
Editorial
Ann Obes Disord. 2017; 2(1): 1018.
Caffeine Consumption and Induction of Obesity in the Developed World
Martins IJ1,2,3*
¹School of Medical Sciences, Edith Cowan University, Australia
²School of Psychiatry and Clinical Neurosciences, University of Western Australia, Australia
³McCusker Alzheimer’s Research Foundation, Hollywood Medical Centre, Australia
*Corresponding author: Ian James Martins, School of Medical Sciences, Edith Cowan University, 270 Joondalup Drive, Joondalup, Western Australia 6027, Australia
Received: January 13, 2017; Accepted: January 19, 2017; Published: January 23, 2017
Keywords
Caffeine; Appetite; Obesity; Adipose tissue; Non alcoholic fatty liver disease; Magnesium
Editorial
The susceptibility of humans to obesity favors the deposition of fat with fat deposition in man much higher than compared with other species. The global increase in Non Alcoholic Fatty Liver Disease (NAFLD) is linked to the obesity epidemic and appetite dysregulation [1-3] is possibly the major defect in man with over eating the major cause of adiposity and NAFLD. Environment and genes have been indicated to play a major role in the global obesity epidemic with alterations in the appetite centre early in life relevant to the defective adipose tissue-liver crosstalk [4]. Induction of NAFLD may favor adipogenesis with consumption of high calorie diets, lack of essential nutrients, palmitic acid rich diets, nuclear receptor inhibitors, magnesium deficiency and bacterial lipopolysaccharides all relevant to liver inflammation and steatosis (Figure 1).
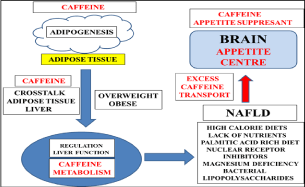
Figure 1: Caffeine consumption in overweight and obese individuals has
become important to maintain the adipose tissue-liver crosstalk that is
important to maintain lipid metabolism in adipocytes and hepatocytes. Obese
individuals with NAFLD should be carefully monitored with relevance to diet,
lifestyle and environment interactions that may prevent NAFLD induction
and defective liver caffeine metabolism. Caffeine is an appetite suppressant
but obesity and NAFLD may allow excessive caffeine transport to the brain
with induction of brain disorders that are connected to the adipose-tissue
crosstalk.
After consumption of a fat meal fatty acids are transported to the adipose tissue for storage or metabolism. The liver is involved with rapid clearance of lipoproteins that increase in the blood plasma after a fat meal with fatty acids rapidly metabolized by mitochondria in hepatocytes [1]. The crosstalk between the adipose tissue and the liver for storage and metabolism of fat is important to prevent adiposity and adipogenesis [5-7] and this interplay between the adipose tissue and liver becomes defective in overweight individuals early in life. Overweight is defined with a Body Mass Index (BMI) between 25 and 30 with obesity defined as a BMI > 30 (weight in Kg/[height m]2). Interests in caffeine and adipogenesis has accelerated with relevance to treatment of overweight/obese individuals and the prevention of adipocyte dysfunction early in life linked to caffeine ingestion important to the sympathetic nervous system, lipolysis and lipid oxidation [8-12].
Coffee contains caffeine and other components with the effects of other components also relevant to the reduced risk for obesity [10].
Coffee but not caffeine determines the expression of the Peroxisome Proliferator-Activated Receptor γ (PPAR γ) a transcription factor controlling the differentiation of adipocytes with PPAR γ [11] also closely linked to other nuclear receptors associated with fat metabolism in the adipose tissue and liver [13]. Caffeine in humans has become important to appetite regulation and is now considered to act as an appetite suppressant [14]. The dose of caffeine used in obesity [15] has become important with relevance to the NAFLD epidemic [16- 22] and pharmacokinetics of caffeine [23,24] completely impaired in the liver (NAFLD) in overweight/obese individuals. Inducing factors for NAFLD (Figure 1) override the beneficial effects of caffeine on adipocyte/liver fat metabolism [25,26].
With excess transport of caffeine to the brain and induction of Type 3 diabetes [24,27]. The effects of brain and interactions between sympathetic/parasympathetic innervation (autonomic nervous system) are closely connected to fat metabolism in the adipocyte and liver with defective interactions (brain, liver and adipocytes) induced by excess brain caffeine in overweight/obese individuals with relevance to appetite dysregulation, circadian rhythm abnormalities, accelerated brain aging and Type 3 diabetes [2,3,28-30].
With excess transport of caffeine to the brain and induction of Type 3 diabetes [24,27]. The effects of brain and interactions between sympathetic/parasympathetic innervation (autonomic nervous system) are closely connected to fat metabolism in the adipocyte and liver with defective interactions (brain, liver and adipocytes) induced by excess brain caffeine in overweight/obese individuals with relevance to appetite dysregulation, circadian rhythm abnormalities, accelerated brain aging and Type 3 diabetes [2,3,28-30].
The effects of caffeine treatment to prevent adipocyte dysfunction is superseded in overweight/obese individuals by magnesium deficiency in overweight and obese individuals [31-35]. Magnesium deficiency may occur by inadequate intake in children and young adults and may induce obesity and brain disorders with relevance to defective brain and peripheral organ (adipose tissue, liver, and pancreas) interactions. Caffeine may induce magnesium deficiency (Figure 1) [24] and the plasma concentrations of caffeine and magnesium should be carefully assessed in individuals with NAFLD [16-22] to prevent hyperglycemia induced mitochondrial apoptosis [36-39]. LPS as an inducing factor for obesity [40,41] has become of major concern with its effects overriding the beneficial effects of caffeine with LPS involved in magnesium deficiency [36,42,43]. Various caffeine sources for caffeine consumption include coffee, coca-cola, herbs and plants and excessive intake should be avoided to prevent caffeine toxicity to the liver and brain in childhood obesity.
Conclusion
Humans as a species are more susceptible to obesity and NAFLD with low magnesium intake connected to mitochondrial apoptosis in adipocytes/hepatocytes relevant to impaired mitochondrial oxidative capacity and systemic insulin sensitivity. Caffeine intake to improve lipid metabolism in obesity should be carefully controlled with relevance to defective caffeine metabolism in obese individuals with NAFLD. Defective caffeine metabolism in obese individuals can allow excessive caffeine transport to the central nervous system with effects on appetite regulation and induction of Type 3 diabetes. Connections between LPS and magnesium deficiency may override the beneficial effects of caffeine on the maintenance of the adipocyte-liver crosstalk important to the prevention of NAFLD in obesity.
Acknowledgement
This work was supported by grants from Edith Cowan University, the McCusker Alzheimer’s Research Foundation and the National Health and Medical Research Council and the CRC.
References
- Martins IJ. Appetite Control with Relevance to Mitochondrial Biogenesis and Activation of Post-Prandial Lipid Metabolism in Obesity Linked Diabetes. Ann. Obes. Disord. 2016; 1: 1-3.
- Martins IJ. Lambert BOOK APPETITE ISBN 978-3-659-40372-90. Appetite dysregulation and obesity in Western Countries. Ebook; 2015. First edited by Emma Jones; LAP LAMBERT Academic Publishing. ISBN: 978-3-659-40372-9/2013.
- Martins IJ. Nutritional diets accelerate amyloid beta metabolism and prevent the induction of chronic diseases and Alzheimer’s disease. Photon ebooks. 2015.
- Martins IJ. Unhealthy Nutrigenomic Diets Accelerate NAFLD and Adiposity in Global communities. J Mol Genet Med. 2015; 9: 1-11.
- Camp HS, Ren D, Leff T. Adipogenesis and fat-cell function in obesity and diabetes. Trends Mol Med. 2002; 8: 442-447.
- Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007; 56: 1517-1526.
- McGregor RA, Choi MS. microRNAs in the regulation of adipogenesis and obesity. CurrMol Med. 2011; 11: 304-316.
- Su SH, Shyu HW, Yeh YT, Chen KM, Yeh H, Su SJ. Caffeine inhibits adipogenic differentiation of primary adipose-derived stem cells and bone marrow stromal cells. ToxicolIn Vitro. 2013; 27: 1830-1837.
- Kim AR, Yoon BK, Park H, Seok JW, Choi H, Yu JH, et al. Caffeine inhibits adipogenesis through modulation of mitotic clonal expansion and the AKT/GSK3 pathway in 3T3-L1 adipocytes. BMB Rep. 2016; 49: 111-115.
- Nordestgaard AT, Thomsen M, Nordestgaard BG. Coffee intake and risk of obesity, metabolic syndrome and type 2 diabetes: a Mendelian randomization study. Int J Epidemiol. 2015; 44: 551-565.
- Aoyagi R, Funakoshi-Tago M, Fujiwara Y, Tamura H. Coffee inhibits adipocyte differentiation via inactivation of PPARγ. Biol Pharm Bull. 2014; 37: 1820-1825.
- Romijn JA, Fliers E. Sympathetic and parasympathetic innervation of adipose tissue: metabolic implications. CurrOpinClinNutrMetab Care. 2005; 8: 440-444.
- Martins IJ. Increased Risk for Obesity and Diabetes with Neurodegeneration in Developing Countries. J Mol Genet Med. 2013; S1: 1-8.
- Jessen A, Buemann B, Toubro S, Skovgaard IM, Astrup A. The appetite-suppressant effect of nicotine is enhanced by caffeine. Diabetes ObesMetab. 2005; 7: 327-333.
- Abernethy DR, Todd EL, Schwartz JB. Caffeine disposition in obesity. Br J ClinPharmacol. 1985; 20: 61-66.
- Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010; 51: 679-689.
- Patell R, Dosi R, Joshi H, Sheth S, Shah P, Sarfaraz S. Non-Alcoholic Fatty Liver Disease (NAFLD) in Obesity.JClinDiagn Res. 2014; 8: 62-66.
- Wong RJ, Ahmed A. Obesity and non-alcoholic fatty liver disease: Disparate associations among Asian populations. World J Hepatol. 2014; 6: 263-273.
- Targher G, Byrne CD. Obesity: Metabolically healthy obesity and NAFLD. Nat Rev GastroenterolHepatol. 2016; 13: 442-444.
- Ezzat WM, Ragab S, Ismail NA, Elhosary AY, NourEldinAbdElBaky AM, Farouk H, et al. Frequency of non-alcoholic fatty liver disease in overweight/obese children and adults: Clinical, sonographic picture and biochemical assessment. Journal of Genetic Engineering and Biotechnology. 2012; 10: 221-227.
- Suano de Souza FI, SilverioAmancio OM, SaccardoSarni RO, Sacchi Pitta T, Fernandes AP, Affonso Fonseca FL, et al. Non-alcoholic fatty liver disease in overweight children and its relationship with retinol serum levels. Int J VitamNutr Res. 2008; 78: 27-32.
- Kim NH, Kim JH, Kim YJ, Yoo HJ, Kim HY, Seo JA, et al. Clinical and metabolic factors associated with development and regression of nonalcoholic fatty liver disease in nonobese subjects. Liver Int. 2014; 34: 604-611.
- Acheson KJ, Gremaud G, Meirim I, Montigon F, Krebs Y, Fay LB, et al. Metabolic effects of caffeine in humans: lipid oxidation or futile cycling? Am J ClinNutr. 2004; 79: 40-46.
- Martins IJ. Caffeine consumption with Relevance to Type 3 diabetes and accelerated brain aging. Research and Reveiws: Neuroscience. 2016; 1: 1-5.
- Kennedy OJ, Roderick P, Poole R, Parkes J. Coffee, caffeine and non-alcoholic fatty liver disease? TherapAdvGastroenterol. 2016; 9: 417-418.
- Chen S, Teoh NC, Chitturi S, Farrell GC. Coffee and non-alcoholic fatty liver disease: brewing evidence for hepatoprotection? J GastroenterolHepatol. 2014; 29: 435-441.
- Martins IJ. Food intake and caffeine determine amyloid beta metabolism with relevance to mitophagy in brain aging and chronic disease. European Journal of Food Science and Technology. 2016; 4: 11-17.
- Jensen KJ, Alpini G, Glaser S. Hepatic nervous system and neurobiology of the liver. Compr Physiol. 2013; 3: 655-665.
- Gimble JM, Sutton GM, Ptitsyn AA, Floyd ZE, Bunnell BA. Circadian rhythms in adipose tissue: an update. CurrOpinClinNutrMetab Care. 2011; 14: 554-561.
- Buijs RM, Escobar C, Swaab DF. The circadian system and the balance of the autonomic nervous system. HandbClin Neurol. 2013; 117: 173-191.
- Farhanghi MA, Mahboob S, Ostadrahimi A. Obesity induced magnesium deficiency can be treated by vitamin D supplementation. J Pak Med Assoc. 2009; 59: 258-261.
- Huerta MG, Roemmich JN, Kington ML, Bovbjerg VE, Weltman AL, Holmes VF, et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care. 2005; 28: 1175-1181.
- Nielsen FH. Magnesium, inflammation, and obesity in chronic disease. Nutr Rev. 2010; 68: 333-340.
- Kurpad AV, Aeberli I. Low serum magnesium and obesity--causal role or diet biomarker? Indian Pediatr. 2012; 49: 100-101.
- Takaya J, Higashino H, Kobayashi Y. Intracellular magnesium and insulin resistance. Magnes Res. 2004; 17: 126-136.
- Martins IJ. Magnesium Therapy Prevents Senescence with the Reversal of Diabetes and Alzheimer’s Disease. Health. 2016; 8: 694-710.
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. ToxicolApplPharmacol. 2006; 212: 167-178.
- Boudina S, Graham TE. Mitochondrial function/dysfunction in white adipose tissue. Exp Physiol. 2014; 99: 1168-1178.
- Lee S, Hudson R, Kilpatrick K, Graham TE, Ross R. Caffeine ingestion is associated with reductions in glucose uptake independent of obesity and type 2 diabetes before and after exercise training. Diabetes Care. 2005; 28: 566-572.
- Boutagy NE, McMillan RP, Frisard MI, Hulver MW. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie. 2016; 124: 11-20.
- Machado MV, Cortez-Pinto H. Diet, Microbiota, Obesity, and NAFLD: A Dangerous Quartet. Int J Mol Sci. 2016; 17: 481.