Review Article
Austin J Obstet Gynecol. 2014;1(4): 7.
Hysteroscopy for Infertile Women: A Review
Aarathi Cholkeri-Singh*
Department of Ob/Gynecology, University of Illinois at Chicago, USA
*Corresponding author: Aarathi Cholkeri-Singh, Department of Ob/Gynecology, University of Illinois at Chicago, the Advanced Gynecologic Surgery Institute, 120 Olser Dr, Suite 100 - Naperville, IL 60540, Chicago, USA
Received: July 31, 2014; Accepted: August 31, 2014; Published: September 02, 2014
Abstract
Objective: To review the role of hysteroscopy in infertile women.
Design: Review of peer-reviewed, published literature in PubMed and Cochrane databases on uterine intracavitary pathology, proximal tubal occlusion, failed in vitro fertilization procedures, and first trimester miscarriages of infertile women.
Results: The role of hysteroscopy is well documented in infertile women. Diagnostic and operative hysteroscopy are effective when evaluating and treating uterine intracavitary pathology, proximal tubal occlusion, failed in vitro fertilization procedures, and first trimester miscarriages of infertile women.
Conclusion: In conclusion, hysteroscopy may play an important role prior to or in conjunction with assisted reproductive techniques to help infertile women and couples achieve their goals of a pregnancy and live birth of a child.
Keywords: Hysteroscopy; Infertile women; Recurrent pregnancy loss
Introduction
In 2002, about 2.1 million women in the U.S were infertile. According to the National Center for Health Statistics, it was tabulated that between 2006 and 2010, rates of infertility were to range 8-30% for married women ages 15-44 years [1]. Assisted reproductive technology has been used to treat many of these women. However, hysteroscopy has been proven within peer-reviewed published literature to be a valuable tool for diagnosis and treatment of some infertile women prior to or after undergoing assisted reproductive techniques.
Hysteroscopy indications for infertile women include intracavitary abnormalities, such as sub mucous fibroids, endometrial polyps, uterine septum, adhesions, and retained products of conception. In addition, the role of hysteroscopy in proximal tubal occlusion, failed IVF cycles and first-trimester miscarriages is also valuable.
Intracavitary Pathology
Submucous fibroids
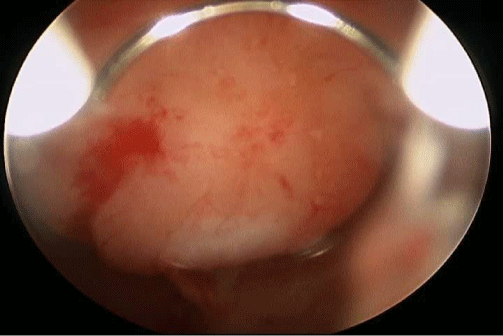
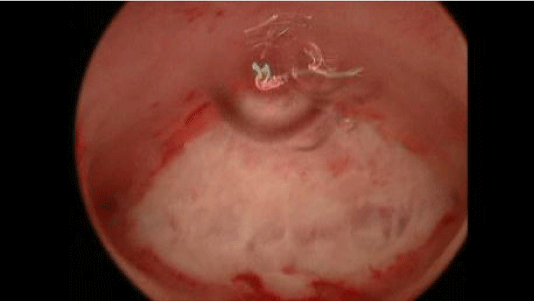
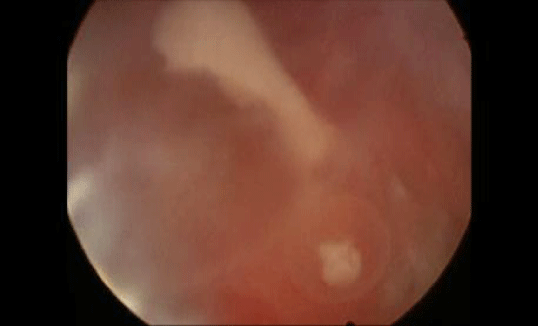
Submucous fibroids are categorized as Type 0, 1, and 2. A Type 0 fibroid is located completely within the uterine cavity; Type 1 fibroid is ≥ 50% within the uterine cavity (Figure 1A); and Type 2 is < 50% within the uterine cavity [2]. Postulated mechanisms by which fibroids cause infertility include the following:
Figure 1A: SubmucousMyoma.
- Interference with normal patterns of endocrine function [3]
- Distortion of the Endometrium [4,5]
- Dysfunctional uterine contractility [6]
- Distortion or obstruction of tubal ostia [7]
- Chronic endometrial inflammation [6]
- Abnormal uterine vascularization [8,9]
- Impaired endometrial receptivity [10]
Pregnancy wastage rates frequently exceed 70% for sub mucosal fibroids [11].
A small, prospective, cohort study, published in 2005 by Shokeir, followed 29 consecutive women with sub mucous fibroids who desired pregnancy. Primary infertility was diagnosed in 14 women, while the remaining 15 had poor obstetrical outcomes. All women were treated with a hysteroscopy myomectomy. Intra operatively, women were found to have a single fibroid with the majority of the fibroids being Type 0 (N=25) and the other four fibroids Type 1, all no larger than 5cm. Twenty-one women (72%) treated achieved 30 pregnancies. Thirteen of these women had live births. The rate of live births increased from 3.8% to 63.2% and the rate of abortions decreased from 61.6% to 26.3% after hysteroscopy myomectomy [12].
In 2008, Pritts et al. published a meta-analysis of 23 studies evaluating women with fibroids and infertility. Nine of the 23 studies looked at sub mucosal fibroids. Six studies were retrospective, 2 prospective and 1 was a randomized control study. When looking at infertile women with sub mucous fibroids in comparison to infertile women without, there were clinically significant differences in pregnancy, implantation and ongoing pregnancy/live birth rates and spontaneous abortion rate. The clinical pregnancy rate was improved amongst women who underwent a hysteroscopy myomectomy compared to women with fibroids left in situ. This study also showed that the pregnancy rates after hysteroscopy myomectomy were comparable to women without evidence of fibroids [13].
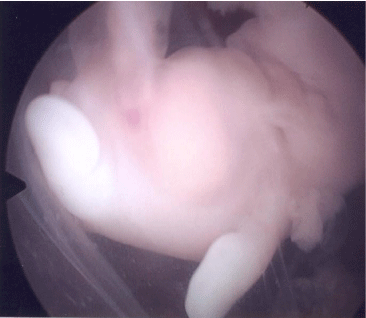
Hysteroscopy myomectomy has been shown to improve fertility rates and is a common method of treatment for sub mucous fibroids (Figure 1B).
Figure 1B: Hysteroscopic resection of submucousmyoma restoring normal cavity architecture.
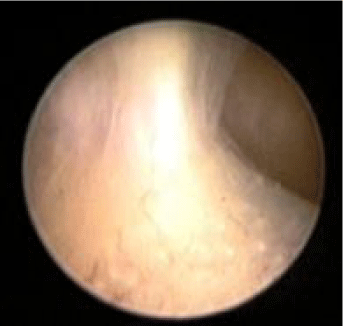
Endometrial Polyps (Figure 2)
Postulated mechanisms by which polyps cause infertility include irregular endometrial bleeding, inflammatory endometrial response, obstructive inhibition of sperm transport, physically obstructing exposure of the embryo to the endometrium, interference with normal patterns of endocrine function and increasing glycodelin which inhibits sperm binding to the zona pelllucida [14,15].
Figure 2: Endometrial Polyp.
In 2005 Pérez-Medina et al published a study evaluating 204 women with ≥ 24 months infertility. Women excluded were those who were <39 years old, anovulatory, diagnosed with uncorrected tubal disease, previous unsuccessful use of recombinant FSH and partners that were azoospermic. The study group (N=101) underwent a hysteroscopy polypectomy versus the control group (N=103) who underwent a hysteroscopy polyp biopsy only. After either procedure, the study participants were to receive up to 4 IUI cycles. However, 65% of the study group participants achieved pregnancies prior to undergoing an IUI cycle, which was a significant finding of this study. There were no significant differences between polyp size and pregnancy conception rates in the study group [16].
Stamatellos I et al. in 2008 not only evaluated size of polyps but also whether number of polyps within the uterine cavity impact fertility. This study included women younger than 35 years with primary or secondary infertility for greater than 12months. The participants were separated into 2 groups, those with either a polyp ≤1cm and those with a polyp >1cm or multiple polyps. The findings of this study correlated with Pérez-Medina's study as polyp size and number of polyps did not significantly affect pregnancy rates. The pregnancy rate was 61.4% for the entire study population undergoing hysteroscopy polypectomy with no difference in whether the patient had primary or secondary infertility [17].
Therefore, with the understanding that polyps do impact fertility, Yahaihara et al. studied 230 women to determine whether the location of the endometrial polyp made a significant difference. The locations were defined as anterior wall, posterior wall, lateral wall, utero-tubal junction, or multiple polyps. Similar with the previous studies' findings, polyp size in any area of the uterus did not significantly impact pregnancy rates. However, the highest pregnancy rates of 50- 60% were achieved in those who had polyps removed from the utero-tubal junction [18].
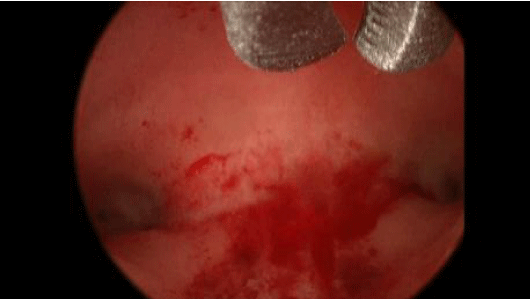
Uterine septum
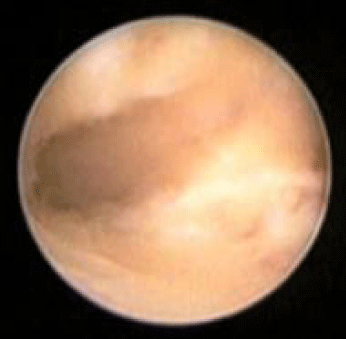
Uterine septum has been associated with pregnancy wastage rates as high as 90%, most likely due to the structural alterations in the endometrium of the septum, which affects implantation (Figure 3A) [19,20]. The American Fertility Association, now known as American Society of Reproductive Medicine, developed a sub classification with 12 variations of uterine septum anatomy [21].
Figure 3A: Uterine septum.
Four prospective trials looked at the effectiveness of hysteroscopy metroplasty (Figure 3B) in reduction of pregnancy wastage rates. Live birth rates for these studies ranged from 30-54% after a hysteroscopy metroplasty was performed [19,23,24].
Figure 3B: Hysteroscopic transection of uterine septum restoring normal anatomy of the cavity.
Mollo et al. studied two groups with unexplained fertility. Both groups were similar for age, duration of infertility and BMI. The control group's pregnancy rate was 20.4% with a live birth rate of 18.9%. The group who underwent a hysteroscopy metroplasty had an increased pregnancy rate of 38.6% with a live birth rate of 34.1% [24].
The Pabuçcu et al. study looked at women between the ages 21- 35 years old 6-24 months who underwent hysteroscopy metroplasty. Five women (8.2%) underwent a repeat surgery for a residual septum >1cm. The outcome of this study was a 41% pregnancy rate with a 29.5% live birth rate. In 13 of the 18 pregnancies that went to term, 2 patients had a total septum and 11 had a subtotal septum resected [22].
As some women have a residual septum after their initial metroplasty, Kormányos et al investigated whether a residual septum impacts fertility. After the first hysteroscopy metroplasty, 1 patient had >1cm residual septum, which was further resected, and 35 patients (37%) had a residual septum of ≤1cm. If no pregnancy was conceived in 24months for those with a residual septum ≤1cm, then the patients underwent a second hysteroscopy metroplasty for removal of the residual septum. Increase in pregnancy rates after the second hysteroscopy was not significant [9].
Looking further at septum lengths, Shokeir et al. studied women with septums measuring ≥2.5cm and compared them with women having<2.5cm of septum length. Of those 42 women (47.7%) who achieved pregnancy, all women were <40 years of age with <3 years of infertility. Eight percent of these pregnancies were spontaneous, with a pregnancy rate of 66.7% in those with septums ≥2.5cm and 42.8% in those with septums <2.5cm. Overall, live birth rate was 40.1% [23].
A retrospective study published in 2009 by Ban-Frangež H et al. also compared septum length and impact on pregnancy loss. When comparing women with a large (>1.5cm) or small partial (1.3-1.5cm) septum to women without a septum, there was a significantly higher pregnancy loss rate in those with septums. When both septum groups underwent a hysteroscopy metroplasty, both groups had comparable pregnancy loss rates to the control group [25].
Other studies, although not prospective, also confirmed the significant impact of septum with infertility. Grimbizis et al reviewed six studies prior to 2001 that showed a live birth rate of 6.1% in those women with intact septums versus 82% in those women who underwent a hysteroscopic metroplasty [26]. Nouri et al. also performed a more recent literature search which noted live birth rates ranging from 26-73% with a cumulative rate at 45% status post hysteroscopy metroplasty [27].
Intrauterine adhesions
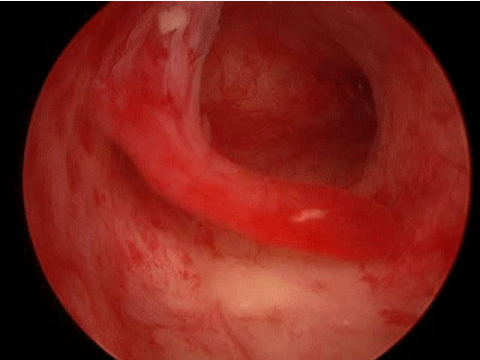
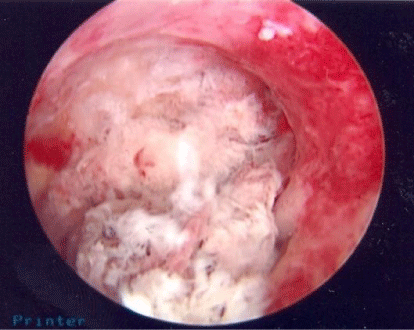
Intrauterine adhesions are caused by postsurgical or infectious damage to the basalis layer of the Endometrium. This can cause granulation tissue which can create tissue bridges leading to cavity obliteration (Figure 4A). This is associated with increased risks of ectopic pregnancy, recurrent miscarriage, preterm labor and abnormal placentation. Pregnancy wastage rates have been reported as high as 90% [28-33].
Figure 4A: Intrauterine adhesions blocking the right tubal ostia.
The severity of intrauterine adhesions is defined as follows: mild = less than 25% of the uterine cavity contains thin or filmy adhesions; moderate = 25-75% of the cavity containing adhesions causing partial occlusion of ostium and upper fundus; severe = greater than 75% of the cavity with agglutination of walls or thick bands [34].
Studies have shown that patients with mild adhesive disease had pregnancy rates between 58-88% after the adhesions were treated by hysteroscopy adhesiolysis. Similarly, women who underwent hysteroscopy adhesiolysis for moderate (Figure 4B) and severe intrauterine adhesions had pregnancy rates of 30-75% and 14-33%, respectively [32,35-37].
Figure 4B: Hysteroscopic lysis of adhesions restoring normal uterine cavity anatomy.
When looking at severe adhesions, Cappella-Allouc and Fernandez found a live birth rate of about 32% after undergoing a hysteroscopy adhesiolysis, which was consistent with the literature. These two studies also found a significant difference between women aged less than 35 years old and greater than 35 years old after hysteroscopy treatment of severe intrauterine adhesions. Pregnancy rates from both studies were 62-67% for women less than 35 years old versus 16-24% for those greater than 35 years old [38,39].
It is important to note that all these studies used a combination of 1-3 months of estrogen and progesterone hormonal therapy with or without a distended intrauterine foley bulb for 3-5 days or IUD postoperatively. At the completion of the hormonal therapy, most patients either underwent a hysterosalpingogram and/or hysteroscopy to assess and treat recurrent adhesions if necessary.
Retained Products of Conception
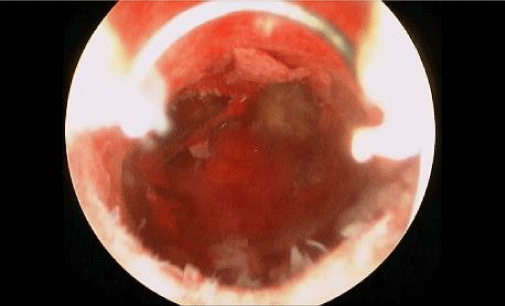
Retained products of conception can lead to an inflammatory state within the uterine cavity, thus, potentially causing intrauterine adhesions and infertility (Figure 5). Typically, a diagnosis can be made with a history of a pregnancy loss with or without undergoing a D&C, complaints of irregular vaginal bleeding and abnormal cavity appearance on trans vaginal ultrasound, hysterosonogram or hysterosalpingogram [40].
Figure 5: Retained products of conception.
Cohen et al examined patients who were diagnosed with residual trophoblastic tissue. Patients either underwent a hysteroscopy or dilatation and curettage (D&C) for removal of retained tissue. The time from pregnancy termination to surgery was not significant between the two groups. The time to a subsequent conception after management was significantly shorter, 7 versus 11 months, for those who underwent hysteroscopy resection versus a D&C. Five of the 24 patients, 20.8%, who underwent a D&C initially, had to undergo hysteroscopy resection due to persistent residual trophoblastic tissue [39].
A more recent study in 2011 by Rein et al studied patients who underwent dilatation and evacuation (D&E) with ultrasound guidance versus hysteroscopy resection for removal of residual trophoblastic tissue. A second look hysteroscopy was performed at 3 months to evaluate intrauterine adhesions. There were significantly more patients with mild adhesions who underwent an ultrasound guided D&E (30.8%) compared to hysteroscopy resection (4.2%), and one patient was diagnosed with severe adhesions. Conception rates and time to conception were also found to be significantly different between the two groups. Those in the D&E group had a conception rate of 59.9% versus 68.8% in the hysteroscopy resection group and the time to conception was 14.5 versus 11.5 months, respectively [41].
Two studies evaluated conception and live birth rates after hysteroscopy resection of residual trophoblastic tissue. Conception rates were 76% and 82% with a live birth rate of 70% and 75% [42,43]. One of the studies noted women ≤35 years old had a conception rate of 88% versus 66% for women>35 years old.
Proximal tubal occlusion
Proximal tubal occlusion occurs in 10-25% of infertile women leading to an obstruction of the anatomical pathway for fertilization. Tubal spasms, mucus plugs, debris, salpingitis isthmica nodosa, chronic salpingitis, intratubal endometriosis, tubal polyps and hypoplasia are all known causes of proximal tubal occlusion. False positive diagnosis has led to unnecessary tubal resection in 16-50% of women [44-47].
A meta-analysis, published in 1999 by Honoré G et al., examined 7 articles for those patients undergoing tubal micro- and macro surgery, 4 articles with selective salpingography and 4 articles for hysteroscopy management of proximal tubal occlusion. The average pregnancy rates were higher for those women managed by hysteroscopy tubal recanalization, 48.9%, when compared to tubal micro- and macro surgery, 38%, and selective salpingography, 28.8% [46].
A more recent review in 2010 by Allahbadia GN et al. evaluated success and pregnancy rates of tubal recanalization with hysteroscopy. Success rates ranged from 57-88% with partially occluded tubes or with complete occlusion in the cornual, proximal and intramural/ interstitial portions of the fallopian tube. One study that was reviewed, found a success rate of 13.3% for those tubes occluded in the distal portion of the tube after undergoing hysteroscopy tubal cannulation [43].
Failed In vitro fertilization
The use of hysteroscopy for women unable to conceive a pregnancy after undergoing in vitro fertilization (IVF) has been shown to have a two-fold effect. First, hysteroscopy will allow the diagnosis and treatment of intrauterine pathology, as described above, for those who may have had a normal hysterosalpingogram prior to their IVF cycle. Second, uterine instrumentation with hysteroscopy through the cervical canal may facilitate future embryo transfers as well as trigger an immunological mechanism in the endometrium, which may improve pregnancy rates [48,49].
Four prospective trials were found looking at the impact of hysteroscopy on those women with ≥2 failed IVF cycles. All of these patients had a normal hysterosalpingogram prior to their IVF cycles. The most common findings were polyps and intrauterine adhesions amongst all of the studies. Other hysteroscopy findings were fibroids, endometritis, hyperplasia and uterine septum. Oliveira et al. showed significantly higher pregnancy rates of those who had intrauterine pathology found and treated during hysteroscopy versus those who had a normal hysteroscopy. This is consistent with all the findings of the literature reviewed in this paper. Interesting to note, however, Demirol et al and Raju et al both found that those patients undergoing hysteroscopy even with normal findings had significantly higher pregnancy rates than those patients who never underwent hysteroscopy prior to their IVF cycle, approximately 32-44% versus 21-26%, respectively [50,51].
First trimester miscarriages
Approximately 15-20% of pregnancies result in spontaneous abortions with approximately 60-70% of these miscarriages secondary to chromosomal abnormalities. Performing hysteroscopy during the first trimester after confirming the pregnancy failure is called embryoscopy. Embryoscopy may allow the physician to evaluate embryonic mal development for those genetic lesions currently undetectable by cytogenetic techniques (Figures 6A and 6B). A direct biopsy of the demised embryo through embryoscopy improves cytogenetic testing by avoiding maternal blood/tissue contamination. Genetic counseling for those with chromosomal abnormalities may be offered to reduce the risk of abortion recurrence [52-54].
Figure 6A: Pregnancy at 7 weeks gestation with failure to develop beyond 5 weeks. Image is of fetus and yolk sac.
Figure 6B: 2 vessel cord of aborted fetus at 8 weeks gestation.
In 2003, Ferro et al evaluated 68 women that experienced first trimester abortions. These women underwent direct biopsy followed by suction curettage. Biopsies were successful in 97.2% of the gestational sacs. Karyotyping was accomplished in 79.7% of the cases. Of the 36 cases where cytogenic results were available from both the biopsy and curettage, it was noted that 12 (33%) of the cases had maternal contamination and in 8 (22%) of the cases only maternal tissue was found in the curettage specimen [55].
Another study in 2003 by Philipp et al, performed embryoscopy in 233 women with missed abortions. Visualization was successful in all women and karyotyping successful for 221 women. Mean age of the study population was 35.2 years with 89.7% being nulliparous. This population of women mainly conceived with assisted reproductive techniques (75.4%) and the remainder had a history of ≥ 2 previous spontaneous abortions. Of those identified with normal morphology that underwent karyotyping (n=31), 48.4% had an abnormal karyotyping. Abnormal morphology defined as growth disorganization, combined defects, and isolated defects, was detected in 200 (86%) of the fetuses. Normal karyotype was the result for 30% of fetuses with growth disorganization, 14% with combined defects and 40% with isolated defects. Three cases with normal morphology and karyotyping had amniotic band syndrome.
Although cytogenetics provides information of chromosomal causes for pregnancy, embryoscopy helps to reduce maternal contamination to provide a higher yield of fetal chromosomes as well as allows direct visualization of morphologic causes of a pregnancy failure. This information is valuable to for an infertile couple and their treating physician to help understand probable cause of miscarriage and to be able to take steps to reduce the chances of another unsuccessful outcome.
Conclusion
In conclusion, hysteroscopy may play an important role prior to or in conjunction with assisted reproductive techniques to help infertile women and couples achieve their goals of a pregnancy and live birth of a child.
References
- Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat 23. 2005; : 1-160.
- Bajekal N, Li TC. Fibroids, infertility and pregnancy wastage. Hum Reprod Update. 2000; 6: 614-620.
- Deligdish L, Loewenthal M. Endometrial changes associated with myomata of the uterus. J Clin Pathol. 1970; 23: 676-680.
- Casini ML, Rossi F, Agostini R, Unfer V. Effects of the position of fibroids on fertility. Gynecol Endocrinol. 2006; 22: 106-109.
- Richards PA, Richards PD, Tiltman AJ. The ultrastructure of fibromyomatous myometrium and its relationship to infertility. Hum Reprod Update. 1998; 4: 520-525.
- Yoshino O, Hayashi T, Osuga Y, Orisaka M, Asada H, Okuda S, et al. Decreased pregnancy rate is linked to abnormal uterine peristalsis caused by intramural fibroids. Hum Reprod. 2010; 25: 2475-2479.
- Oliveira FG, Abdelmassih VG, Diamond MP, Dozortsev D, Melo NR, Abdelmassih R. Impact of subserosal and intramural uterine fibroids that do not distort the endometrial cavity on the outcome of in vitro fertilization-intracytoplasmic sperm injection. Fertil Steril. 2004; 81: 582-587.
- Check JH, Choe JK, Lee G, Dietterich C. The effect on IVF outcome of small intramural fibroids not compressing the uterine cavity as determined by a prospective matched control study. Hum Reprod. 2002; 17: 1244-1248.
- Hart R, Khalaf Y, Yeong CT, Seed P, Taylor A, Braude P. A prospective controlled study of the effect of intramural uterine fibroids on the outcome of assisted conception. Hum Reprod. 2001; 16: 2411-2417.
- Rackow BW, Taylor HS. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril. 2010; 93: 2027-2034.
- Ezzati M, Norian JM, Segars JH. Management of uterine fibroids in the patient pursuing assisted reproductive technologies. Womens Health (Lond Engl). 2009; 5: 413-421.
- Shokeir TA. Hysteroscopic management in submucous fibroids to improve fertility. Arch Gynecol Obstet. 2005; 273: 50-54.
- Pritts EA, Parker WH, Olive DL. Fibroids and infertility: an updated systematic review of the evidence. Fertil Steril. 2009; 91: 1215-1223.
- Richlin SS, Ramachandran S, Shanti A, Murphy AA, Parthasarathy S. Glycodelin levels in uterine flushings and in plasma of patients with leiomyomas and polyps: implications for implantation. Hum Reprod. 2002; 17: 2742-2747.
- Oehninger S, Coddington CC, Hodgen GD, Seppala M. Factors affecting fertilization: endometrial placental protein 14 reduces the capacity of human spermatozoa to bind to the human zona pellucida. Fertil Steril 1995; 63: 377-383.
- Pérez-Medina T, Bajo-Arenas J, Salazar F, Redondo T, Sanfrutos L, Alvarez P, et al. Endometrial polyps and their implication in the pregnancy rates of patients undergoing intrauterine insemination: a prospective, randomized study. Hum Reprod. 2005; 20:1632-1635.
- Stamatellos I, Apostolides A, Stamatopoulos P, Bontis J. Pregnancy rates after hysteroscopic polypectomy depending on the size or number of the polyps. Arch Gynecol Obstet. 2008; 277: 395-399.
- Yanaihara A, Yorimitsu T, Motoyama H, Iwasaki S, Kawamura T. Location of endometrial polyp and pregnancy rate in infertility patients. Fertil Steril. 2008; 90: 180-182.
- Kormányos Z, Molnár BG, Pál A. Removal of a residual portion of a uterine septum in women of advanced reproductive age: obstetric outcome. Hum Reprod. 2006; 21: 1047-1051.
- Fedele L, Bianchi S, Marchini M, Franchi D, Tozzi L, Dorta M. Ultrastructural aspects of endometrium in infertile women with septate uterus. Fertil Steril. 1996; 65: 750-752.
- Gubbini G, Di Spiezio Sardo A, Nascetti D, Marra E, Spinelli M, Greco E, et al. New outpatient subclassification system for American Fertility Society Classes V and VI uterine anomalies. J Minim Invasive Gynecol. 2009; 16: 554-561.
- Pabuçcu R, Gomel V. Reproductive outcome after hysteroscopic metroplasty in women with septate uterus and otherwise unexplained infertility. Fertil Steril. 2004; 81: 1675-1678.
- Shokeir T, Abdelshaheed M, El-Shafie M, Sherif L, Badawy A. Determinants of fertility and reproductive success after hysteroscopic septoplasty for women with unexplained primary infertility: a prospective analysis of 88 cases. Eur J Obstet Gynecol Reprod Biol. 2011; 155: 54-57.
- Mollo A, De Franciscis P, Colacurci N, Cobellis L, Perino A, Venezia R, et al. Hysteroscopic resection of the septum improves the pregnancy rate of women with unexplained infertility: a prospective controlled trial. Fertil Steril. 2009; 91: 2628-2631.
- Ban-Frangez H, Tomazevic T, Virant-Klun I, Verdenik I, Ribic-Pucelj M, Bokal EV. The outcome of singleton pregnancies after IVF/ICSI in women before and after hysteroscopic resection of a uterine septum compared to normal controls. Eur J Obstet Gynecol Reprod Biol. 2009; 146: 184-187.
- Grimbizis GF, Camus M, Tarlatzis BC, Bontis JN, Devroey P. Clinical implications of uterine malformations and hysteroscopic treatment results. Hum Reprod Update. 2001; 7: 161-174.
- Nouri K, Ott J, Huber JC, Fischer EM, Stögbauer L, Tempfer CB. Reproductive outcome after hysteroscopic septoplasty in patients with septate uterus--a retrospective cohort study and systematic review of the literature. Reprod Biol Endocrinol. 2010; 8: 52.
- Robinson JK, Colimon LM, Isaacson KB. Postoperative adhesiolysis therapy for intrauterine adhesions (Asherman's syndrome). Fertil Steril. 2008; 90: 409-414.
- Valle RF, Sciarra JJ. Intrauterine adhesions: hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol. 1988; 158: 1459-1470.
- Valle RF, Sciarra JJ. Intrauterine adhesions: hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am J Obstet Gynecol. 1988; 158: 1459-1470.
- Orhue AA, Aziken ME, Igbefoh JO. A comparison of two adjunctive treatments for intrauterine adhesions following lysis. Int J Gynaecol Obstet. 2003; 82: 49-56.
- Klein SM, García CR. Asherman's syndrome: a critique and current review. Fertil Steril. 1973; 24: 722-735.
- Goldenberg M, Sivan E, Sharabi Z, Mashiach S, Lipitz S, Seidman DS. Reproductive outcome following hysteroscopic management of intrauterine septum and adhesions. Hum Reprod. 1995; 10: 2663-2665.
- March C, Hysteroscopy for infertility. In: Baggish M, editor. Chicago: Year Book Medical. 1989: 136-44.
- Pabuçcu R, Atay V, Orhon E, Urman B, Ergün A. Hysteroscopic treatment of intrauterine adhesions is safe and effective in the restoration of normal menstruation and fertility. Fertil Steril. 1997; 68: 1141-1143.
- Roy KK, Baruah J, Sharma JB, Kumar S, Kachawa G, Singh N. Reproductive outcome following hysteroscopic adhesiolysis in patients with infertility due to Asherman's syndrome. Arch Gynecol Obstet. 2010; 281: 355-361.
- Yu D, Li TC, Xia E, Huang X, Liu Y, Peng X. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman's syndrome. Fertil Steril. 2008; 89: 715-722.
- Capella-Allouc S, Morsad F, Rongières-Bertrand C, Taylor S, Fernandez H. Hysteroscopic treatment of severe Asherman's syndrome and subsequent fertility. Hum Reprod. 1999; 14: 1230-1233.
- Fernandez H, Al-Najjar F, Chauveaud-Lambling A, Frydman R, Gervaise A. Fertility after treatment of Asherman's syndrome stage 3 and 4. J Minim Invasive Gynecol. 2006; 13: 398-402.
- Cohen SB, Kalter-Ferber A, Weisz BS, Zalel Y, Seidman DS, Mashiach S, et al. Hysteroscopy may be the method of choice for management of residual trophoblastic tissue. J Am Assoc Gynecol Laparosc. 2001; 8: 199-202.
- Rein DT, Schmidt T, Hess AP, Volkmer A, Schöndorf T, Breidenbach M. Hysteroscopic management of residual trophoblastic tissue is superior to ultrasound-guided curettage. J Minim Invasive Gynecol. 2011; 18: 774-778.
- Faivre E, Deffieux X, Mrazguia C, Gervaise A, Chauveaud-Lambling A, Frydman R, et al. Hysteroscopic management of residual trophoblastic tissue and reproductive outcome: a pilot study. J Minim Invasive Gynecol. 2009; 16: 487-490.
- Golan A, Dishi M, Shalev A, Keidar R, Ginath S, Sagiv R. Operative hysteroscopy to remove retained products of conception: novel treatment of an old problem. J Minim Invasive Gynecol. 2011; 18: 100-103.
- Allahbadia GN, Merchant R. Fallopian tube recanalization: lessons learnt and future challenges. Womens Health (Lond Engl). 2010; 6: 531-548.
- Thurmond AS. Fallopian tube catheterization. Semin Intervent Radiol. 2008; 25: 425-431.
- Sulak PJ, Letterie GS, Coddington CC, Hayslip CC, Woodward JE, Klein TA. Histology of proximal tubal occlusion. Fertil Steril. 1987; 48: 437-440.
- Honoré GM, Holden AE, Schenken RS. Pathophysiology and management of proximal tubal blockage. Fertil Steril. 1999; 71: 785-795.
- McManus J, McClure N, Traub AI. The effect of cervical dilatation in patients with previous difficult embryo transfer. Fertil Steril. 2000; 74: 159.
- Mansour RT, Aboulghar MA. Optimizing the embryo transfer technique. Hum Reprod. 2002; 17: 1149-1153.
- Demirol A, Gurgan T. Effect of treatment of intrauterine pathologies with office hysteroscopy in patients with recurrent IVF failure. Reprod BioMed Onlie. 2004; 8: 590-594.
- Rama Raju GA, Shashi Kumari G, Krishna KM, Prakash GJ, Madan K. Assessment of uterine cavity by hysteroscopy in assisted reproduction programme and its influence on pregnancy outcome. Arch Gynecol Obstet. 2006; 274: 160-164.
- Philipp T, Feichtinger W, Van Allen MI, Separovic E, Reiner A, Kalousek DK. Abnormal embryonic development diagnosed embryoscopically in early intrauterine deaths after in vitro fertilization: a preliminary report of 23 cases. Fertil Steril. 2004; 82: 1337-1342.
- Philipp T, Philipp K, Reiner A, Beer F, Kalousek DK. Embryoscopic and cytogenetic analysis of 233 missed abortions: factors involved in the pathogenesis of developmental defects of early failed pregnancies. Hum Reprod. 2003; 18: 1724-1732.
- Paschopoulos M, Meridis EN, Tanos V, O'Donovan PJ, Paraskevaidis E. Embryofetoscopy: a new "old" tool. Gynecol Surg. 2006; 3: 79-83.
- Ferro J, Martínez MC, Lara C, Pellicer A, Remohí J, Serra V. Improved accuracy of hysteroembryoscopic biopsies for karyotyping early missed abortions. Fertil Steril. 2003; 80: 1260-1264.