
Research Article
Austin J Obstet Gynecol. 2023; 10(2): 1216.
The rs2304256, a Non-Synonymous Polymorphism in Tyrosine Kinase 2 Gene is Associated with the Risk of Endometriosis
KV Veena¹, Madhavi Latha Manolla¹, Mamata Deenadayal², Sisinthy Shivaji3,4 and Manjula Bhanoori¹*
1Department of Biochemistry, Osmania University, India
2Infertility Institute and Research Centre (IIRC), India
3Centre for Cellular and Molecular Biology (CCMB), India
4Presently at: Director of Research, Brien Holden Eye Research Centre, L V Prasad Eye Institute, India
*Corresponding author: Manjula BhanooriProfessor, Department of Biochemistry, Osmania University, 500007, Hyderabad, India Email: bhanoorim@yahoo.co.in bhanoori@osmania.ac.in
Received: February 02, 2023 Accepted: March 27, 2023 Published: April 03, 2023
Abstract
The objective of the study was to investigate whether the TYK2 gene influences the risk of developing endometriosis in South Indian women. The non-synonymous SNP, rs2304256, in exon8 of the TYK2 gene was tested for association in a case–control study of 150 affected women and 150 women with no evidence of disease. The genotype frequencies of the polymorphism were compared using polymerase chain reaction and restriction fragment length polymorphism. Immunohistochemistry was used to analyze the distribution and expression of TYK2 in the endometrium of women with and without endometriosis. According to codominant, dominant, and recessive genotype models, statistically significant differences were observed in the genotype distribution and allele frequency (P=0.0432) between the cases and controls. The distribution and expression of TYK2 did not vary in the endometrium of cases and controls. In the present study, we could establish an association between the TYK2 rs2304256 non-synonymous polymorphism with the risk of endometriosis in South Indian women, indicating that this polymorphism may lead to significant disease susceptibility.
Keywords: Endometriosis; Tyrosine kinase 2; Polymorphism; South Indian women
Abbreviations: CI: Confidence Interval; Χ²: Chi Square; D': Disequilibrium Coefficient; LD: Linkage Disequilibrium; HWE: Hardy–Weinberg Equilibrium; OR: Odds Ratio; TYK2: Tyrosine Kinase 2; IL: Interleukin; STAT: Signal Transducer and Activator of Transcription; PCR: Polymerase Chain Reaction; SNPs: Single Nucleotide Polymorphisms
Introduction
Endometriosis is a chronic, endocrine gynecologic disease characterized by the implantation of functional endometrial tissue at ectopic positions. It is observed mainly in the pelvic area including the ovaries, peritoneal surfaces and ligaments including bowel and bladder [10]. It affects 10-15% of women in their reproductive age and is responsible for dysmenorrhea, dyspareunia, infertility and chronic pelvic pain. Retrograde menstrual invasion and implantation of endometrial stromal cells into the peritoneum are the widely accepted explanations for this condition [17]. Although retrograde menstruation is common in 70-90% of women, the much lower endometriosis prevalence suggests that there must be other variables that may contribute to endometriosis pathogenesis. We previously looked at the correlation between genetic variants in multiple candidate genes and endometriosis risk in Indian women [4,13-16,32,33], which suggested that the condition is polygenic and multifactorial [31].
A complex network of cytokines mediates the immunomodulatory mechanisms leading to pathogenesis of endometriosis [39]. An altered secretion of Th1 and Th2 specific cytokines have been implicated in the pathogenesis of the disease. In the peritoneal fluid of affected women, there has been a shift in the balance of Th1/Th2 cytokines toward the Th2 response, contributing to the derangement of immunologic defense mechanism [28]. Elevated levels of Th2-specific cytokines such as interleukin IL-4, IL-5, IL-10 and IL-13 impairs T-cell cytotoxicity, enhancing the endometrial cell implantation and growth at the extra uterine sites [11]. Tyrosine Kinase 2 (TYK2) is located on chromosome 19p13.2 [9] and is implicated in signaling from Th2 cells, for example, through IL-10 and IL-13 receptors activating STAT3 and STAT6 signaling pathway [21,34]. TYK2 is widely studied in the pathogenesis of several tumors because of its critical role in tumor immunosurveillance [20].
Earlier, few genetic association studies have been conducted on TYK2 locus to study its impact on several autoimmune diseases, however, the identification has been duplicated in a large number of recent analyses and TYK2 is now considered to be a molecular marker in a variety of autoimmune and inflammatory diseases [12]. A non-synonymous SNP of TYK2rs2304256, is widely studied for its association with diseases like systemic lupus erythematosus, Crohn’s disease, rheumatoid arthritis, type 1 diabetes mellitus, ulcerative colitis, etc. [7,22,26]. This study was undertaken to investigate the association of TYK2 rs2304256 polymorphism with the risk of endometriosis in South Indian women.
Materials and Methods
Study Population
The case-control study was carried out on three hundred women in their reproductive age, recruited at the Infertility Institute and Research Center (IIRC), Hyderabad, India. The study subjects were obtained as described earlier [33].
Tissue collection
The proliferative phase endometrial tissues were collected and fixed as per the method described by Bhanoori et al (2008) [4].
DNA Extraction
Salting out procedure was used to extract the genomic DNA from one milliliter of anticoagulated whole blood.
Molecular Analysis of TYK2
Genotyping of TYK2 gene polymorphism was carried out by Polymerase Chain Reaction (PCR) and Restriction Fragment Length Polymorphism (RFLP) and the results confirmed by Sanger sequencing method as described earlier [16]. The primers and PCR conditions are summarized in (Table 1). The PCR products of TYK2 gene (385 bp) was digested with restriction enzyme (BsmI at 65°C) for 3 h and the DNA fragments were electrophoresed through a 2% agarose gel, stained with ethidium bromide. For the TYK2BsmI C/A SNP, the A allele was represented by DNA band of size 251 and 134bp, the C allele was represented by DNA bands of sizes 385 bp; whereas, the heterozygotes displayed a combination of both alleles (385, 251 and 134bp) (Figure 1).
S.no
Gene
Primers
PCR conditions
(35 cycles)Amplicon
Size1
TYK2
F:5'TCACCAGGCACTTGTTGTCC 3'
R:5'CGGCTTCCAGCATGTGTATG3'
5 min at 95°C, 40 sec at 94°C, 30 sec at 59°C,50 sec at 72°C
and 10min72°C.385
PCR*: Polymerase Chain Reaction
Table 1: Primers and PCR conditions used for TYK2 genotyping.
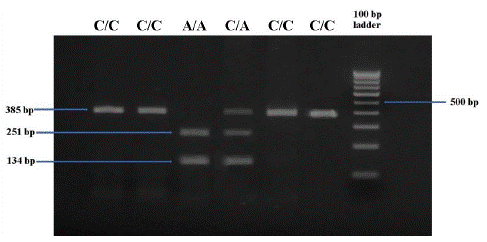
Figure 1: Restriction digestion of TYK2 polymorphism rs2304256 (C/A) by Bsm I.
Immunohistochemical Analysis
Immunohistochemical analysis of TYK2 expression was performed on the proliferative phase endometrial tissue sections from endometriosis patients (n=5) and healthy controls (n=4), using the method described by Bhanoori et al. (2008) [4]. After the incubation of sections with rabbit polyclonal antibody against TYK2 (1:1000, Cell signaling technology, USA) and FITC-conjugated goat anti-rabbit secondary antibody (1:1000, Sigma–Aldrich, USA), they were washed and mounted with an anti-fade mounting medium (Vector Lab, USA) then visualized using an Axioplan 2 epifluorescence microscope (Carl Zeiss, Inc., USA). Negative controls were obtained by substituting TYK2 antibody with the non-immune rabbit serum.
Statistical Analysis
Statistical analysis was performed as per the methods described in our previous study [33].
Results
All subjects (n=300) were successfully genotyped. The genotype distributions of individual SNPs, as well as allele system, were all in Hardy-Weinberg equilibrium (P<0.05) in both the cases and controls. The results were analyzed in a blinded fashion.
Genotyping of TYK2 (rs2304256C/A) Polymorphism
Sequence analysis of TYK2rs2304256 non-synonymous SNP is shown in Figure 2. The homozygotes (C/C) and (A/A) manifested as a single peak, whereas heterozygote (C/A) as double peaks. The genotype and allele (P=0.0432) distribution of TYK2 rs2304256 C/A polymorphism showed significant difference between cases and controls according to codominant, dominant and recessive model (P< 0.05; Table2). The frequencies of allele ’A’ and genotype AA in patients with endometriosis were significantly higher than those in controls, suggesting that allele ‘A’ and genotype AA are associated with endometriosis.
Genotypes/
AllelesCases
(n=150)Controls
(n = 150)P-value
Odds ratio
95% CI
TYK2
rs2304256/
Genotypes
Codominant model
57
71
-
Reference
Reference
CA
80
75
0.2353
1.3287
0.8306-2.1256
AA
Recessive model
AA
CA+CCDominant model
CA+AA
CC13
13
137
93
574
4
146
79
710.01328
0.02461
0.10219
4.0482
3.4635
1.4664
1.2519-13.0907
1.1025-10.8802
0.9259-2.3225
Alleles
C
194
217
Reference
Reference
A
106
83
0.0432
0.5901
0.3988-0.8731
CI: Confidence Interval.
Table 2: Genotype and allele frequencies of TYK2 gene polymorphism in endometriosis cases and controls.
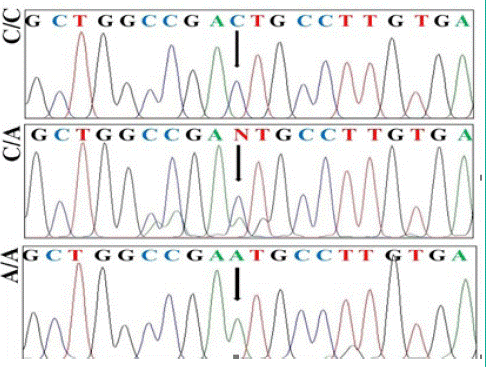
Figure 2: Genotyping of TYK2 gene polymorphism rs2304256 by sequence analysis of the PCR amplifi ed product using a forward primer.
Immunohistochemistry of TYK2
Immunostaining of TYK2 was observed in both glandular epithelial and stromal cells of endometrium. In the endometria of women with and without endometriosis, we observed no significant difference in the expression of TYK2 (Figure 3).
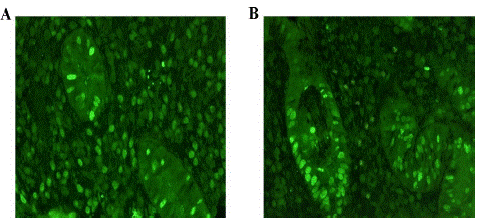
Figure 3: Immunohistochemical analysis of TYK 2 expression in phase-matched (proliferative) endometrium from case (A) and control (B).
Discussion
Endometriosis is a polygenically inherited and multifactorial disease. A variety of genetic association studies have focused on the association between cytokine (TNF-a, IL-2, IL-4, IL-6, IL-10, or IL-16) gene polymorphisms and endometriosis risk [24,25,36,37,38]. However, the studies on the down-stream signaling molecules like JAKs and STATs are very few. It is evident from the literature that the Th2 immune response is associated with endometriosis. [1]. Interleukin IL13, a typical Th2 cytokine is the key regulator of inflammatory and immune responses, that is central to endometriosis and associated abnormalities [8]. Elevated mRNA and protein levels of IL-13 was reported in the peritoneal fluid of endometriosis patients [35]. The same changes are observed in the ectopic endometrium of affected women which could be responsible for the defective immunosurveillance leading to endometriotic tissue overgrowth [8]. TYK2 is required for mediating the biological function of IL-13 in processes associated with Th2 immune response [5,21]. IL-13/TYK2 signaling in B cell proliferation, Ig E and MHC class II expression and Th1-cytokines inhibition are well demonstrated in endometriosis [29]. An aberration in TYK2 can lead to abnormal STAT6 signaling, gaining resistance to apoptosis and escaping the immune surveillance [6,20]. Therefore, TYK2gene may have the crucial importance in regulating immune and/or inflammatory responses in endometriosis.
In the current study, SNP in TYK2 rs2304256 was examined to ascertain whether the polymorphism is associated with endometriosis susceptibility in women from South Indian. The non-synonymous SNP rs2304256, a C to An alteration in exon 8 of TYK2, induces a change of valine to phenylalanine at position 362 in the JAK-homology 4 region of TYK2 [30]. Li et al showed that the allele ‘A’ disrupts a putative exonic splicing enhancer binding motif affecting the pre-mRNA processing of TYK2; thus, promoting the inclusion of exon 8 in the mRNA, which is essential for TYK2 binding to cytokine receptor [23]. At the rs2304256 locus, we observed that the allele ‘A’ and genotype AA occurred at significantly higher frequency in cases than in controls, indicating that the mutant allele 'A' could be a genetic risk factor for endometriosis. Of note, Peluso et al found no correlation between the SNP rs2304256 and endometriosis risk in the Brazilian population [27], which is inconsistent with the present finding. Possible reasons for this inconsistency could be: first, ethnic variation seen in the polymorphism analyzed. In point of fact, drastic difference was observed between the two populations in the distribution of the 'A' allele. In the Brazilian population, the mutant allele 'A' was found in 26.4 percent of cases and 23.6 percent of controls, whereas in the South Indian population, the frequencies were 35.3 percent and 27.6 lymphopercent in the current study. The second possible reason could be due to the difference in the sample size used in the study. However, larger population studies may require validating the exact role of this mutation in endometriosis.
The expression of TYK2 in the eutopic endometrium of women with and without endometriosis did not differ significantly in our study. Although there are conflicting results on TYK2 expression in various cancers, the majority of research found higher expression in tumor tissues when compared to controls [18,19]. Additionally, a strong correlation between TYK2 over-expression and late-stage tumors was observed, indicating that TYK2 over-expression contributes to the aggressiveness of tumors. However, we found no significant difference in TYK2 expression between eutopic endometria of women with and without endometriosis in the current study. To validate the role of TYK2 expression in the pathophysiology of endometriosis, more research with a bigger sample size is needed.
The minor allelic frequency for the SNP evaluated was compared with the mutation frequency data from populations of different ethnic origins, obtained from HapMap, 1000Genomes, Genome Aggregation Database (GnomAD) and EXAC database (dbSNP) (Supplementary Table 1). In the cases with the polymorphism under study, we observed that the minor allelic frequency was close to the values reported for Asian populations in the 1000 Genome Database. The frequency of 'A' allele is more common in Asians, Europeans, and Americans than it is in Africans for the TYK2 gene polymorphism rs2304256. Since Indians are a part of Asian ethnic group, the allelic frequency for the SNP rs2304256 found in the cases were very close with the Asians represented in 1000 Genomes, however, further confirmation requires studies with large sample sizes.
JAK-STAT signaling mediates immune regulatory processes that are central in endometriosis development and progression. We believe that the combined effect of various JAK-STAT mediator polymorphisms may disrupt immune homeostasis leading to the establishment and maintenance of endometrial cells at ectopic locations, as illustrated previously by our team considering IL6 and STAT6 [2,3]. The Discovery of how these genetic polymorphisms interact on endometriosis development may be a crucial step in understanding the pathophysiology of endometriosis.
In conclusion, our study shows that the TYK2 gene polymorphism rs2304256 is significantly associated with endometriosis in South Indian women. Analysis of this polymorphism might help to identify patients at high risk for endometriosis development. Although further work is necessary to understand the molecular mechanism, our findings may lead to new insights into the disease pathogenesis.
Author Statements
Acknowledgements
This work was supported in part by grants from the SERB (DST), India (Lr. No: SR/FT/LS-188/2009) and OU-DST PURSE Programme-II (DST Sanction No. SR/PURSE Phase 2/32(G)), Department of Science and Technology (DST), India to Prof. Manjula Bhanoori.
Conflict of Interests
The authors declare that there is no conflict of interests.
Ethical Approval
The study was approved by the ethical committee and review board of Centre of Cellular and Molecular biology (CCMB), Hyderabad. In the study all the participants were of South Indian origin (Dravidian linguistic group).
Informed Consent
Informed written consent form was obtained from all subjects prior to participation in this study.
Author’s Contribution
KVV: execution of experiments, analysis and interpretation of data, statistical analysis and drafting of manuscript. MLM: Data collection. MD: acquisition of data. SS: analysis and interpretation of data, drafting of manuscript. MB: conception and design of study, analysis and interpretation of data, statistical analysis, drafting of manuscript. All authors will have seen and agreed to the ‘Author Contribution’ statement.
References
- Antsiferova YS, Sotnikova NY, Posiseeva LV, Shor AL. Changes in the T-helper cytokine profile and in lymphocyte activation at the systemic and local levels in women with endometriosis. Fertil Steril. 2005; 84: 1705-11.
- Bhanoori M, Babu KA, Deenadayal M, Kennedy S, Shivaji S. The interleukin-6 -174G/C promoter polymorphism is not associated with endometriosis in South Indian women. J Soc Gynecol Investig. 2005; 12: 365-9.
- Bhanoori M, Deenadayal M, Kennedy S, Shivaji S. The G2964A 3'-untranslated region polymorphism of the signal transducer and activator of transcription 6 gene is associated with endometriosis in South Indian women. Hum Reprod. 2007; 22: 1026-30.
- Bhanoori M, Kameshwari DB, Zondervan KT, Deenadayal M, Kennedy S, et al. The endothelial nitric oxide synthase Glu298Asp polymorphism is not a risk factor for endometriosis in south Indian women. Eur J Obstet Gynecol Reprod Biol. 2008; 139: 53-8.
- Bhattacharjee A, Shukla M, Yakubenko VP, Mulya A, Kundu S, et al. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med. 2013; 54:1-16.
- Borcherding DC, He K, Amin NV, Hirbe AC. TYK2 in Cancer Metastases: Genomic and Proteomic Discovery. Cancers (Basel). 2021; 13: 4171.
- Can G, Tezel A, Gürkan H, Can H, Yilmaz B, et al. Tyrosine kinase-2 gene polymorphisms are associated with ulcerative colitis and Crohn's disease in Turkish Population. Clin Res Hepatol Gastroenterol. 2015; 39: 489-98.
- Chegini N, Roberts M, Ripps B. Differential expression of interleukins (IL)-13 and IL-15 in ectopic and eutopic endometrium of women with endometriosis and normal fertile women. Am J Reprod Immunol. 2003; 49: 75-83.
- Cunninghame Graham DS, Akil M, Vyse TJ. Association of polymorphisms across the tyrosine kinase gene, TYK2 in UK SLE families. Rheumatology (Oxford). 2007; 46: 927-30.
- Foti PV, Farina R, Palmucci S, Vizzini IAA, Libertini N, et al. Endometriosis: clinical features, MR imaging findings and pathologic correlation. Insights Imaging. 2018; 9: 149-172.
- Gallinelli A, Chiossi G, Giannella L, Marsella T, Genazzani AD, Volpe A. Different concentrations of interleukins in the peritoneal fluid of women with endometriosis: relationships with lymphopercent cyte subsets. Gynecol Endocrinol. 2004; 18: 144-51.
- Gonciarz M, Pawlak-Bus K, Leszczynski P, Owczarek W. TYK2 as a therapeutic target in the treatment of autoimmune and inflammatory diseases. Immunotherapy. 2021; 13: 1135-1150.
- Govatati S, Challa K, Reddy SB, Pramod K, Deenadayal M, et al. BRCA1 alterations are associated with endometriosis, but BRCA2 alterations show no detectable endometriosis risk: a study in Indian population. J Assist Reprod Genet. 2015; 32: 277-85.
- Govatati S, Deenadayal M, Shivaji S, Bhanoori M. Mitochondrial NADH:ubiquinone oxidoreductase alterations are associated with endometriosis. Mitochondrion. 2013; 13: 782-90.
- Govatati S, Kodati VL, Deenadayal M, Chakravarty B, Shivaji S, et al. Mutations in the PTEN tumor gene and risk of endometriosis: a case-control study. Hum Reprod. 2014; 29: 324-36.
- Govatati S, Tangudu NK, Deenadayal M, Chakravarty B, Shivaji S, et al. Association of E-cadherin single nucleotide polymorphisms with the increased risk of endometriosis in Indian women. Mol Hum Reprod. 2012; 18: 280-7.
- Hill CJ, Fakhreldin M, Maclean A, Dobson L, Nancarrow L, et al. Endometriosis and the Fallopian Tubes: Theories of Origin and Clinical Implications. J Clin Med. 2020; 9: 1905.
- Ide H, Nakagawa T, Terado Y, Kamiyama Y, Muto S, Horie S. Tyk2 expression and its signaling enhances the invasiveness of prostate cancer cells. BiochemBiophys Res Commun. 2008; 369: 292-6.
- Jia X, Huang C, Hu Y, Wu Q, Liu F, et al. Cirsiliol targets tyrosine kinase 2 to inhibit esophageal squamous cell carcinoma growth in vitro and in vivo. J Exp Clin Cancer Res. 2021; 40: 105.
- Karjalainen A, Shoebridge S, Krunic M, Simonovic N, Tebb G, et al. TYK2 in Tumor Immunosurveillance. Cancers (Basel). 2020; 12: 150.
- Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003; 300: 1527-8.
- Lee YH, Bae SC. Association between TYK2 polymorphisms and susceptibility to autoimmune rheumatic diseases: a meta-analysis. Lupus. 2016; 25: 1307-14.
- Li Z, Rotival M, Patin E, Michel F, Pellegrini S. Two common disease-associated TYK2 variants impact exon splicing and TYK2 dosage. PLoS One. 2020; 15: e0225289.
- Malutan AM, Drugan C, Drugan T, Ciortea R, Mihu D. The association between interleukin-4 -590C/T genetic polymorphism, IL-4 serum level, and advanced endometriosis. Cent Eur J Immunol. 2016; 41: 176-81.
- Matalliotakis M, Zervou MI, Eliopoulos E, Matalliotaki C, Rahmioglu N, et al. The role of IL 16 gene polymorphisms in endometriosis. Int J Mol Med. 2018; 41: 1469-1476.
- Pellenz FM, Dieter C, Duarte GCK, Canani LH, de Souza BM, et al. The rs2304256 Polymorphism in TYK2 Gene Is Associated with Protection for Type 1 Diabetes Mellitus. Diabetes Metab J. 2021; 45: 899-908.
- Peluso C, Christofolini DM, Goldman CS, Mafra FA, Cavalcanti V, et al. TYK2 rs34536443 polymorphism is associated with a decreased susceptibility to endometriosis-related infertility. Hum Immunol. 2013; 74: 93-7.
- Podgaec S, Abrao MS, Dias JA Jr, Rizzo LV, de Oliveira RM, et al. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007; 22: 1373-9.
- Roberts M, Luo X, Chegini N. Differential regulation of interleukins IL-13 and IL-15 by ovarian steroids, TNF-alpha and TGF-beta in human endometrial epithelial and stromal cells. Mol Hum Reprod. 2005; 11: 751-60.
- Tao JH, Zou YF, Feng XL, Li J, Wang F, et al. Meta-analysis of TYK2 gene polymorphisms association with susceptibility to autoimmune and inflammatory diseases. Mol Biol Rep. 2011; 38: 4663-72.
- Tempfer CB, Simoni M, Destenaves B, Fauser BC. Functional genetic polymorphisms and female reproductive disorders: part II--endometriosis. Hum Reprod Update. 2009; 15: 97-118.
- Veena KV, Siddamalla S, Deenadayal M, Shivaji S, BhanooriM . DNMT1 and DNMT3B gene variants and their association with endometriosis in South Indian women. Mol Biol Rep. 2022a; 49: 321-329.
- Veena KV, Siddamalla S, Deenadayal M, Sisinthy S, Bhanoori M. Histone deacetylase 1, Sirtuin 1, and Sirtuin 3 single-nucleotide polymorphisms and the risk of endometriosis in South Indian women. J Obstet Gynaecol. 2022b; 42: 3230-3235.
- Verma R, Balakrishnan L, Sharma K, Khan AA, Advani J, et al. A network map of Interleukin-10 signaling pathway. J Cell Commun Signal. 2016; 10: 61-7.
- Wang XM, Ma ZY, Song N. Inflammatory cytokines IL-6, IL-10, IL-13, TNF-a and peritoneal fluid flora were associated with infertility in patients with endometriosis. Eur Rev Med Pharmacol Sci. 2018; 22: 2513-2518.
- Wang XQ, Hu M, Chen JM, Sun W, Zhu MB. Effects of gene polymorphism and serum levels of IL-2 and IL-6 on endometriosis. Eur Rev Med Pharmacol Sci. 2020; 24: 4635-4641.
- Yasri S, Wiwanitkit V. Tumor necrosis factor-a gene-1031T/C promoter polymorphism and endometriosis. Horm Mol Biol Clin Investig. 2020; 41.
- Zhang X, Hei P, Deng L, Lin J. Interleukin-10 gene promoter polymorphisms and their protein production in peritoneal fluid in patients with endometriosis. Mol Hum Reprod. 2007; 13: 135-40.
- Zhou WJ, Yang HL, Shao J, Mei J, Chang KK, et al. Anti-inflammatory cytokines in endometriosis. Cell Mol Life Sci. 2019; 76: 2111-2132.