
Research Article
Austin J Obstet Gynecol. 2025; 12(1): 1230.
Live Birth from the Oocytes having Granulation in Perivitelline Space-A Case study
Nisha Patel*
Consulting Obstetrics and Gynecologist, Gynob Sonoscan Center, Dev ART IVF- Test Tube Baby Center and Shachi Women’s Hospital, Ahmedabad, Gujarat, India
*Corresponding author: Nisha Patel, Consulting Obstetrics and Gynecologist, Gynob Sonoscan Center, Dev ART IVF- Test Tube Baby Center and Shachi Women’s Hospital, Ahmedabad, Gujarat, India Email: drnishapatel2022@gmail.com
Received: April 05, 2025 Accepted: April 18, 2025 Published: April 23, 2025
Abstract
Assisted Reproductive Technology success is influenced by multiple factors, with oocyte quality and controlled ovarian stimulation being critical determinants of embryo development. A specific concern in ART is the presence of granules in the perivitelline space, which is debated for its potential impact on oocyte quality and ART outcomes. While some studies link PVS granularity to reduced fertilization rates and poor embryo quality, others find no significant correlation. This study reports a case of a 29-year-old woman with primary infertility who underwent in vitro fertilization after four unsuccessful intrauterine insemination cycles. During ovarian stimulation and oocyte retrieval, all mature oocytes displayed severe PVS granularity. Despite this, intracytoplasmic sperm injection was performed, resulting in the development of high-quality embryos. Two embryos were transferred during the second thaw embryo transfer cycle, leading to a successful pregnancy and live birth of a healthy female infant. This case challenges the prevailing notion that PVS granularity necessarily predicts poor reproductive outcomes, highlighting the need for further research to explore its impact. It emphasizes the importance of individualized treatment approaches and comprehensive assessment of oocyte quality, rather than solely relying on morphological markers like PVS granularity.
Keywords: Perivitelline space; Granulation; ART; IVF; Intracytoplasmic sperm injection
Introduction
Assisted Reproductive Technology (ART) success is influenced by multiple factors, from folliculogenesis to live birth. Oocyte quality, particularly its maturity, plays a crucial role in embryo development, and controlled ovarian stimulation is key to optimizing oocyte quality. However, the significance of perivitelline space (PVS) granularity remains unclear—whether it reflects a physiological or pathological process, such as apoptosis, is not well understood. Some evidence suggests PVS granules may originate from remnants of coronal cell processes, which retract during meiotic resumption [1]. While various factors, including improper ovulation induction, poor culture conditions, advanced maternal age, and genetic mutations, impact oocyte quality, the specific link between PVS granularity and ART outcomes is debated. Some studies associate PVS granules with reduced fertilization rates, poor embryo quality, or implantation failure, while others find no such correlation. Granules may be linked to factors such as high doses of human menopausal gonadotropins (hMG), abnormal zona pellucida formation, and oocyte aging [2]. However, the impact of PVS granules on ART success has not been thoroughly studied. This study aims to explore live birth outcomes from oocytes with PVS granulation through a prospective observational case report [3,4].
Methodology
Patient History
A 29-year-old woman presented with primary infertility, having previously undergone diagnostic laparoscopy which showed normal results. Her ovarian reserve was found normal. Despite undergoing 4 cycles of intrauterine insemination (IUI) without success, patient was advised to proceed with invitro fertilization (IVF) as the next step of the treatment.
Ovarian Stimulation and ART Procedure
A self-cycle stimulation was started for the patient using an antagonist protocol for ovarian stimulation. Menopur 150 HP, a Human Menopausal Gonadotropin (HMG) injection was administered for first 5 days. After that, Cetrorelix acetate 0.25 mg was added to the HMG regimen and continued for an additional 5 days, during these periods the growth of the follicle was monitored. After ten days of stimulation cycle, the growth of most of the follicles were reached a size of 17 mm. At this point, Decapeptyl was given as trigger. After 35 hours of trigger, ovum pickup was carried out using a Cook ovum aspiration unit (set to 120 mmHg pressure) and a single-lumen ovum aspiration needle from Kitazato. This ovum pick up was carried out under general anesthesia. The serum estradiol level was 4874 pg/ml. During the ovum pickup procedure, an oocytecumulus complexes was collected using HEPES media with human serum albumin from Vitromed. Follicular fluid was then transferred to a 14 ml test tube and then to IVF lab. Scanning of follicular fluid was performed using a 60 X a5 mm size Petri dish and a glass pipette under a stereo zoom microscope.
After the oocyte-cumulus complexes (OCC) were retrieved, they were placed into a ml of one step media in central well dish without an oil overlay and incubated in a CO2 Heracell 150i incubator until intracytoplasmic sperm injection (ICSI) was performed. ICSI was performed 39-40 hours post-trigger using Falcon ICSI dish with 7% PVP from Vitromed for sperm immobilization and HEPES media droplets to maintain the oocyte during the procedure. Vitromed injecting and holding needle were used and the procedure was conducted with a Narishige 71 micromanipulator in conjunction with an Olympus. The semen parameters were within normal ranges, with sperm concentration 62 million/ml, motility of 35 % and morphology of 4 %. The semen was prepared using a double-layer density gradient method. Total 26 oocyte-cumulus complexes were retrieved. Amongst them, 21 were at the M2 stage and 5 were GV after denudation. Diluted Hyalase from Vitromed was used for denudation, with Denupet sizes of 150 and 140, respectively. All mature oocytes showed severe granulation in the perivitelline space (Figure 1).
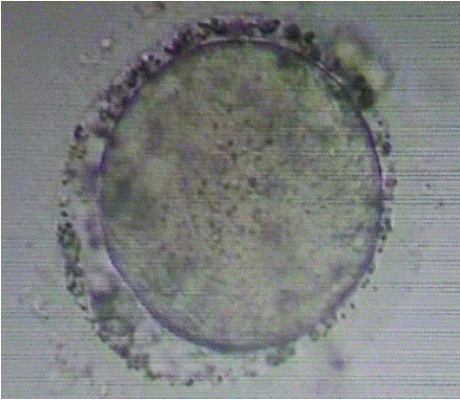
Figure 1: Granularity in PVS.
ICSI was performed on all M2 oocytes, followed by incubation in one-step culture media (Group Culture) from Vitromed. The media were incubated 7 to 8 hours prior to OPU. At day-01, total of 18 oocytes showed 2PN and 2 polar bodies. Out of these 18, 14 oocytes reached the 8-cell stage on day 3 (72 hours post insemination), with 15% fragmentation. Out of these 14, a total of 8 embryos were formed. On day-04, 6 embryos were frozen, and on day-05, 2 embryos were frozen. Kitazato Vitrification media and standard protocol of Kitazato were used for vitrification. Cryolock served as the vitrification device, and the embryos were stored in liquid nitrogen at -196 °C. the embryo has been graded as follows. The day-04, 6 embryo was frozen at the 15% fragmented compacted morula phase. Two embryos were frozen with a total grade of 4AB and 4BB on day five. Figure 2(a) and Figure 2(b) showed the ET Thawed Embryo on day 4 and day 5, respectively.
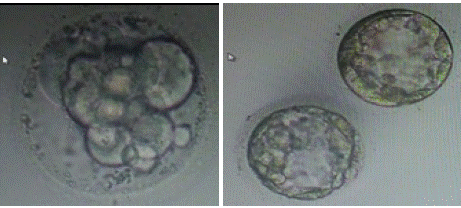
Figure 2: (a) ET thawed embryo on day 4; (b) ET thawed embryo on day 5.
Result
The first trial of thaw embryo transfer was conducted after 1.5 months, with an endometrial thickness of 7.9 mm. Two embryos were transferred on day 5 after being thawed on the same day. The BhCG values were 246 mIU/ml and 365 mIU.ml on the 15th and 17th days, respectively. But it was a slow growing embryo that resulted into a missed abortion.
The second trial of thaw embryo transfer was carried out after 2 months. Two embryos were transferred and thawed on the same day, with the endometrium thickness measured at 8.8 mm. After 15 days of embryo transfer, the BhCG value was 849 mIU.ml, and a single gestational sac measuring 4.41 mm was detected in the ultrasound. Subsequent NT scan, fetal anomaly scan, and NIPT (Noninvasive prenatal testing) were appears to be normal. An elective lower segment Caesarean section was performed at full term, 37 weeks of gestation, resulting in the birth of a normal female child weighing 2.520 kg. Figure 3 presents the graphical representation of oocyte to embryo growth, while Figure 4 illustrates the progression from embryo number to live birth.
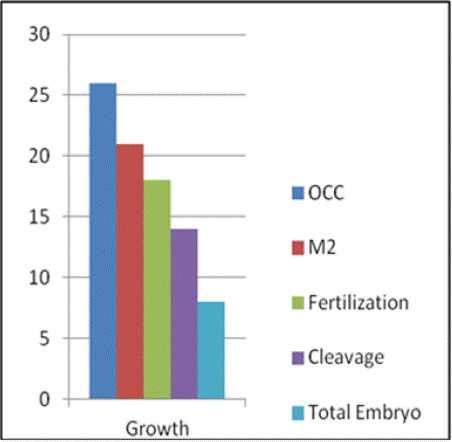
Figure 3: Growth of oocyte to embryo.
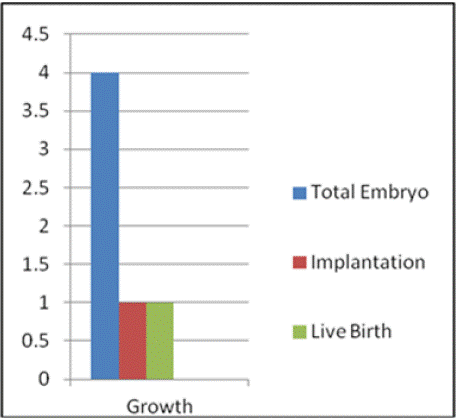
Figure 4: Growth from no. of embryo to Live birth.
Discussion
This case study presents an intriguing scenario wherein a patient with primary infertility achieved a successful live birth following IVF, despite all retrieved oocytes exhibiting severe perivitelline space (PVS) granularity. Traditionally, PVS granularity has been linked to reduced oocyte quality, lower fertilization rates, and diminished pregnancy outcomes. Studies such as those by Farhi et al. [5] and Zanetti et al. [6] provide significant insights into these negative associations.
Farhi et al. [5] conducted a study analyzing 623 oocytes, of which 418 were fertilized, and 246 embryos were transferred. Despite comparable IVF cycle characteristics between the study and control groups, the outcomes differed notably. The pregnancy rate per cycle in the study group, where coarse granules were present in the PVS, was only 10.7%, compared to 32.3% in the control group without granulation. Similarly, the implantation rate per embryo was 5.7% in the study group, while the control group showed 11.5%. This study strongly suggests that coarse granulation in the PVS negatively impacts embryonic viability and success rates in IVF-ICSI cycles, making this case's outcome particularly remarkable.
Zanetti et al. [6] further supported these findings, investigating the role of PVS abnormalities, including PVS granularity, on intracytoplasmic sperm injection (ICSI) outcomes. They studied 9,752 oocytes and found that cases where both large PVS and PVS granularity were present showed significantly lower implantation and pregnancy rates compared to controls, despite similar fertilization rates. These findings reinforced the notion that combined PVS abnormalities compromise reproductive outcomes. However, isolated PVS granularity alone did not significantly affect clinical outcomes, leaving open the possibility that success can still be achieved under specific conditions.
The study by Bulgurcuoglu-Kuran et al. [7] used transmission electron microscopy (TEM) to evaluate the ultrastructure of oocytes with coarse PVS granules, revealing irregular microvilli structures and denser lipid droplets within the PVS and cumulus cells. The study also linked coarse granules to lower-quality embryos and higher rates of embryo arrest and miscarriage. Interestingly, this study highlighted that higher body mass index (BMI) was associated with a greater likelihood of PVS granularity, which adds an additional layer to the understanding of this abnormality’s impact on fertility.
In another study, Hassa et al. [8] analyzed the developmental potential of embryos from oocytes with PVS abnormalities in ICSI cycles. They found that oocytes with PVS abnormalities had lower rates of Grade I embryo development (68.5%) compared to normal oocytes (82.1%), and higher rates of Grade III embryo development (7.7% versus 0%, respectively). This further suggests that PVS abnormalities negatively influence embryo quality and developmental potential.
However, this case challenges the conventional understanding of the impact of PVS granularity on reproductive success. Despite severe granularity in all oocytes, the patient achieved a successful pregnancy and live birth, suggesting that PVS granularity may not be an absolute determinant of poor outcomes. Some oocytes may possess compensatory mechanisms that allow for normal fertilization and development despite the granularity. Oocytes with granularity may still have the potential to develop into viable embryos, depending on other inherent qualities. For example, Bulgurcuoglu-Kuran et al. [7] found that while coarse granules were associated with lower-quality embryos and higher miscarriage rates, individual oocytes may still overcome these challenges, particularly with expert handling during IVF procedures.
Precise techniques during oocyte retrieval, denudation, and ICSI might have reduced the impact of morphological abnormalities. As reported by Hassa et al., [8] careful handling during these stages can influence embryo development and pregnancy outcomes, even when oocytes exhibit PVS abnormalities. Another critical factor that may have contributed to the successful outcome is the patient’s semen quality. In this case, semen parameters such as concentration, motility, and morphology were all within normal ranges, which likely supported the fertilization process.
This case study is limited by its nature as a single case report, meaning that its findings cannot be generalized to all patients with PVS granularity. While the outcome is promising, larger studies are needed to fully understand the implications of PVS abnormalities on IVF success rates. Research with a broader patient population is essential to confirm the positive outcome in study. Despite its limitations, this case offers hope for patients with oocytes exhibiting PVS granularity. It highlights the importance of individualized patient management, suggesting that traditional markers of oocyte quality, such as granularity, should not always be used as definitive predictors of IVF failure. Clinicians should consider a range of factors, including semen quality and technical expertise, before making decisions about embryo viability. While existing literature has consistently shown a negative association between PVS abnormalities and pregnancy success rates, this case demonstrates that successful outcomes are still possible. It calls for further research to explore the full impact of PVS granularity on oocyte and embryo development, and to develop strategies for improving IVF success in cases where this morphological abnormality is present. Importantly, clinicians should avoid discarding oocytes solely based on the presence of granularity and instead adopt a more comprehensive approach to assessing oocyte quality and potential.
References
- Hassan-Ali H, Hisham-Saleh A, El-Gezeiry D, Baghdady I, Ismaeil I, Mandelbaum J. Perivitelline space granularity: a sign of human menopausal gonadotrophin overdose in intracytoplasmic sperm injection. Hum Reprod. 1998; 13: 3425-3430.
- Khalili MA, Mojibian M, Sultan AM. Role of oocyte morphology on fertilization and embryo formation in assisted reproductive techniques. Middle East Fertil Soc J. 2005; 10: 72-77.
- Coticchio G, Dal Canto M, Fadini R, Mignini Renzini M, Guglielmo MC, Miglietta S, et al. Ultrastructure of human oocytes after in vitro maturation. MHR Basic Sci Reprod Med. 2016; 22: 110-118.
- Xia P. Intracytoplasmic sperm injection: correlation of oocyte grade based on polar body, perivitelline space and cytoplasmic inclusions with fertilization rate and embryo quality. Hum Reprod. 1997; 12: 1750-1755.
- Farhi J, Nahum H, Weissman A, et al. Coarse granulation in the perivitelline space and IVF-ICSI outcome. J Assist Reprod Genet. 2002; 19: 545-549.
- Zanetti BF, Braga DP, Setti AS, Figueira RC, Iaconelli A Jr, Borges E Jr. Is perivitelline space morphology of the oocyte associated with pregnancy outcome in intracytoplasmic sperm injection cycles?. Eur J Obstet Gynecol Reprod Biol. 2018; 231: 225-229.
- Bulgurcuoglu-Kuran S, Altun A, Karakus FN, Kotil T, Ozsait-Selcuk B. Ultrastructure of coarse granules in the perivitelline space and association with ovulation induction protocols. JBRA Assist Reprod. 2023; 27: 660-667.
- Hassa H, Aydin Y, Taplamacioglu F. The role of perivitelline space abnormalities of oocytes in the developmental potential of embryos. J Turk Ger Gynecol Assoc. 2014; 15: 161-163.