
Research Article
Ann Obes Disord. 2016; 1(1): 1004.
Guaraná Supplementation Modulates Tryglicerides and Some Metabolic Blood Biomarkers in Overweight Subjects
Suleiman L¹, Barbisan F³, Ribeiro EE², Moresco RN³, Bochi G³, Marta Duarte MMF³, Antunes KT¹, Mânica-Cattani MF¹, Unfer TC¹, Azzolin VF³, Griner J¹ and Da Cruz IBM¹*
¹Programa De Pós-Graduação Em Bioquímica Toxicológica, Universidade Federal De Santa Maria, Brazil
²Universidade Aberta Da Terceira Idade, Universidade Do Estado Do Amazonas, Brazil
³Programa De Pós-Graduação Em Farmacologia, Universidade Federal De Santa Maria, Brazil
*Corresponding author: Da Cruz IBM, Programa De Pós-Graduação Em Bioquímica Toxicológica, Centro De Ciências Naturais E Exatas, Universidade Federal De Santa Maria, Av. Roraima 1000, Prédio 19, Santa Maria- RS, Brazil
Received: April 05, 2016; Accepted: April 26, 2016; Published: May 03, 2016
Abstract
Background: Guaraná (Paullinia cupana) commonly used in energetic beverage presents some functional properties including antiobesogenic effects. However, it is an open question if guaraná presents effect on atherosclerosis risk blood biomarkers (glycaemia, lipid, nutritional, oxidative-inflammatory), similar to other foods rich in catechins and caffeine such as green tea and coffee.
Material and Methods: We conducted a randomized, controlled, singleblind, clinical trial to test if a daily minimal guaraná supplementation (90 mg) could affect lipid, glucose and other metabolic blood biomarkers. Guaraná´s effects on oxidative markers were also evaluated. A total of 14 healthy overweight volunteers were selected considering similar lifestyle and clinical aspects. Fasting blood collection was collected before intervention and volunteers were oriented to intake capsules guaraná power (90mg/day) during 14 days. Blood collection after 7 and 14 days were performed in order to evaluate guarana´s effect on blood variables. In this period, volunteers were advised to abstain from any weight-loss program and to not drink caffeinate beverages. The same subjects also consumed placebo capsule during 14 days after a 45 days of washout.
Results: Guaraná supplementation significantly lowered the effect on triglycerides levels, uric acid, and oxidative blood biomarkers. On the contrary, an increase in blood albumin and total protein levels was also observed.
Conclusion: The present study suggests which short-term use of guaraná supplementation could causes positive effects on some human blood cardiovascular risk biomarkers, mainly blood triglycerides and this effect seems to be independent of weight loss.
Keywords: Guaraná; Cardiovascular; Ischemic; Triglycerides; Inflammation; Oxidative metabolism
Introduction
Obesity has become a major worldwide health mainly, because it increases cardiovascular risk through risk factors, such as increased fasting plasma triglycerides, high LDL cholesterol, low HDL cholesterol, elevated blood glucose and insulin levels, and high blood pressure [1]. Evidence suggests that foods rich in polyphenolic and alkaloids compounds, like caffeine, catechins and Epigallocatechin- 3-Gallate (EGCG), as coffee, green tea and yerba mate, present antiobesogenic effect, as well as action on lipid and inflammatory metabolism [1-4].
Guaraná, an Amazon native fruit, has gained popularity in developed countries, like the U.S., because is used to provide caffeine or energy boosts, such as energy and sports drinks [5-7]. This plant contains about twice the caffeine found in coffee beans (about 2-4.5% caffeine in guaraná seeds compared with 1–2% for coffee beans).In addition, guaraná chemical matrix also that presents other methylxanthines such as theobromine, theophylline. Guaraná also has saponins, catechins, epicatechins, and proanthocyanidols, along with other compounds, in trace concentrations in this nutritional matrix [8,9].
Despite the use as caffeine source, some previous investigations also suggested that guarana isused in some herbal preparations, presents positive effects on weight loss, increasing basal energy expenditure [10-13]. Moreover, previous investigations have suggested that guaraná could also positively affect lipid metabolism [14,15].
To test this hypothesis we performed a controlled study to test if the minimum daily guaraná dose (100 mg/day) supplementation could modulate blood biomarkers associated with lipid, glucose and oxidative blood biomarkers of healthy overweight adult.
Subjects and Methods
The study was undertaken in 14 volunteers (six male and eight female) with a mean age of 37.1 years old. All participated voluntarily in the study after giving their consent. The study was approved by the Ethics Committee of the Universidade Federal de Santa Maria (UFSM, No 23081.015838/2011-10). They were recruited in Rosário do Sul city, localized in the Southern Brazilian State (Rio Grande do Sul). They were not non-smokers, recreationally active, with Body Mass Index (BMI) > 23 and < 30 kg/m and without chronic morbidities. Body weight, height BMI, waist circumference and blood pressure were measured according to previously described by Krewer et al. [14].
A crossover, controlled intervention protocol was performed and divided in 4 consecutive periods (Figure 1). The protocol started with an initial washout period (7 days) during which the participants were instructed not to deviate from their regular habits and to maintain their normal diet and exercise level, avoiding medications and alcohol intake. The volunteers were advised to not ingest foods rich in caffeine such as coffee, tea, chimarrao, a traditional yerba mate beverage, and other nutritional supplements that could have an influence on biochemical variables analyzed. In the second period, the volunteers were supplemented with guaraná capsules during 14 days. The third wash-out period (75 days), the volunteers did not receive any supplementation, and they were advised to follow their everyday lifestyle. In the fourth period, the volunteers were supplemented again during 14 days with placebo capsules. The volunteers were informed that they would receive guaraná supplementation along with placebo. However, it was not informed to them whether supplementation with guaraná would occur in the first or second phase of the intervention. Fasting blood samples were also collected at baseline and after each period. At baseline and after each intervention period, a medical examination and a nutrient intake and physical activity, along with structured questionnaires were made.
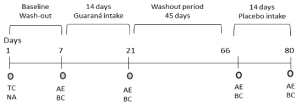
Figure 1: Experimental design. TC: Volunteer inclusion; Term consent sign;
NA: Nutritional Advisement. The volunteers were orientated to maintain
the same dietary pattern, with exception of caffeine and antioxidant foods
and supplement intake that were prohibited in the intervention periods; AE:
Anthropometric Examination; BC: Blood Collection. All volunteers were
supplemented with guaraná capsule (90 mg) and placebo capsule. The
randomization was performed to determine if the first intervention was made
with guaraná or placebo intake. The wash-out period is the interval where the
volunteers did not receive any treatment or dietary intervention.
In order to perform the trial, a health history, a physical examination and basic laboratory indices were collected on all subjects before their inclusion in the study protocol. The guaraná powder was packaged in capsules of 90mg to facilitate administration and ensure the correct bioavailability. The subjects took one capsule (90mg) every day between 8:00 AM and 9:00 AM during the intervention period (Figure 1).
The guaraná powder was supplied by EMBRAPA, Eastern Amazonia (Agropecuary Research Brazilian Enterprise) governmental sector that control the guaraná production in the Amazonas State. The bioactive compounds present in guaraná powder was used as a supplement in this study was previously determined by Bittencourt et al. [16] and bromatological characteristics were informed by EMBRAPA. We chose the minimal dose guaraná supplementation of 90 mg per day. Previous determination of caffeine (12.24%), theobromine (6.7%) and catechin (4.3%) were performed in the guaraná powder used in this study [17].
From this analysis, it was estimated that a daily ingestion of 22 mg of methylxanthines (mainly compounded by caffeine) from guaraná supplementation. This methylxanthines daily dose from guaraná supplementation is within the range recommended by ANVISA (15 to 70 mg), a Brazilian regulatory agency that has similar action that US Food & Drug Administration (FDA). This dose also decreased the probability to occurrence of cafeinism symptoms (Higgins et al., 2010) in the volunteers that could lead to a discontinuity of subjects during the treatment period.
Blood samples were collected after 12 h overnight fasting by venous puncture into Vacutainers® tubes (BD Diagnostics, Plymouth, UK). Fasting glucose, total cholesterol, HDL cholesterol, triglycerides, uric acid, creatinine, total proteins and albumin were evaluated using standard methods on Cobas MIRA® automated analyzer (Roche Diagnostics, Basel, Switzerland). Low-density lipoprotein cholesterol was estimated with the Friedewald equation. High-sensitivity C Reactive Protein (hs-CRP) was measured by nephelometry (Dade Behring, Newark, DE, EUA). Serum Ischemic Modified Albumin (IMA) used as an ischemic marker was measured by a colorimetric assay reported in Absorbance Units (ABSU).
The biomarkers of oxidative metabolism spectrophotometrically analyzed here were: total polyphenol content, lipid peroxidation quantified by measuring the formation of Thiobarbituric Acid Reactive Substances (TBARS) using method described by Jentzsch et al. [18,19]. Protein carbonyls were measured according to the method described by Morabito et al. [20]. Results were expressed as nanomoles of carbonyl groups per mg protein. The Catalase (CAT) enzyme was determined in whole blood using method described by Aebi [21] by measuring the rate of decomposition of H2O2 at 240 nm and Superoxide Dismutase Enzymes (SOD) activity using the method described by McCord and Fridovich [22]. Thiol groups were determined, as described by Ellman [23]. Total polyphenols were spectrophotometrically determined in plasma by reading the absorbance at 750 nm (Folin–Ciocalteau method) and using gallic acid as a standard, as described by Chandra and de Mejia [24]; Advanced Oxidative Protein Products (AOPP) was spectrophotometrically measured according Selmeci et al. [25] and Nitric Oxide (NO) according Tatsch et al. [26,27].
The statistical analyses were performed using SPSS software (Version 19.0). Initially, a Kolmogorov-Smirnov and Shapiro- Wilk tests were conducted to determine normality of variables. The values from data that did not present normal distribution were log transformed before statistical analysis. Data comparison baseline 1, 7 after 14 days of guaraná supplementation, baseline 1, 7 and after 14 days of placebo supplementation was compared by one-way analysis of variance followed by Bonferroni post hoc test. An additional multivariate analysis of variance was performed to evaluate the potential influence of sex and age in the results. The p values were two-tailed and the differences were considered to be statistically significant at p≤ 0.05.
Results
The baseline characteristics of all parameters evaluated in the volunteers that participated of the study are presented in (Table 1). Sex and age had not significant influence on the biochemical variables analyzed here.
Variables
Mean ± SD
95% CI
Age (years)
37.0 ± 8.3
32.1-41.8
BMI (Kg/m2)
27.1 ± 3.5
25.0-29.1
Waist circumference (cm)
93.7 ± 11.6
87.5-102.7
Systolic blood pressure (mmHg)
118.9 ± 11.8
112.1-125.7
Diastolic blood pressure (mmHg)
72.9 ± 7.3
68.7-77.1
Glucose (mg/dL)
86.1 ± 12.3
79.0-93.2
Cholesterol total (mg/dL)
192.8 ± 29.0
176.0-209.6
Triglycerides (mg/dL)
146.0 ± 36.7
103.6-188.4
LDL- cholesterol (mg/dL)
113.9 ± 27.2
98.2-129.6
HDL- cholesterol (mg/dL)
49.2 ± 11.1
42.7-51.8
Total protein (g/dL)
45.2 ± 4.9
42.4-48.1
Albumin (mg/dL)
4.5 ± 0.3
3.2-4.9
IMA (ABSU)
0.75 ± 0.13
0.67-0.82
Uric acid (mg/dL)
4.9 ± 1.2
4.0-5.8
HS-CRP (mg/L)
4.2 ± 4.3
1.7-7.5
Total Polyphenols (mgdL)
2.7 ± 0.3
2.52.9
Thiols (µmolmL plasma)
154.1 ± 28.2
137.7-170.3
Protein carbonyl (mg/dL)
0.47 ± 0.09
0.7-0.8
TBARS (nmol/mL erit)
16.3 ± 12.1
9.3-23.3
SOD (uSOD)
32.7 ± 11.9
25.7-39.5
Catalase (lmol /mL)
38.1 ± 3.4
31.4-36.6
NO (mmol/L)
140.9 ± 141.6
89.1-252.6
AOPP (mmol/L)
179.1 ± 42.2
154.6-203.6
SD: Standard Deviation; CI: Confidence Interval
Table 1: Baseline characteristics of adult healthy volunteers.
In the intervention period, no significant changes in BMI, waist circumference and blood pressure were observed in the volunteers. However, some biochemical parameters were affected by guaraná daily supplementation. The guaraná supplementation decreased the triglycerides levels (102.7 ± 38.5 mg/dL, p=0.015) and uric acid (4.3 ± 1.2 mg/dL, p=0.042) when compared to baseline values (Figure 2). On the other hand, guaraná daily intake increased the albumin levels (4.8 ± 0.23 mg/dL, p<0.0001) as well as total proteins (74.3 ± 4.1 g/dL, p= 0.0005) when compared to baseline values. When the volunteers were supplemented just placebo, these results were not observed, indicating guaraná modulated these variables (Figure 2).
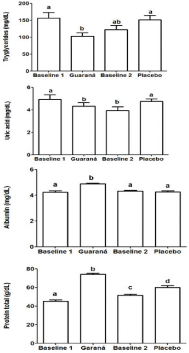
Figure 2: Guaraná and placebo effect on blood triglycerides, albumin, uric
acid and total protein levels (mean ± standard deviation). Different letters
indicate statistical differences determined by analysis of variance followed by
Bonferroni post hoc test at p< 0.05.
Some oxidative metabolism markers were also significantly affected by guaraná supplementation (Figure 3). A lowering in proteic, non proteic thiols (p=0.048) and SOD was observed after guaraná supplementation (p=0.0009). On the contrary, an increase in the catalase activity (p=0.001) was observed when volunteers intake guaraná supplementation (Figure 3).
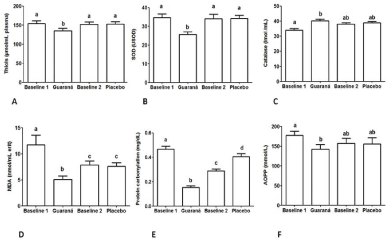
Figure 3: Guaraná and placebo effect on oxidative metabolism variables thiols total, superoxide dismutase, catalase, lipoperoxidation (TBARS/MDA), protein
carbonilation and Advance Oxidative Protein Products (AOPP) presented as mean ± standard deviation. Different letters indicate statistical differences determined
by analysis of variance followed by Bonferroni post hoc test at p< 0.05.
The follow blood markers related with oxidative stress decreased their levels in the guaraná supplementation: lipoperoxidation measured by TBARS (p=0.0015), protein carbonylation (p <0.0001) and AOPP (p=0.025). The glucose, other lipid parameters, hsCRP, IMA and NO were not influenced by the placebo treatment.
Multivariate analysis showed that these results were independent of sex and age of the volunteers.
Discussion
The present investigation described guarana’s effectson particular metabolic molecules despite a short time of supplement ingestion. Overweight volunteers presented 20% lowering in triglycerides levels when compared to basal values. This effect is according of previous investigations that described guaraná effects on lipid metabolism. An in vitro and in vivo protocols performed by Portella et al. [15] showed that guaraná, had some effect on LDL oxidation that could partially explain the protective effects of this food in cardiometabolic diseases.
A recent investigation performed in hypercholesterolemic rat’s evaluated 30 days guaraná treatment on purinergic and inflammatory molecules: ecto-nucleoside triphosphate diphosphohydrolase and ecto-adenosine deaminase activity in lymphocytes. Three guaraná concentrations were tested (12.5, 25 and 50 mg/kg/day) and the results showed that in the highest guaraná concentration was able to decrease ecto-adenonosine deaminase activity, total cholesterol, LDL-cholesterol and inflammatory process [17].
The results described here also corroborate a previous eight weeks randomized study performed by Boozer et al. [11] that examined the efficacy for weight loss of an herbal supplement containing Ma Huang, guaraná and other ingredients. The authors reported triglycerides lowering in subjects supplemented with herbal mixture containing guaraná.
The guaraná effects on triglycerides is probably related to a chemical composition that presents high caffeine concentrations and other methylxanthines such as theobromine, theophylline. This plant contains about twice the caffeine found in coffee beans (about 2-4.5% caffeine in guaraná seeds compared with 1-2% for coffee beans) [18]. Guaraná also has saponins, catechins, epicatechins, and proanthocyanidols, along with other compounds, in trace concentrations in this nutritional matrix [19]. Probably, lowering guaraná effects on triglycerides is associated with catechins detected in the guaraná powder. Experimental in vitro and in vivo studies have suggested that catechins are able to inhibit the intestinal absorption of dietary lipids. These molecules also inhibit the glycerol-3- phosphate dehydrogenase that catalyses the β-Nicotinamide Adenine Dinucleotide (NADH)-dependent reduction of Dihydroxyacetone Phosphate (DHAP) to yield glycerol-3-phosphate, which serves as one of the major precursors of triacylglycerols [28,29].
Other results described here showed guaraná influence on albumin and total protein. Serum albumin, the most abundant protein in the blood, plays an important role in the reversible binding of many compounds, being the most important carrier of dietary flavonoids [30]. However, we are not able to identify studies showing the potential stimulation of foods rich in catechin and/or caffeine on albumin levels. Therefore, complementary investigations need to be performed to understand the nature of the association between guaraná ingestion and blood albumin levels.
We also found a decrease in plasmatic uric acid levels after 14 days of guaraná powder supplementation. Hyperuricaemia may result from an increased production of uric acid, decreased renal excretion, or both. Serum uric acid has been found to be related not only to a risk of clinical gout, but also to hypertension, diabetes mellitus and cardiovascular diseases. Previous studies as performed by Kiyohara et al. [31] had 2240 Japanese male, self-defense officials, describe that the consumption of a beverage rich in caffeine, such as coffee and green tea ,were related to lower serum uric acid concentrations. The authors hypothesized that as caffeine has a diuretic action probably associated with an increase in renal blood flow, caffeine-rich beverages might increase the renal excretion of uric acid, thereby lowering serum uric acid concentrations. The guaraná seed is a very rich caffeine source, therefore, we can hypothesize that a similar effect described in coffee and green tea beverages could be found in a guaraná powder supplementation. Additionally, the increase in total protein and albumin concentration could also be related to the diuretic effect related to caffeine present in guaraná powder.
Guaraná supplementation presented an important effect on the blood concentration of the most oxidative metabolism biomarkers studied here. Oxidative biomarkers like lipoperoxidation, protein carbonylation and AOPP decreased the levels after 14 days of guaraná supplementation. However, we also observed a decreasing in enzymatic antioxidant markers (SOD and catalase) as well as in total thiois levels. These results are opposed by Bittencourt et al. [17] that found an increase in SOD levels when embryonic fibroblast culture (NIH-3T3 cells) were exposed to Sodium Nitroprusside (SNP), that generate high intracellular Nitric Oxide (NO) levels, with and without guaraná supplementation.
On the other hand, in vitro investigations as performed by Zeidán-Chuliá [32] showed that treated human neuronal SH-SY5Y cells with caffeine, taurine and guaraná,found that these compounds reduced SOD and catalase activities. These contradictory data could indicate that guaraná affects the SOD activity but this effect is dependent of experimental conditions and the guaraná concentration. The authors also found that excessive removal of intracellular ROS, to nonphysiological levels could cause important in vitro toxicity induced by these drugs.
The present study has some methodological concerns which including low numbers of subjects and short guaraná treatment time. However, we are looking for volunteers to participate of the study that presented some uniformity in lifestyle and clinical terms. As habitual diet of Western populations is rich in caffeine beverage, we opted to perform a short-time investigation to guarantee that volunteer’s did not consume concomitantly other caffeinate products that could present some influence in the results of this investigation. Despite methodological limitations, the study is in line with previous investigations that suggested positive effects of functional foods rich in polyphenols and caffeine on cardiometabolic risk factors associated with atherogenesis [33].
Conclusion
In conclusion, these results suggests that daily low dose intake of guaraná (90 mg) may exert beneficial and short-time effects on some variables as triglycerides, uric acid, and oxidative parameters related to cardiometabolic physiology without weight loss. In addition, we cannot discard the guaraná effect on other blood metabolic variables after more time of supplementation.
Acknowledgment
The authors thank Mr. Sérgio Mesquita Dantas from Laboratório Proanálise (Rosário do Sul, Brasil) and Centro Vérdi-Fisioterapia, Hidroterapia e Nutrição for help in blood and anthropometric data collection. Financial support: this research was supported by the Brazilian government research agencies: FAPERGS, FAPEAM and CNPq. The authors declare no conflict of interest.
References
- Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013; 5: 1218-1240.
- Heckman MA, Weil J, Gonzalez de Mejia E. Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci. 2010; 75: 77-87.
- Bøhn SK, Ward NC, Hodgson JM, Croft KD. Effects of tea and coffee on cardiovascular disease risk. Food Funct. 2012; 3: 575-591.
- Bracesco N, Sanchez AG, Contreras V, Menini T, Gugliucci A. Recent advances on Ilex paraguariensis research: minireview. J Ethnopharmacol. 2011; 136: 378-384.
- Smith N, Atroch AL. Guaraná's Journey from Regional Tonic to Aphrodisiac and Global Energy Drink. Evid Based Complement Alternat Med. 2010; 7: 279-282.
- Schimpl FC, da Silva JF, Gonçalves JF, Mazzafera P. Guarana. Revisiting a highly caffeinated plant from the Amazon. J Ethnopharmacol. 2013; 150: 14-31.
- Higgins JP, Tuttle TD, Higgins CL. Energy beverages. Content and safety. Mayo Clin Proc. 2010; 85: 1033-1041.
- Bempong DK, Houghton PJ. Dissolution and absorption of caffeine from guarana. J Pharm Pharmacol. 1992; 44: 769-771.
- Angelo PC, Nunes-Silva CG, Brigido MM, Azevedo JS, Assunção EN, Sousa AR, et al. Guaraná (Paullinia cupana var. sorbilis), an anciently consumed stimulant from the Amazon rain forest: the seeded-fruit transcriptome. Plant Cell Rep. 2008; 27: 117-124.
- Andrews KW, Schweitzer A, Zhao C, Holden JM, Roseland JM, Brandt M, et al. The caffeine contents of dietary supplements commonly purchased in the US: analysis of 53 products with caffeine-containing ingredients. Anal Bioanal Chem. 2007; 389:231-239.
- Boozer CN, Nasser JA, Heymsfield SB, Wang V, Chen G, Solomon JL. An herbal supplement containing Ma Huang-Guarana for weight loss: a randomized, double-blind trial. Int J Obes Relat Metab Disord. 2001; 25: 316-324.
- Opala T, Rzymski P, Pischel I, Wilczak M, Wozniak J. Efficacy of 12 weeks supplementation of a botanical extract-based weight loss formula on body weight, body composition and blood chemistry in healthy, overweight subjects--a randomised double-blind placebo-controlled clinical trial. Eur J Med Res. 2006: 11: 343-350.
- Lima WP, Carnevali LC, Eder R, Costa Rosa LF, Bacchi EM, Seelaender MC. Lipid metabolism in trained rats: effect of guarana (Paullinia cupana Mart.) supplementation. Clin Nutr. 2005; 24: 1019-1028.
- Krewer Cda C, Ribeiro EE, Ribeiro EA, Moresco RN, da Rocha MI, Montagner GF, et al. Habitual intake of guaraná and metabolic morbidities: an epidemiological study of an elderly Amazonian population. Phytother Res. 2011; 25: 1367-1374.
- Portella Rde L, Barcelos RP, da Rosa EJ, Ribeiro EE, da Cruz IB, Suleiman L, et al. Guaraná (Paullinia cupana Kunth) effects on LDL oxidation in elderly people: an in vitro and in vivo study. Lipids Health Dis. 2013; 12: 12.
- Ruchel JB, Rezer JF, Thorstenberg ML, Dos Santos CB, Cabral FL. Hypercholesterolemia and Ecto-enzymes of Purinergic System: Effects of Paullinia cupana. Phytother Res. 2016; 30: 49-57.
- Krewer CC, Suleiman L, Duarte MMM, Ribeiro EE, Mostardeiro CP, Montano MAE, et al. Guaraná, a supplement rich in caffeine and catechin, modulates cytokines: evidence from human in vitro and in vivo protocols. Euro Food Res Techn. 2014; 239: 49-57.
- Bittencourt LS, Machado DC, Machado MM, Dos Santos GF, Algarve TD, Marinowic DR, et al. The protective effects of guaraná extract (Paullinia cupana) on fibroblast NIH-3T3 cells exposed to sodium nitroprusside. Food Chem Toxicol. 2013; 53: 119-125.
- Jentzsch AM, Bachmann H, Fürst P, Biesalski HK. Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med. 1996; 20: 251-256.
- Morabito F, Cristani M, Saija A, Stelitano C, Callea V, Tomaino A, et al. Lipid peroxidation and protein oxidation in patients affected by Hodgkin's lymphoma. Mediators Inflamm. 2004; 13: 381-383.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105: 121-126.
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969; 244: 6049-6055.
- ELLMAN GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82: 70-77.
- Chandra S, Gonzalez de Mejia E. Polyphenolic compounds, antioxidant capacity, and quinone reductase activity of an aqueous extract of Ardisia compressa in comparison to mate (Ilex paraguariensis) and green (Camellia sinensis) teas. J Agric Food Chem, 2004; 52: 3583-3589.
- Selmeci L, Seres L, Antal M, Lukács J, Regöly-Mérei A, Acsády G. Advanced oxidation protein products (AOPP) for monitoring oxidative stress in critically ill patients: a simple, fast and inexpensive automated technique. Clin Chem Lab Med. 2005; 43: 294-297.
- Tatsch E, Bochi GV, Pereira Rda S, Kober H, Agertt VA, De Campos MM, et al. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem. 2011; 44: 348-350.
- Harrold JA, Hughes GM, O'Shiel K, Quinn E, Boyland EJ, Williams NJ, et al. Acute effects of a herb extract formulation and inulin fibre on appetite, energy intake and food choice. Appetite. 2013; 62: 84-90.
- Bérubé-Parent S, Pelletier C, Doré J, Tremblay A. Effects of encapsulated green tea and Guarana extracts containing a mixture of epigallocatechin-3-gallate and caffeine on 24 h energy expenditure and fat oxidation in men. Br J Nutr. 2005; 94: 432-436.
- Kao CC, Wu BT, Tsuei YW, Shih LJ, Kuo YL, Kao YH. Green tea catechins: inhibitors of glycerol-3-phosphate dehydrogenase. Planta Med. 2010; 76: 694-696.
- Pal S, Saha C. A review on structure-affinity relationship of dietary flavonoids with serum albumins. J Biomol Struct Dyn. 2014; 32: 1132-1147.
- Kiyohara C, Kono S, Honjo S, Todoroki I, Sakurai Y, Nishiwaki M, et al. Inverse association between coffee drinking and serum uric acid concentrations in middle-aged Japanese males. Br J Nutr. 1999; 82: 125-130.
- Zeidán-Chuliá F, Gelain DP, Kolling EA, Rybarczyk-Filho J, Ambrosi P, Resende Terra S, et al. Major components of energy drinks (caffeine, taurine, and guarana) exert cytotoxic effects on human neuronal SH-SY5Y cells by decreasing reactive oxygen species production. Oxi Med Cell Long. 2013; 2013: 791-795.
- Baboota RK, Bishnoi M, Ambalam P, Kiran KK, Sarma SM, Boparai RK, et al. Functional food ingredients for the management of obesity and associated co-morbidities - A review. J Funct Foods. 2013; 5: 997-1012.