. In both compulsive exercise maybe an additional behavior strategy for maintaining or losing weight. Diagnostic criteria for BN are distinct from those for AN mainly as they do not include low body weight or amenorrhea. Because the mortality rate associated with this syndrome is significant it warrants close attention. The mortality rate is estimated to be around 6% [1]. Some studies have found that most patients recover and that there is not increase in mortality [2,3]. Initially it was thought to occur in young white middle to upper class females under 25 but now apparently it occurs in all socioeconomic groups with 0.5-1% young females being involved [4].
Bulimia Nervosa (BN)
Two subtypes again described-purging and non purging
Clinical features: Restrictive AN include weight loss, which frequently dates from a specific event like an illness, insensitive comments, rebuke or loss. Amenorrhea typically precedes weight loss which begins with dieting and specific restriction of fat intake. Affected women often admit fatigue, nausea, early satiety or bloating after meals. They exhibit a distorted body image, denial &disordered thinking and frequently use exercise as an additional weight control strategy. In women with AN the physical examination may reveal hypotension, bradycardia, low body temperature, dry skin [5] and lanugo (fine soft hair on the back ,buttocks and extremities).Thus while BN exhibits impulsive and addictive qualities on their behaviors, an inability to control binge eating and purging and frequently used cigarettes, alcohol and other drugs. Many have irregular menses, but not amenorrhea, and in most, weight fluctuates but is not abnormally low. Those with BN have parotid gland hypertrophy and erosion of their teeth enamel from frequent vomiting. Metabolic abnormalities associated with AN reflect dysfunctional hypothalamic regulation of appetite thirst, temperature, sleep, autonomic balance and endocrine secretion. Clinical consequences can be severe and even life threatening. The associated endocrine abnormalities include low FSH/LH/E2/IGF1, leptin concentrations and increased cortisol levels, prolactin, TSH and T4 levels are normal but the T3 levels low and Reverse T3 (r T3) high (r T3 is an isomer of T3 derived from T4 which binds but does not activate thyroid hormone receptors). With a gain all of metabolic abnormalities resolve [6]. Even though normal gonadotropin secretion may be restored with appropriate wt gain approximately 1/3rd remain amenorrheic likely reflecting persistent hypothalamic dysfunction [7].
Response Inhibition in ED’s
Behavior and personality characteristics vary in Eating Disorder (ED) patients depending on subtypes. Patients binge eating or purging behavior like AN, binge eating/purging type & or BN often show compulsive and disinhibited personality characteristics. While those with AN restricting type [8-10] often show a restrictive or overly controlled behavior style.
BN Is characterized by disinhibition & impulsivity related to eating behaviors [11,12]. Because of this vital hallmark of BN, DSMIV diagnostic description includes the disinhibited (out of control) characteristic [13]. Impulsitivity may also extend into other areas of life besides binge eating or purging [9]. Like patients with BN also show alcohol and drug abuse, self harm, sexual disinhibition and shoplifting [12]. Few data suggest that basis of cognitive and behavioral disinhibition in BN may also be related to serotonin dysregulation [14], while neuropsychiatric studies have found evidence of disinhibition at neurocognitive level in affected individuals. As compared to healthy subjects individuals with BN who used laxatives were seen to make greater errors of commissioning go/no go task for impulsive behaviors on a self report assessment [11]. Cognitive research in inhibition processing using a motor stop signal paradigm and a motor stroop task found that patients with a restrictive type AN displayed superior response inhibition. Overall fewer impulsive errors occur in patients with the binge eating/purging type [15]. AN patients with binge eating/purging behavior also made more response errors on a modified version of the Hayling sentence completion task relative to patients with restrictive subtype [15,16]. Thus behavior of similarities in impulsive cognitive styles between AN, binge eating/ purging type and BN they were grouped together as a single category of binge eating /purging type eating disorder in order to compare these patients with individuals in a restrictive type AN group and a healthy comparison group for this study of cognitive inhibitory control. Neural difference in the executive functioning related to inhibitory control maybe associated with the cognitive and clinical symptoms in BN [17-19]. Only few functional imaging studies have examined inhibition and disinhibition in BN till date. Marsh et al. studied response inhibition in adult patients with BN. They found that adult patients with BN responded more impulsively and made more errors on a response inhibition task (Simon task) [20], as compared to healthy comparison subjects and patients with the most severe symptoms made the most errors. Correct responding on incongruent trials, patients failed to activate frontostriatal circuits to the same degree as healthy comparison subjects including the bilateral inferior frontal gyrus, lenticular, caudate nuclei and the Anterior Cingulate Cortex (ACC). Marsh et al. concluded that diminished activity in these regions may contribute to the loss of control in eating behaviors of patients with BN. In contrast to patients with BN, clinical report show preservative, obsessive and rigid thinking styles in patients with AN. Patients with AN are commonly perfectionist and report obsessive compulsory personality traits and Obsessive Compulsive Disorders (OCD) in childhood [8]. Also studies show that patients who had AN but though recovered still showed traits of anxiety, perfectionism, inflexible thinking and over concern support/the likely importance of examining inhibition/disinhibition in adolescents with eating disorders. More work showed abnormal functioning of the inferior frontal and anterior cingulate gyrus is likely to be associated with impulsitivity and disinhibition in AN pts who binge eat and purge but not in those with the restrictive subtype [11]. Examination of response inhibition using the functional MRI in adolescents with ED’s specifically comparing those with restrictive type AN with those who had B Nor AN, Binge eating/purging type gives an opportunity to explore these processes in the developing brain and to distinguish patients with those subtypes from each other on a neural basis as well as from healthy comparison subjects. Examining neural correlates of inhibitory control in an adolescent population which is not severely emaciated and not chronically ill may help to distinguish these features associated with the onset of ED’s as opposed to the secondary effects associated with starvation and prolonged disease. Hence Lock et al. conducted a first study regarding brain activation associated with response inhibition in adolescents with ED’S and compared 13 female adolescents with binge eating and purging behavior (i.e. BN or AN binge eating /purging type). 14 with AN restricting type & 13 healthy normal controls who performed a rapid jittered event related go/nogo task. fMRI images were collected using a 3 Tesla GE scanner and a spiral pulse sequence. A whole brain 3 group analysis of variance in SPMS was used to identify significant activation associated with the main effect of group for the comparison of correct no go versus go trial. The mean activation in these clusters was extracted for further comparison in SPSS. They found that the binge eating/ purging group showed a markedly greater activation than the healthy comparison group in the bilateral precentral gyrus, ACC, middle and superior temporal gyrus as well as greater activation relative to both comparison and restrictive AN subjects in the hypothalamus and right dorsolateral prefrontal cortex. Within group analysis found that only the restrictive type AN group showed a positive correlation between the percent correct on go trials and activation in post visual and inferior parietal cortex regions. Thus they concluded that the study provided initial evidence that during adolescence, ED subtypes maybe distinguishable in terms of neural correlates of inhibitory control. This distinction is consistent with difference in behavior or impulsivity in this patient groups [21].
Altered Reward Processing in AN
Wagner et al. based on previous findings of altered striatal dopamine binding in AN tried to assess the response of the anterior ventral striatum to reward and loss in this disorder. They studied striatal responses to a simple monetary reward task using event related functional MRI. They compared 13normal women &13 women who had recovered from resting type AN and had 1 year of normal weight and regular menstrual cycles without binge eating or purging. They found that recovered women showed a significant positive relationship between trait anxiety and the percentage changes in haemodynamic signal in the caudate during either wins or loses .In contrast in the anterior ventral striatum comparison women distinguished positive and negative feedback whereas recovered women had similar responses to both conditions. Thus they concluded that individuals who have recovered from AN may have difficulties in differentiating positive and negative feedback. The exaggerated activation of the caudate, a region involved in linking action to outcome may constitute an attempt at ‘’strategic’’ (as opposed to hedonic) means of responding to reward stimuli. Thus they hypothesized that individuals of AN have an imbalance in information processing with impaired ability to identify the emotional significance of a stimulus but increased traffic in neurocircuits concerned with planning and consequences [22]. Figure 1 for neurocircuits associated with hedonic and goal directed behaviors.
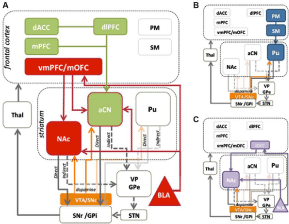
Figure 1: Cortico-striatal circuits involved in instrumental conditioning [34]. (A) Evaluative learning processes, shown in red, are mediated by bilateral connections
between the medial Orbitofrontal Cortex (mOFC) and Basolateral Amygdala (BLA), which are relayed to the anterior Caudate Nucleus (aCN). Contingency learning
processes, shown in green, are thought to occur in the medial Prefrontal Cortex (mPFC) and are relayed to the aCN to mediate control of action selection. Reward
information is also relayed to the Nucleus Accumbens (NAc) to provide motivational drive for the performance of instrumental behaviors. The dlPFC and dorsal
anterior cingulate cortex (dACC) play a role in comparing action values and can exert a modulator influence over circuits involving prefrontal and aCN activity.
Together, the contingency and evaluative circuits allow for the acquisition of goal-directed behaviors. (B) Stimulus-response associations, or habits, are mediated
by projections from Premotor (PM) and Sensori Motor Cortices (SM) to the posterior Putamen (Pu). (C) The lateral Orbitofrontal Cortex (lOFC) and the BLA encode
the value assigned to reward predictive stimuli, which the NAc uses to mediate instrumental performance. Mid-brain dopamine modulates plasticity in the dorsal
striatum, and is associated with motivational processes in the ventral striatum. The balance between striatal output to the Direct (D1) and indirect (D2) pathways
serves to promote or inhibit behavior, respectively.
Further Fladung et al. examined activity in the ventral striatum system in response to disease specific stimuli in women with acute AN. They examined 14 women with AN and 14 matched healthy comparison women who underwent fMRI during evaluation of visual stimuli depicting a female body with underweight and overweight canonical whole body features as per standardized BMI’s. All subjects were needed to process every stimulus in a self referring way. Ratings for each weight category were used as the control task. They found that behaviorally females with AN gave markedly higher positive ratings in response to underweight stimuli than in response to normal weight stimuli while normal comparison women showed greater preference for normal weight relative to underweight stimuli. Functionally ventral striatal activity showed a highly significant group by stimulus interaction for underweight and normal weight stimuli. In women with AN activation was higher during processing of underweight stimuli compared with normal weight stimuli. The reverse pattern was seen in healthy controls. Thus they concluded that these findings were consistent with predictions in animal studies of the important role of the human reward system in AN and thus support theories of starvation dependence in maintenance of this disorder [23].
Berridge et al. reviewed brain mechanisms known to generate “liking” or “wanting” for foods and evaluated their further interaction and regulatory mechanism of hunger and satiety, relevant to clinical issues. Liking mechanism include hedonic circuits which connect together cubic millimeter hotspots in forebrain limbic structures such as nucleus accumbens and ventral pallidum (where opioid/ endocannabinoid/orexin signals can amplify sensory pleasure). Wanting mechanisms include larger opioid networks in nucleus accumbens, striatum or amygdale which extend beyond the hedonic hotspots as well as mesolimbic dopamine systems and corticolimbic glutamate signals that interact with these systems. Further role in ED’s has been reviewed by Berridge et al. [24].
Further the role of serotonergic mechanisms in reward processing which has been neglected was reviewed by Kranz 2010. The evidence for this is gained from electrophysical and pharmacological as well as genetic and imaging studies. Primate research using single unit recordings of neurons within dorsal raphe nucleus argues for a serotonergic mediation of reward value whereas studies using intracranial self stimulation point to an important role of serotonin modulating motivational aspects of rewarding brain stimulation. Pharmacological studies using agonists and antagonists for a serotonin receptor subtypes and approaches investigating an increase or decrease of the extracellular level of serotonin offer strong evidence for a serotonergic mediation ranging from aversion to pleasure. Kranz et al. reviewed why serotonin should be considered as a basic mediator of emotional, motivational and cognitive aspects of rewarding representation which makes it possibly as important as dopamine in reward processing [25].
Gut and brain are closely connected and that this interaction plays an important part not only in GIT function but also in certain feeling states and in intuitive decision making is deeply rooted in our language. Current neurobiological insights into this gut - brain crosstalk have revealed a complex, bidirectional communication system which not only ensures the proper maintenance of GIT homeostasis and digestion but is likely to have multiple effects on affect, motivation and higher cognitive function including intuitive decision making. Further disturbances of this system have been implicated in a wide range of disorders including functional and inflammatory GIT disorders, obesity & ED [26].
Further Avena et al. reviewed the neurochemical evidence of reward related brain dysfunctions obtained through animal models of binge eating, BN or AN. They reported that findings suggested that alterations in Dopamine (DA), Acetylcholine (Ach) and opioid systems in reward related brain areas occur in response to binge eating of palatable foods. Moreover animal models of BN suggest that while bingeing of palatable foods release DA, purging attenuates release of Ach, which might otherwise signal satiety. Animal models of AN suggest that restricted access to food enhances the reinforcing effects of DA when animal does eat. The activity based anorexia model suggests alterations in mesolimbic DA and serotonin occur as a result of excessive wheel running. These findings with animal models complement data obtained through neuroimaging and pharmacotherapy studies of clinical populations. Information on the neurochemical consequences of the behavior association with these ED’s will be useful in understanding these complex disorders or may inform future therapeutic approaches [27].
Although neurobiological abnormalities as a consequence of starvation are controversial, still there is evidence regarding abnormalities in the reward system of patients as well as recovered individuals. Still an explanation for reward abnormalities observed in AN and their relevance to symptoms of the illness remain illunderstood. Theories explaining reward dysfunction have mostly focused on anhedonia, which describe that patients have an impaired ability to experience reward or pleasure. Keating et al. reviewing taste reward literature proposed that patients reduced response to conventional taste reward tasks may reflect a fear of weight gain associated with the caloric nature of the taste as compared to an impaired ability to experience reward. Thus they proposed that patients are capable of liking hedonic taste stimuli (e.g identifying them) however they do not want or feel motivated for the stimuli in the same way that healthy control report. Current brain imaging data on more complex reward processing tasks provide insight into frontostriatal neural circuit dysfunction related to altered reward processing in AN that challenge the relevance of anhedonia in explaining reward dysfunction in AN. In this way altered activity in the ACC and striatum could explain patients pathological engagement in behaviors they consider rewarding (e.g self starvation) that are otherwise aversive or punishing to those without the ED. Such evidence for altered patterns of brain activity associated with reward processing tasks in patients and recovered individuals may provide information about mechanism underlying symptoms of AN, future investigations and development of therapeutic approaches [28].
Monteleone 2013 reviewing the role of leptin, ghrelin, BDNF and endocannabonoids dysfunction in ED’s. Till recently the role of most appetite modulators in the control of eating behavior was conceptualized solely in terms of their influence on homeostatic control of energy balance. But now it is becoming more evident that appetite modulators also affect non homeostatic cognitive emotional rewarding component of food intake as well as non food related mechanisms. Therefore the possibility exists observed changes in appetite modulators in acute AN and BN represent not only homeostatic adaptations to malnutrition, but also contribute to the development and/or the maintenance of aberrant non homeostatic behaviors such as self starvation and binge eating. They gave evidence supporting a role of leptin, ghrelin BDNF and endocannabinoids in the homeostatic and non homeostatic dysregulation of patients with AN and BN. The literature reviewed is highly suggestive that changes in the physiology of these modulators may play a pivotal role in the pathophysiology of ED by providing a possible link between motivated behaviors, reward processing, cognitive functions and energy balance [29].
O’Hara examined neurobiological and psycho physiological evidence supporting a role for altered reward processes in the development and maintenance of AN. In AN ,there does not appear to be a generalized inability to experience reward. Rather data suggest that a reluctance to gain weight leads to an aversive appraisal of food and taste related stimuli. As a result cues compatible with the aberrant mode of thinking become rewarding for the individual. Also there is evidence that attribution of motivational salience to such cues promotes anorectic behaviors. These findings are consistent with modes in which interactions between cognition and reward are important in eliciting the anorectic habit. Hence they proposed a model which is consistent with elements of other theoretical frameworks but differs in that it emphasized towards neural overlap between AN and addiction. It is consistent with AN being a reward based learned behavior in which aberrant cognitions related to eating and shape alter functioning of central reward systems. It proposes that the primary neural problem responsible for the development, maintenance and treatment resistance is centered in the striatal reward system .This helps shift the emphasis of aetiological models towards processing, particularly in the context of illness –compatible cues. Furthermore it suggests that continuity to explore the utility and valued nature of AN in the patient’s life would be useful in inclusion in treatment and prevention models [30].
Treatment
It is disappointing that despite the impressive studies on AN there is no specific or new therapy available. This only serves to emphasize the need for early recognition to allow psychological intervention before the syndrome is entrenched in its full severity. Cognitive behavioral therapy to help patients cope with their feelings has been shown to be effective in randomized clinical trials. A team approach utilizing the primary clinician, a psychiatrist and a nutritionist is most effective. In addition treatment with antidepressants is worthwhile. Clinicians and parents should pay special attention to weight and diet in young women with amenorrhea. Even amenorrheic adolescents of normal or above normal body weights, disordered eating patterns (fasting and purging) are often present as an underlying stressful disorder [31].
A careful and gentle revelation to the patient of the relationship between amenorrhea and the low body weight is often all that is needed to see the patient frequently become involved in a programme of daily calorie counting of (minimum intake of 2600 calories) in order to break the patients established eating habits. If progress is slow, hormonal therapy should be initiated. In an adult weighing less than 100pounds continued weight loss requires psychiatric consultation. Some would argue that any patient with an eating disorder requires psychiatric intervention [32].
Going away to school or the development of a relationship with a male friend often are turning points for young women with mildmoderate AN. A failure to respond to these life changes is relatively ominous predicting a severe problems with a protracted course.
Deep Brain Stimulation (DBS) has been applied to a circuit based neuropsychiatric disease such as Parkinson’s disease and major depression with promising results. Lipman et al. aimed to assess the safety of DBS to modulate the activity of limbic circuits and to examine how this might affect the clinical features of AN. They did a phase 1 prospective trial of subcallosal cingulate DBS in 6 patients with chronic severe and treatment refractory AN. Eligible patients were aged 20-60 yrs, had been diagnosed with restrictive or binge eating AN and showed evidence of chronicity or treatment resistance. Patient underwent medical optimization preoperatively and had baseline BMI, psychometric and neuroimaging investigations followed by implantation of electrodes and pulse generation continuous delivery of electrical stimulation. Patients were followed up for 9mths after DBS activation and the primary outcome of side effects associated with surgery or stimulation was monitored at every follow up visit. Repeat psychometric assessment, BMI measurements and neuroimaging investigations were also done at various intervals. This trial is registered with clinical trials gov.No.NCT01476540. They found that DBS was associated with various side effects only one of which (seizure during programming roughly 2wks after surgery) was serious. Other related side effects were panic attack during surgery, nausea, air embolus and pain. After 9mths 3/6 patients had achieved and maintained a BMI>than their historical baseline. DBS was associated with improvement in mood, anxiety, affective regulation and AN related assessment and compulsion in 4 patients and with improvement in quality of life in 3 patients after 6mths of stimulation. These clinical benefits were accompanied by changes in cerebral glucose metabolism (seen in a comparison of composite PET scans at baseline and 6mths) which were consistent with a reversal of the abnormalities seen in the anterior cingulate, insula and parietal lobe in the disorder. Thus they interpreted that subcallosal cingulate DBS seems to be generally safe in the sample of patients with chronic refractory AN [33].
Conclusion
Thus reward processing is impaired in AN with emphasis on role of altered liking and wanting and cognitive behaviors with forced starvation becoming rewarding. There is importance in understanding how both obesity and AN can be compared to neurocircuits dysfunction similar to addiction and try to use it as a target for therapy.
References
- Sullivan PF. Mortality in anorexia Nervosa. Am J Psychiatr. 1995; 152: 1073.
- Wentz E, Gillberg C, Gillberg IC, Rastam M. Ten year follow up of adolescent onset anorexia nervosa: psychiatric disorders and overall functioning scales. J Child Psychol Psychiatr. 2001; 42: 613.
- Korndorfer SR, Lucas AR, Suman VJ, Crowson CS, Krahn LE, Melton LJ. Longterm survival of patients with anorexia nervosa: a population based studyin Rochester. Minn. Mayo Clinic Proc. 2003; 78: 278.
- Halmi KA. Eating disorder research in the past decade. Ann N Y AcadSci. 1996; 789: 67.
- Warren MP, Vande Wiele RL. Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol. 1973; 117: 435.
- Kims MB. Eating disorders in a general practice population. Prevalence, characteristics and follow up at 12 and 18mths. Psychiatr Med. 1989; 14: 1-34.
- Kochar Kaur Kulvinder, Allahbadia GN, Singh M. Hypothalamic Amenorrheaan Update on Aetiopathogenesis, Endocrine Profile and Management. J Gynaecol. 2016; 1: 1-18.
- Anderluch MB, Tchanturia K, Rabe-Hesketh S, Treasure JL. Childhood obsessive–compulsive personality traits in adult women with eating disorders: defining a broader eating disorder phenotype. Am J Psychiatr. 2003; 160: 242-247.
- Wagner A, Barbarich–Marstellar N, Frank GK, Bailer U, Wonderlich S, Crosby R, et al. Personality traits after recoveryfrom eating disorders: Do subtypes differ? Int J Eat Disord. 2006; 39: 276-284.
- Strober M, Freeman R, Lampert C, Diamond J. The association of anxiety disorders and obsessive compulsive personslity disorders with anorexia nervosa: evidence from a family study of discussion of nosological and neurodevelopmental implications. Int J Eat Disord. 2007; 40: 546-551.
- Bruce KR, Koerner NM, Steiger H, Young SN. Laxative misuse and behavioral disinhibitionin bulimia nervosa. Int J Eat Disord. 2003; 33: 92-97.
- Rosval L, Steiger H, Bruce K, Israel M, Richardson J, Aubut M. Impulsitivity in women with eating disorders: problems of response inhibition, planning or at. Int J Eat Disord. 2006; 39: 590-593.
- Wolfe RE, Metzger ED, Levine JM, Finkelstein DM, Cooper TB, Jimerson DC. Serotonin function following remission from bulimia nervosa. Neuropharmacology. 2000; 22: 257-263.
- Jimerson DC, Wolfe RE, Metzger ED, Finkelstein DM, Cooper TB, Levine JM. Decreased serotonin function in bulimia nervosa. Arch Gen Psychiatry. 1997; 55: 529-534.
- Southgate L. Response inhibition in anorexia nervosa and bulimia nervosa: an exploration of neuropsychological functions and their association with personality traits and behaviors (PhD thesis) King College London, Institute of Psychiatry. 2005.
- Roberts ME, Tchanturia K, Stahl D, Southgate L, Treasure J. A systematic review and metaanalysis of set shifting ability in eating disorders. Psychol Med. 2007; 37: 1075-1084.
- Gordon CM, Dougherty DD, Fishman AJ, Emans SJ, Grace E, Lamm R, et al. Neural substrates of anorexia nervosa: a behavioral challenge study with positron emission tomography. J Pediatr. 2001; 139: 51-57.
- Marsh R, Stein glass JE, Gerber AJ, Graziano O’Leary K, Wang Z, Murphy D, et al. Deficient activity in the neural systems that mediate self regulatory controlin bulimia nervosa. Arch Gen Psychiatry. 2009; 66: 51-63.
- Zasirow A, Kaiser S, Stippich C, Walther S, Herzog W, Tchanturia K, Beiger A, et al. Neural correlates of impaired cognitive behaviorsal flexibility in anorexia nervosa. Am J Psychiatr. 2009; 166: 608-616.
- Peterson BS, Karne MJ, Alexander GM, Lacadie C, Skidiarski P, Leong HC, et al. An event related functional MRI study comparing interference effects in the Simon and Stroop tasks .Brain Res Cogn Brain Res. 2002; 13: 427-440.
- Lock J, Garret A, Been hakker J, Reiss AL. Aberrant brain activation during a response inhibition task in Adolescent Eating Disorder Subtypes Am J Psychiatr. 2011; 168: 55-64.
- Wagner A, Aizenstein H, Venkatraman VK, Fudge J, Mazukewicz L, Frank GK, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatr. 2007; 164: 1842-1849.
- Fladung AK, Gron G, Grammer K, Hernbeger B, Schilly E, Grasteit S, et al. A Neural signature of Anorexia Nervosa in the Ventral Striatal Reward System. Am J Psychiatr. 2010; 167: 206-212.
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesiy and eating disorders. Br Res. 2010; 1350: 43-64.
- Kranz GS, Lanzenberger KR. Reward and the serotonergic system. Neuroscience. 2010; 166: 1023-1035.
- Mayer EA. Gut feelings: the emerging biology of gut-braincommunication. Nature Reviews. 2011; 12: 453-466.
- Avena NM, Bocarsly ME. Dysregulation of brain reward systems in eating disorders: Neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology. 2012; 63: 87- 96.
- Keating C, Tilbrook AJ, Rossell SL, Emticoti PG, Fitzgerald PB. Reward processing in Anorexia Nervosa. Neuropsychologia. 2012; 50: 567-575.
- Monteleone P, Maj M. Dysfunctions of leptin, ghrelin, BDNFand endocannabinoids in eating disorders: Beyond homeostatic controlof food intake. Psychoneuroendocrinology. 2013; 38: 312-330.
- O’Hara CB, Campbell IC, Schmidt U. Areward centred model of Anorexia Nervosa: A focussed narrative review of the neurological and psychophysiologicalliterature. Nuroscience and Neurobehavioral Reviews. 2015; 52: 131-152.
- Selzer R, Caust J, Hibbert M, Bowes G, Patton G. The association between secondary Amenorrheaand common eating disordered weight controlpractices in adolescent population. J Adolesc Health. 1996; 19: 56.
- American Psychiatric Association. Practice guidelines for the treatment of patients with eating disorders(revision). Am J Psychiatr. 2000; 157: 1.
- Lipman N, Woodside B, Giacobbe P, Hamani C, Carter JC, Norwood SJ, et al. Subcallosal cingulated deep brain stimulation for treatment refractory anorexia nervosa: a phase 1pilot trial; Lancet. 2013; 381: 1361-1370.
- Griffiths KR, Morris RW. Translational studies of goal directed action as a framework for classifying deficits across psychiatric disorders. Front Syst Neurosci. 2014; 8: 101.