Research Article
Austin J Obstet Gynecol. 2014;1(1): 4.
Mice Peritoneal Macrophage Activation Response to Surgical Suture Material
Hice J, Penrose L, Yandell RB and Prien S*
Department of Obstetrics and Gynecology, Texas Tech University Health Sciences Center, USA
*Corresponding author: Prien S, Department of Obstetrics and Gynecology, Texas Tech University Health Sciences Center, 3601-4th Street, Stop 3601, Lubbock, TX, USA
Received: May 20, 2014; Accepted: June 03, 2014; Published: June 06, 2014
Abstract
Objective: Studies have suggested that closure of the peritoneum at the time of cesarean may reduce adhesion formation postoperatively. Basic science implicates the macrophage as one of the primary initiators of the inflammatory response. Activated macrophages secrete inflammatory cytokines and other proteins noted to contribute to adhesion formation. The goal of this study is to identify which suture materials cause the least peritoneal macrophage activation as an indicator of inflammatory response and predictor of adhesion formation.
Methods: Peritoneal macrophages were harvested from euthanized mice. The harvested cells were placed in culture for 12 hours prior to exposure to suture, PDS, 3–0 Vicryl, 3–0 Plain gut, 3–0 Chromic, or 3–0 Monocryl. Macrophage activation rates were documented at 24 hrs and 72 hrs for each treatment. The macrophage activation rate was calculated by counting the number of activated cells compared to partially activated or non–activated cells in the microscope field, then dividing each activation classification by the total number of cells in the field and multiplying by 100.
Results: There is a direct correlation between the suture exposure and inflammatory response mediated by macrophages. Monofilament suture causes the least macrophage activation. Monocryl is predicted to cause less activation than PDS.
Conclusion: The magnitude of inflammatory response mediated by macrophage activation in peritoneal exposure may be minimized by suture selection. Thus, choosing the correct suture in closure of the human peritoneum at the time of cesarean may reduce the inflammatory response and subsequently reduce adhesion formation.
Keywords: Macrophages; Adhesions; Peritoneal Closure; Suture Material
Introduction
Cesarean delivery has become one of the mainstays of obstetrical practice over the last 20 years. In 2007 the cesarean delivery rate in the US was reported to be 31.7%, an increase of nearly 50% in ten years [1]. The most recent data from 2012 reports the US Cesarean delivery rate at 32.8% which suggests a slower rate of increase over the past 5years [2]. Much of the current push to increase VBAC acceptance among patients and attempts to decrease primary cesarean rates is grounded in the evidence–based finding of maternal comorbidities and longterm sequelae of cesarean delivery [3]. Chief among the potential negative outcomes associated with multiple cesarean deliveries is the risk of future surgery [4]. Further, adhesions formed as a consequence of prior surgery can increase the risk of complications during pelvic and abdominal surgery. Given that Cesarean section has become so common, it would appear prudent to identify the best surgical practices to minimize post–surgical adhesive disease.
Closing the peritoneum was considered part of standard cesarean technique until the 1990s, at which time the idea was proposed that skipping this step may cut down on surgical time, comorbidity, wound infection, postoperative pain, and adhesion formation [5]. Subsequently there have been a series of mixed studies with conflicting results evaluating the benefit of peritoneal closure to minimize adhesions [6–8]. However, since this great debate began there has not been a clear, evidence based, advantage reported for either technique. Lyell published one of the major prospective randomized trials that concluded that peritoneal closure has a protective affect against adhesion formation [8]. These findings were supported by the meta– analysis completed by Cheong in 2009 [6]. A recent randomized prospective trial published in 2012 concluded that there was no significant difference in adhesion formation, but failed to address visceral peritoneal closure [7]. However, this study identifiedseveral inconsistencies in the previous meta–analysis and reviews by highlighting use of different suture types to close the peritoneum and using different techniques [7].
It would appear evident that any debate over the technique of peritoneal closure and its effect on adhesion formation should include an evaluation of the process of adhesion formation as a subset of the inflammatory cascade. The pathophysiology of the condition implicates that inflammatory factor concentration and severity of peritoneal disruption can be correlated to the subsequent amount⁄severity of adhesive disease [9]. Studies suggest the inciting agents would include: L collagen I, fibronectin, matrix metalloproteinase 1 (MMP–1), tissue inhibitor of metalloproteinases (TIMP–1), transforming growth factor– beta (TGFB1 and TGIFB2), cyclooxygenase–2 (COX–2), and interleukin –10 (IL–10) [9], all of which are manifested in the process of macrophage activation. This would suggest a technique is needed to evaluate whether surgical materials, such assuture, contribute tomacrophage activation. Such information could subsequently be used to determine the suture and other surgical materials that would be optimal for closing the peritoneum. Further, the standard use of a low inflammatory index suture for peritoneal closure studies could then be used to establish a clear advantage in the closure vs non–closure debate for caesarian section surgery. The objective of the present study was to determine if a previously described macrophage culture system [10] could be used to evaluate suture materials commonly used in peritoneal closure by determining their ability to activate macrophages in vitro. Such data might prove useful in predicting the adhesion formation potential of such materials during use in surgical procedures.
Materials and Methods
Two studies were completed using the previously described macrophage model [10]. In the first study, macrophages were obtained from previously euthanized research grade mice (CB6F1; Charles Rivers, Willington, MA) under an approved IACUC protocol. Macrophages were harvested by rinsing the peritoneal cavity of 6 males and 2 female mice with phosphate buffered saline solution (PBS; Irvine Scientific; Santa Ana, CA). The harvested cells were placed in Ham’s F–10 culture media supplemented with human serum and antibiotics (Irvine Scientific)and transferred to a standard 24–culture plate, and then incubated for 12 hours at 37oC, 5% CO2 and 95% relative humidity prior to exposure to suture materials to allow the macrophages to adhere to the culture plate (Falcon; Bedford, MA). At the end of the initial culture period, the plates were rinsed with PBS to remove non–adherent cells and fresh media added using sterile technique. Sterile suture materials were prepared one hour prior to the end of the initial culture period of the suture samples to limit possible culture contamination. Study suture materials were cut into 5 mm segments using sterile technique. Each row of the 24–well culture plate represented one animal source. Cells in the first row were used as a control. In the first experiment, the subsequent rows were then exposed to 5 mm segments of either PDS, 3–0 Vicryl, 3–0 Plain gut, or 3–0 Chromic (Ethicon, Inc.; Bridgewater, NJ) by placing the sterile suture segments in the wells using sterile gloves and sterile pickups. Once the entire suture was appropriately placed, the plates were returned to culture for an additional 72 hours, with data collection occurring after 24 and 72 hrs of suture exposure. At the collection time points, wells were first inspected for evidence of visual contamination and those wells were excluded. The cells in the remaining wells were then scored for macrophage activation percentage on an inverted microscope at 630X magnification (LeitzDiavert Inverted Microscope; Leica Microsystems Inc., Buffalo Grove, IL) by three trained observers. Each observer counted 100 cells per well (a total of 1200 cells/treatment) using a scoring system previously defined [10]. In brief, cells were characterized as activated (globular 3–dimensional), non–activated (flat with long podocyte projections), or partially activated (thickened 3–dimensional with few projections, tubular appearance). Data for each cell type (activated, partially activated, or non–activated) were then expressed as a percentage of total cells counted per well creating 12 replicates per treatment (4 wells x 3 observes) for statistical analysis. After confirming no differences between observers, final data were expressed as a mean of the 12 observations per treatment per time point.
In the second study, the process was repeated using macrophages from 3 females and the three most common suture types used by surgeons during cesarean section: plain gut, Monocryl and Vicryl, plus the control. The same protocol described above was utilized with the exception of suture preparation taking place in the operating room and then transported to the clean area in sterile containers. Further, because of contamination issues in the first culture experiment, 100 units of Penn–Strep (Fisher Scientific; Pittsburg, PA) ⁄mL of media was added to the base culture media for this second experiment.
All data were analyzed using the Statistical Package for the Social Sciences (SPSS ver. 12; Chicago, IL). The basic analysis was a one–way analysis of variance of treatment at each time point using a p value of 0.05 for significance, with Tukey’s mean separation as appropriate.
Results and Discussion
The initial study was designed as a pilot study with two plates of cells. One plate became contaminated with a fungal infection and was removed from the study. The remaining plate contained the macrophages of three animals. Table 1 displays the results of macrophage activation after 24 hrs of exposure. It is apparent that all suture materials induced some level of macrophage activation compared to the control (P < 0.01). Further, there were distinct patterns of activation between the suture groups with Vicryl, Plain gut, Chromic inducing significantly more activation than the PDS. As expected, the rate of cellular activation increased from 24 to 72 hrs. However, the same general pattern of activation continued between the four treatments groups (P < 0.001; Table 2). Further, while over half the macrophages in the control remained unactivated, 75 to almost 100% of the macrophages exposed to one of the four suture types demonstrated at least partial activation after 72 hrs.
Suture Type
Not Activated
Activated
Partial Activation
Control
55
20
23
PDS
25
35
39
Vicryl
4.7
78.3
17.2
Chromic
0.1
88
11.2
Plain gut
4.5
83
11.5
Table 1: Pilot Study-Macrophage activation after 24 hr of Exposure to 4 suture materials.
Suture Type
Not Activated
Activated
Partial Activation
Control
53
37
10
PDS
24.5
54
24
Vicryl
0.5
88
12
Chromic
0.1
97
3
Plain gut
0.5
92.5
7
Table 2: Pilot Study-Macrophage activation after 72 hr of Exposure to 4 suture materials.
In the second trial, using suture materials found commonly in cesarean section procedures, additional care was taken to prevent infection of the culture wells.
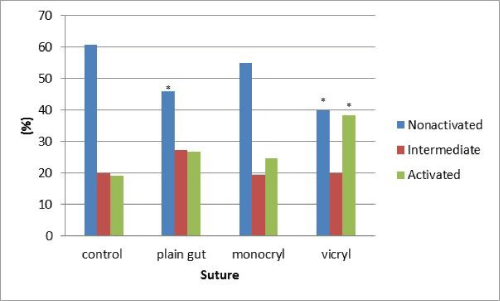
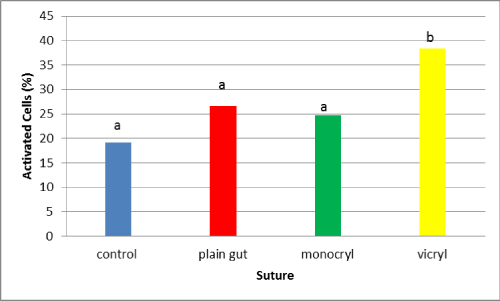
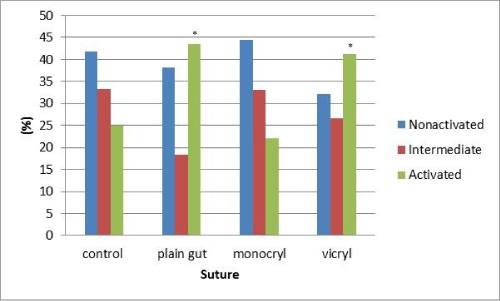
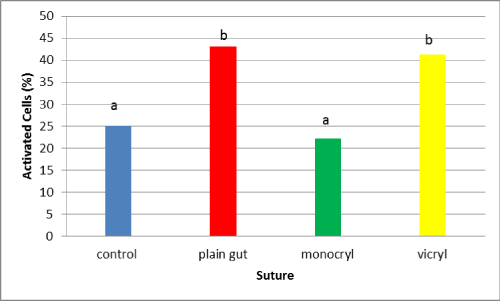
Resulting data demonstrated increased activation of cells exposed to Vicryl compared to the control at 24 hrs (Figure 1; P < .001). Both Plain gut and vicryl had fewer non–activated cells than the control (P < .001). Further, when comparing suture types to each other, Monocryl and plain gut appear to have fewer activated macrophages than Vicryl and were in a range statistically similar to the control (Figure 2). As in the original pilot study, activation continued to increase over time and at 72 hrs both Plain Gut and Vicryl appeared to have more activated macrophages than the control (Figure 3; P < .001) However, by 72 hrs only Monocryl had activated cell counts in a range statistically similar to the control (Figure 4).
Figure 1: State of activation of macrophages after 24 hrs of exposure to various suture materials in vitro. *- indicates means different from the control for that state activation (P <0.001).
Figure 2: Comparison of macrophage activation after 24 hrs of exposure to various suture materials. Bars with different subscripts are significantly different (P < .001).
Figure 3: State of activation of macrophages after 72 hrs of exposure to various suture materials in vitro. *- indicates means different from the control for that state activation (P < 0.001).
Figure 4: Comparison of macrophage activation after 24 hrs of exposure to various suture materials. Bars with different subscripts are significantly different (P < .001).
Conclusion
The debate over closure technique during cesarean section continues [6–8]. Presently it remains unclear if closure of the peritoneum has more beneficial or detrimental effects on long–term patient morbidity. However, there can be little debate that materials that induce macrophage activation may have significant influence on subsequent adhesion development and issues that might develop during subsequent surgical procedure [9].
The present study reexamines a model of in vitro macrophage activation [10] and its usefulness for evaluating surgical agents for their potential adhesion– inducing properties. The most salient point in this study is that monocryl macrophage activation is statistically similar to the control macrophage activation in the cell culture model. Therefore it can be concluded that, of the suture types described in this study, Monocryl is the least activating. The extrapolation is that Monocryl is the least inflammatory and therefore would demonstrate the least adhesion– generating potential.
This finding maybe extremely important to the original question inspiring the study and suggests studies investigating the benefit of closing the peritoneum at the time of cesarean delivery to minimize adhesions at the time of future abdominopelvic surgery can now be undertaken with the use of a consistent minimally inflammatory suture. Currently, the protocol and IRB are being developed for a prospective randomized control trial. Another application of this result is that additional minimally inflammatory devices and suture selections can be developed based on what is known about the construction of Monocryl. Additional larger studies using the animal model described above will also be useful in solidifying the potentially beneficial qualities of Monocryl and other monofilament synthetic suture types such as PDS.
Limitations of the animal model utilized consist of keeping the cell cultures free of contamination, the time allocation for counting and calculating macrophage activation, inter–observer bias, and potential environmental effect on activation. Supplementation of the culture media with antibiotic solution additive may minimize the risk of cell plate bacterial contamination, but promote fungal contamination. It is difficult to prevent all contamination without risk of potentially affecting the results. Even strict sterile technique is dependent on the environmental constraints. Airborne microbes can contaminate the plates each time they are opened. Better protocol techniques to minimize contamination without altering the baseline activation amount will be invaluable to the utility of future studies.
The counters that collected the data received 30 minutes to 1 hour of training for recognizing and counting the cells. However with multiple hours of counting, the possibility of error exists secondary to fatigue and inter–observer differences. To evaluate this effect, comparison of each counter’s data at the conclusion of each plate being counted was conducted, demonstrating relative consistency among the counters. There is no way to reconcile the limitation of the fixed amount of time required to count 100 cells from each well sample population. In order to have an increased study size the number of counters must be increased as well. Lastly, the limitation of an animal model is inherent in the inability to replicate the human cell environment in a synthetic cell plate. There are hormones and cytokines that maternal macrophages are exposed to during pregnancy that have inflammatory effects and could affect macrophage activation. This limitation can only be overcome with human subject clinical trials.
Other meaningful findings include a clear and significant increase in macrophage activation, both at 24 hrs and 72hrs of exposure to vicryl. Both Plain Gut and Vicryl demonstrated macrophage activation rates around 40 % at 72hrs. It is reasonable to extrapolate that Vicryl, Plain Gut and likely chromic will cause increased inflammation and more adhesion forming potential. Therefore it would be preferable to avoid using these suture materials when closing the layers of the abdominal wall including the peritoneum.
While these data suggest the macrophage model represents a potential means for evaluating the adhesion– inducing properties of surgical agents, more study is needed with larger cell populations to fully assess its usefulness. These studies should include both molecular (measure of culture cytokines) and functional (ability of macrophages for phagocytosis) aspects as well as the morphological changes currently described. However, once proven, the model may be a simple and inexpensive means of assessing the adhesion– forming potential of new materials prior to their use in surgical procedures.
A new phenotype, known as cerebriform nevus sebaceous, has been described [15]. Cerebriform nevus sebaceous is characterized by large, pedunculated or verrucous, pink, hairless, nodules or tumors in newborn infants [15].
Nevus sebaceous is usually an isolated finding. Syndromes associated with nevus sebaceous include SCALP syndrome and linear sebaceous nevus syndrome (Schimmelpenning syndrome). SCALP syndrome is characterized by sebaceous nevus, central nervous system malformation, aplasia cutis congenita, limbal dermoid, and pigmented nevus. Linear sebaceous nevus syndrome is characterized by nevus sebaceous which is often extensive and distributed along Blaschko’s lines, central nervous system abnormalities, epilepsy, mental retardation, ocular abnormalities, musculoskeletal defects, cardiovascular abnormalities, and urologic abnormalities.
Diagnosis
The diagnosis is usually clinical based on the characteristic features. A tissue biopsy or referral to a dermatologist should be considered if the diagnosis is in doubt. Prenatal diagnosis is feasible by ultrasonography for large and exophytic lesions [16].
Differential diagnosis
The differential diagnosis includes epidermal nevus, aplasia cutis congenita, solitary mastocytoma, and juvenile xanthogranuoloma.
Complications
The lesion can be aesthetically unappealing, especially when it occurs on the face. Large or extensive lesions may be associated with developmental deficits [9]. Centrofacial lesions have an increased association with underlying neurological involvement. The latter may present as mental retardation, seizures, and hemiparesis.
Nevus sebaceous may be complicated by the development of benign and malignant nevoid tumors in the original nevus [1]. The incidence of these tumors increases with age, particularly after puberty [11]. Neoplasms occur mostly in the fourth decade of life in approximately 10 to 30% of lesions [3,5]. Majority of these tumors are benign; less than 1% of nevus sebaceous is complicated by malignant tumors [17]. Malignancy is suggested by the acute appearance of a large, discrete, ulcerating nodule within the lesion [3]. The most common benign tumors are syringocystadenoma papilliferum followed by trichoblastoma [3,13]. Many trichoblastoms were misdiagnosed in the past as basal cell carcinomas [4]. Other benign tumors include trichilemmoma trichoepithelioma, sebaceous adenoma, seborrheic keratosis, sebaceous epithelioma, keratoacanthoma, apocrine cystadenoma, apocrine hidrocystoma, nodular hidradenoma, follicular poroma, spiradenoma, and syringoma [5,11]. The most common malignant tumor is basal cell carcinoma [4,10]. Other malignant tumors include squamous cell carcinoma, apocrine carcinoma, ductal adenocarcinoma, porocarcinoma, anaplastic adnexal carcinoma, trichilemmal carcinoma, syringomatous carcinoma, and sebaceous carcinoma [4,5,11].
Management
Excision of the lesion may be considered at any age for cosmetic reasons. Other than for the sake of cosmesis, some authors suggest watchful observation of the lesion [18]. However because of the potential of malignant transformation, other authors recommend prophylactic full–thickness, complete excision of the lesion with minimum 2 to 3 mm margins [1,2,4]. Because the incidence of malignant transformation in childhood is low, prophylactic excision of the lesion (and the possible need for general anesthetic) prior to puberty may not be justified [13]. Ablative laser and photodynamic therapy may be considered for the occasional cases with inoperable lesions. Any new “bump” within a nevus sebaceous should be examined and biopsy considered.
References
- Cunningham F, Leveno K, Bloom S, Hauth J, Rouse D, Spong C. Williams Obstetrics 23rd edn. Columbus, OH: McGraw Hill. 2010.
- Center for Disease Control.
- Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006; 107: 1226-1232.
- Awonuga AO, Fletcher NM, Saed GM, Diamond MP. Postoperative adhesion development following cesarean and open intra-abdominal gynecological operations: a review. Reprod Sci. 2011; 18: 1166-1185.
- Holmdahl L, Risberg B. Adhesions: prevention and complications in general surgery. Eur J Surg. 1997; 163: 169-174.
- Cheong YC, Premkumar G, Metwally M, Peacock JL, Li TC. To close or not to close? A systematic review and a meta-analysis of peritoneal non-closure and adhesion formation after caesarean section. Eur J Obstet Gynecol Reprod Biol. 2009; 147: 3-8.
- Kapustian V, Anteby EY, Gdalevich M, Shenhav S, Lavie O, Gemer O. Effect of closure versus nonclosure of peritoneum at cesarean section on adhesions: a prospective randomized study. Am J Obstet Gynecol. 2012; 206: 56.
- Lyell DJ, Caughey AB, Hu E, Daniels K. Peritoneal closure at primary cesarean delivery and adhesions. Obstet Gynecol. 2005; 106: 275-280.
- Alpay Z, Saed GM, Diamond MP. Postoperative adhesions: from formation to prevention. Semin Reprod Med. 2008; 26: 313-321.
- Prien SD, Dunn C, Messer RH. Adhesion-promoting properties of dyes routinely used during fertility surgeries. J Assist Reprod Genet. 1995; 12: 136-140.