Case Report
Austin J Obstet Gynecol. 2014;1(3): 3.
Pelvic Actinomycosis Mimicking Ovarian Cancer: A Report of 3 Cases and Review of the Literature
King Man Wan1, Yoon Ji Jina Rhou2, Trevor T Berges2, Neil Campbell1, Jonathan Carter2,3, Lyndal Anderson3,4 and Selvan Pather2,3*
1Department of Women's and Babies, Royal Prince Alfred Hospital, Australia
2Department of Women's and Babies, Royal Prince Alfred Hospital, Australia
3Sydney Medical School, University of Sydney, Australia
3Department of Anatomical Pathology, Royal Prince Alfred Hospital, Australia
*Corresponding author: Selvan Pather, Lifehouse Gynaecologic Oncology, 119 Missenden Road, Camperdown, 2050, Australia
Received: July 30, 2014; Accepted: August 22, 2014; Published: August 25, 2014
Abstract
Pelvic actinomycosis is an uncommon infection usually associated with the use of the intrauterine contraceptive device. This may present to the gynaecologist with clinical features suggestive of pelvic malignancy. We present 3 cases of patients presenting to a gynaecologic oncology unit with features suggestive of ovarian cancer and discuss the diagnosis and management of this disease.
Introduction
Pelvic Actinomycosis is a rare, chronic infection that clinically can be difficult to differentiate from true gynaecological malignancy. The opportunistic anaerobic bacterium Actinomyces israelii is a normal part of the human flora in the oropharynx, gastrointestinal and genital tract [1]. More than 50% of actinomycosis infections occur in the craniofacial region with pelvic infection accounting for 20% of human cases [2]. The ability for actinomycosis to secrete proteolytic enzymes, disrupt tissue planes and compress surrounding tissue makes their appearance similar to a malignant process [3]. Furthermore, due the slow growing nature of actinomyces, the nonspecific clinical presentation and subsequent extensive spread before diagnosis, the diagnosis is often overlooked. Past or current use of the intrauterine contraceptive device (IUD) is the most important risk factor for the development of pelvic actinomycosis in women. Pre-operative diagnosis is difficult and a diagnosis is often made retrospectively after extensive surgery for removal of tissue. We review 3cases of pelvic actinomycosis that were managed at the Sydney Gynaecologic Oncology Unit and review the management of this condition.
Materials and Methods
Case 1
A 56 year old woman presented with acute onset left iliac fossa pain, fevers and heavy menstrual bleeding. She had a copper intrauterine device (IUD) removed 2 months previously without complication. A computed tomography (CT) scan showed a large fibroid uterus, a cystic lesion in the liver, a left adnexal mass measuring 5.4 x 5 x 4.5cm, and moderate left hydronephrosis that required ureteric stenting. The serum Ca125 level was elevated at 56 ku/l (normal < 32ku/L). She subsequently underwent an elective total abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO) for her left adnexal mass on the presumption that this was an ovarian malignancy. Macroscopically the left adnexa were thickened and contained numerous locules of caseous, yellow material. Histology showed marked chronic salpingitis with foamy macrophages and plasma cells intermixed with actinomycosis organisms, typical of an actinomycotic infection (Figure A and Figure B)She was commenced on a 4 week course of high dose intravenous Benzyl penicillin, followed by oral amoxicillin for 6 months duration and had complete resolution of her disease including the hepatic deposit.
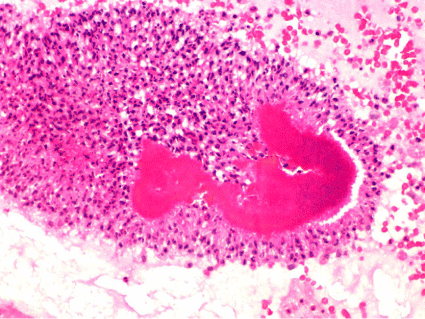
Figure 1: Haematoxyln and Eosing stain showing a sulphur granule with a mycelium-like structure containing clusters of neutrophils and actinomyces (x40).
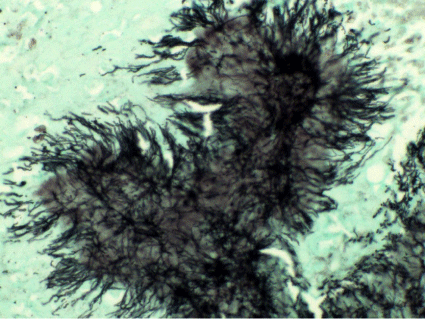
Figure 2: Gomori methenamine-silver showing the filaments of Actonomycosis sp. (x40).
Case 2
A 70 year old woman was referred with a complex pelvic mass found on investigation for vague abdominal pain. 22 years prior she had a vaginal hysterectomy for heavy menstrual bleeding and uterine prolapse. The histology at that time showed a normal uterus, cervix and mild focal adenomyosis. She had not previously used a copper IUD and was systemically well. Ultrasound and CT showed a large 8 cm complex right pelvic mass and the serum CA125 level was 32 (normal < 32ku/L). The preoperative diagnosis was a borderline or early malignant ovarian lesion. She underwent a laparotomy and bilateral salpingo-oophorectomy with left ureterolysis and the intraoperative findings confirmed a left tubal ovarian inflammatory mass. Microscopically there was central necrosis and Actinomyces sp organisms were subsequently cultured from the tissue. The right fallopian tube and ovary was normal. She was initially treated with intravenous ceftriaxone and metronidazole followed by oral therapy for 5 days. Despite confirmation of the actinomycosis she chose not to have further antibiotics and has been well after 18 months of follow up.
Case 3
A 37 year old woman presented to the emergency department with a 4 week history of increasing shortness of breath, intermittent sharp left iliac fossa pain, diarrhoea and fevers. A copper IUD had been inserted 13 months prior but was removed after 8 months due to persistent vaginal bleeding. Ultrasound demonstrated a large bulky uterus and left adnexal mass measuring 6,3x4,0x5,0 cm with solid and cystic components. A CT confirmed the findings but also located an irregularity of the right rectal mucosa measuring 22 mm in diameter. She was treated with intravenous ceftriaxone and metronidazole and subsequently underwent a laparotomy 3 days later a suspected ovarin neoplasm. A large left tubo-ovarian abscess was noted at laparotomy and a left salpingo-oophorectomy was performed. A large indurated mass was noted anterior to the rectum in the rectovaginal pouch and biopsies were taken. Histology showed a chronic left tubo-ovarian abscess with several colonies of filamentous organisms consistent with Actinomyces sp. The rectal biopsy showed inflammation with normal rectal mucosa. After confirmation of actinomycosis she was admitted for high dose IV Benzyl penicillin for 2 weeks followed by 6 months of oral outpatient amoxycillin3g daily. A subsequent CT scan was normal with no evidence of the previously noted rectal mass.
Discussion
We noted in our series that presentation with a complex pelvic mass, raised ca125 level and ureteric obstruction may occur with pelvic actinomycosis and strongly mimics an advanced stage ovarian cancer. The absence of preoperative diagnosis resulted in all 3 cases having extensive surgery and removal of pelvic organs. The intraoperative findings of caseous, yellow material is often noted and should alert the surgeon to the possibility of an inflammatory condition. Failure to diagnose this intraoperatively may lead to extensive debulking surgery as is commonly performed for ovarian cancer with morbidity and loss of organ function. Pelvic actinomycosis has previously been noted to mimic ovarian malignancies with the presence of omental thickening, colon adherent to the inflammatory mass and hydronephrosis. It has also been noted in the cervix, rectum, bladder and urachus mimicking cancer [4-6]. Hepatic involvement as noted in case 1 is uncommon and has only been reported in 2 previous patients [7,8]. Hepatic lesions have previously been noted to respond well to systemic medical therapy, as noted in our case.
The diagnosis of pelvic actinomycosis is challenging with only 10% of cases diagnosed preoperatively [2]. Pelvic actinomycosis can occur at any age and in a reported large cohort of 92 patients, the mean age at diagnosis was 37 years [9]. Despite this, 2 of the patients in this series were in the postmenopausal age group. The presenting features usually include fevers, pelvic pain or the incidental findings of suspicious pelvic masses on imaging. Ultrasound and CT are the most commonly used imaging modalities for diagnosing pelvic actinomycosis; however findings are usually nonspecific and thus unhelpful in assisting in the differential diagnosis. CT findings in women with abdominal actinomycosis show predominantly solid masses with focal areas of reduced attenuation or thick-walled cystic masses [3]. Magnetic resonance imaging (MRI) of actinomycosis have noted relatively low signal intensity on T2 weighted sequences in association with extensive pelvic infiltration that is atypical for a malignant ovarian tumour [10].
The association of pelvic actinomycosis and IUD is strong and it appears the risk increases after 2 years of use although most patients have had longer use. Two large previous series noted the mean duration of use of the IUD in patients with pelvic actinomycosis to be 7-8 years. The presence of the IUD strings is thought to allow ascension of the Actinomyces sp into the uterus where focal necrosis of the endometrium by the IUD is the breach required for disease to occur [11]. Other rarer risk factors that have been reported include direct trauma, endoscopic manipulation and previous surgery. One of our patients had no previous history of IUD use and had indeed had a hysterectomy previously. It is likely that she had a chronic infection from previous instrumentation.
The gold standard for diagnosis is culture from the tissue, pus or IUD itself. Actinomyces sp is a fastidious anaerobic bacterium that requires 2-3 weeks to culture with a failure rate of more than 50% [2]. Therefore, most diagnoses are made histologically with the presence of sulphur granules, yellowish particles with a mycelium-like structure containing clusters of neutrophils and actinomyces (Figure A and Figure B). Sulphur granules may be scant or absent in some patients [12] and their presence is not pathognomonic of Actinomycosis infection [2].
Whilst Actinomycosis commonly co- exists with gram negative organisms and other anaerobic bacteria such as Actinobacillus sp, Aikenella sp and Bacteroides sp, single agent therapy is usually successful. Penicillin in a dose of 18 to 24 million units intravenously for two to six weeks, followed by oral therapy with amoxicillin is recommended [2]. The total duration of therapy though remains controversial. A prolonged treatment regime has been recommended due to the poor penetration of antibiotic into the inflammatory fibrotic tissue. A previous series of 20 patients, however, noted that despite 13 patients having 3 months or less of antibiotic treatment, there was no documented recurrence after a median follow up of 37.5 months [13]. One of our patients had only a 3 month course of antibiotics while another patient only had a 5 day course but both still had full resolution of their disease. This may be due to the fact that the surgery decreased the burden of disease and thus treatment outcomes are still successful with shorter antibiotic regimens. Ceftriaxone, tetracycline, minocycline, erythromycin and clindamycin can also be used to treat actinomyces in patients allergic to penicillin.
Antibiotics can cure very extensive pelvic disease, including masses; however there is still a role for surgical management for exclusion of malignancy, resolving obstruction of surrounding organs and for patients that have failed to clinically respond to antibiotic therapy. Furthermore, surgery is often required for diagnosis by obtaining tissue for histopathological examination and culture. Regardless, the prognosis of pelvic actinomycosis is excellent and the treatment outcome with antibiotic is usually complete resolution.
References
- Lippes J. Pelvic actinomycosis: a review and preliminary look at prevalence. Am J Obstet Gynecol. 1999; 180: 265-269.
- Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ. 2011; 343: d6099.
- Ha HK, Lee HJ, Kim H, Ro HJ, Park YH, Cha SJ, et al. Abdominal actinomycosis: CT findings in 10 patients. AJR Am J Roentgenol. 1993; 161: 791-794.
- Hoffman M. Advanced actinomycotic pelvic inflammatory disease simulating gynecologic malignancy. A report of two cases. J reprod med. 1991; 36: 543-545.
- Akhan SE, Dogan Y, Akhan S, Iyibozkurt AC, Topuz S, Yalcin O, et al. Pelvic actinomycosis mimicking ovarian malignancy: three cases. Eur J Gynaecol Oncol. 2008; 29: 294-297.
- Lim KT, Moon SJ, Kwon JS, Son YW, Choi HY, Choi YY, et al. Urachal actinomycosis mimicking a urachal tumor. Korean J Urol. 2010; 51: 438-440.
- Kim HS, Park NH, Park KA, Kang SB. A case of pelvic actinomycosis with hepatic actinomycotic pseudotumor. Gynecol Obstet Invest. 2007; 64: 95-99.
- O'Kelly K. Pelvic actinomycosis with secondary liver abscess, an unusual presentation. Eur J Obstet and Gynecol. 2012; 163: 239-240.
- Fiorino AS. Intrauterine contraceptive device-associated actinomycotic abscess and Actinomyces detection on cervical smear. Obstet Gynecol. 1996; 87: 142-149.
- Hawnaur JM, Reynolds K, McGettigan C. Magnetic resonance imaging of actinomycosis presenting as pelvic malignancy. Br J Radiol. 1999; 72: 1006-1011.
- Choi MH, Hong DG, Seong WJ, Lee YS, Park IS. Pelvic actinomycosis confirmed after surgery: single center experience. Arch Gynecol Obstet. 2010; 281: 651-656.
- Brown JR. Human actinomycosis. A study of 181 subjects. Hum Pathol. 1973; 4: 319-330.
- Choi MM, Baek JH, Lee JN, Park S, Lee WS. Clinical features of abdominopelvic actinomycosis: report of twenty cases and literature review. Yonsei Med J. 2009; 50: 555-559.