
Case Report
Austin J Obstet Gynecol. 2015;2(1): 1034.
Fatal Case of Bilateral Luteinized Thecoma with Sclerosing Peritonitis in a 33-Year-Old Woman
Juan Jesús Fernández-Alba1*, Ángel Vilar Sánchez1, Carmen González-Macías1, Raquel León del Pino1, Ángela Henz Pérez2, Raquel Perea3, Luis Javier Moreno Corral1 and Rafael Torrejón Cardoso1
1Department of Obstetrics and Gynecology, University Hospital of Puerto Real, Spain
2Department of Pathology, University Hospital of Puerto Real, Spain
3Department of Radiodiagnostic, University Hospital of Puerto Real, Spain
*Corresponding author: Juan Jesús Fernández-Alba, Department of Obstetrics and Gynecology, University Hospital of Puerto Real, Nacional IV Km 665, 11500 Puerto Real, Spain
Received: January 04, 2015; Accepted: March 17, 2015; Published: April 01, 2015
Abstract
Thecomas are sex-cord stromal tumors, included in the thecoma- fibroma group by the World Health Organization. Sclerosing peritonitis has characteristic peritoneal alterations and microscopic findings that can lead to intestinal occlusion. The coexistence of thecoma and sclerosing peritonitis is very rare: 25 cases have been reported in the literature, with most patients treated surgically by bilateral oophorectomy. The operative morbidity and mortality is estimated to be as high as 50%, varying by etiology: from 6% in young patients with idiopathic disease, 28% in adults, and up to 80% in patients undergoing peritoneal dialysis. We present a 33-year-old Caucasian woman who presented to the general emergency department of our hospital with abdominal pain and the sensation of abdominal fullness and distention. She underwent exploratory laparotomy, which revealed copious ascitic fluid and a 15-cm mass, twisted and necrotic, dependent from the left ovary. A similar 10-cm tumor was found in the pouch of Douglas, dependent from the right ovary. The peritoneum was thickened. The omentum was remarkably retracted and had the hard consistency suggestive of tumor infiltration. Bilateral salpingo-oophorectomy with peritoneal and omental biopsies was performed. Despite the high suspicion for malignancy, histology revealed acellular ascitic fluid. Histology of the ovarian sections showed a lax, edematous cellular substrate, with a few dense areas of spindle cells with elongated nuclei and very few mitoses.
Inmediatly after surgery, the patient developed recurrent ascites, intestinal obstruction, and renal failure. Computed tomography revealed intense peritoneal involvement, so we started treatment with high-dose dexamethasone. Unfortunately, the treatment was unsuccessful and the patient died of multiorgan failure.
Sclerosing peritonitis associated with ovarian thecoma is a rare entity. Clinicians should suspect this condition when bilateral ovarian tumors are accompanied by large amounts of ascites and severe peritoneal involvement, particularly when peritoneal histology reveals no malignancy.
Introduction
Thecomas are sex-cord stromal tumors that the World Health Organization includes in the thecoma- fibroma group. Histologically, they consist of large, rounded cells with characteristics similar to theca interna cells, with abundant vacuolated cytoplasm rich in lipids. These tumors account for less than 1% of ovarian neoplasms [1]. The vast majority occur in postmenopausal women, and only 10% occur in women under 30 years of age. More than 70 instances have been reported in the literature, of which 4 were found to be malignant and 25 associated with an entity known as sclerosing peritonitis [2-10]. The largest case series was described in 1982 [11]. The association between thecoma and sclerosing peritonitis was first described in 1994 by Clement [12].
Sclerosing peritonitis is also called “peritonitis chronica fibrosa incapsulata,” a term introduced by Owtschinnikov in 1907. The condition may be either primary or secondary and describes an entity comprising peritoneal alterations and microscopic findings. The condition causes abdominal adhesions, leading ultimately tointestinal occlusion. In 1968, Black et al. [13], described sclerosing peritonitis in an 8-year-old girl, probably related to hemoperitoneum after abdominal trauma. In 1974, sclerosing peritonitis was etiologically associated with the use of beta-blockers [14,15]. In 1977, Jackson presented 6 surgical patients who had a history of practolol use. Finally, in 1980, sclerosing encapsulating peritonitis was linked to the use of continuous peritoneal dialysis [16]. The condition also has reported associations with spontaneous bacterial peritonitis, [17] placement of LeVeen peritoneovenous shunts in patients with liver cirrhosis and ascites, [18] primary peritoneal sarcoidosis, [19] carcinoid syndrome, familial Mediterranean fever, and asbestosis [13].
Case Report
A 33-year-old nulliparous Caucasian woman presented to our general emergency department with a 2-week history of abdominal pain that had intensified over the last 3 days. She also described a sensation of abdominal fullness that intensified after eating. Over the previous 2 weeks, she developed progressive swelling of the face, hands, legs, and abdomen. The edema was initially attributed to an adverse reaction to ramipril, which she took for 1 week to treat her hypertension. This medication was stopped and she was given a tapering dose of deflazacort, starting at 30 mg. Besides hypertension, her medical history was significant for hypothyroidism treated with thyroxine, mixed hyperlipidemia, bronchial asthma, and polycystic ovarian syndrome, for which she took ethinyl estradiol and cyproterone acetate.
Clinical examination revealed diffuse abdominal pain and distension, and bimanual examination demonstrated a large pelvic mass. The uterus had decreased mobility and was painful with movement. Transvaginal ultrasound revealed a hypoplastic uterus and 2 pelvic masses. One of the masses, about 10 cm in diameter and homogenous, was located in the right adnexa. The other, about 15 cm in diameter, was located in the left adnexa and consisted of both solid and cystic areas. Abdominal ultrasound revealed copious ascites. Computed tomography confirmed the presence of 2 solid lesions. The first was located adjacent to the right posterior uterus, measuring 76 × 85 × 91 mm. A thin septum was present, and the lesion demonstrated rough and irregular peripheral uptake of contrast. The second lesion was located anterior to the uterus and superior to the bladder, measuring 140 × 100 × 170 mm. This mass had a lobed outline and appeared to have a similar makeup to the first lesion, although the peripheral contrast uptake was more tenuous (Figure 1). Particularly striking was the large amount of free fluid throughout the abdominal cavity. No retroperitoneal or mesenteric lymph nodes of significant size were identified (Figure 2).
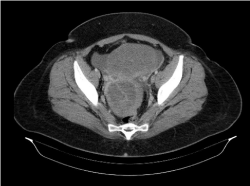
Figure 1: Computed tomography. A solid lesion, 76 × 85 × 91 mm, is present
posterior to the uterus on the right and contains a thin septum. The peripheral
contrast uptake is irregular. A second solid lesion, 140 × 100 × 170 mm, is
present anterior to the uterus and superior to the bladder and demonstrates
tenuous peripheral contrast uptake.
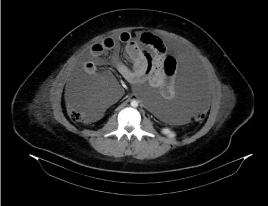
Figure 2: Computed tomography showing a large amount of free fluid. No
retroperitoneal or mesenteric lymph nodes of significant size are identified.
The vascular pedicle, limited contrast enhancement, and pain described by the patient suggested possible torsion. We therefore performed an emergency exploratory laparotomy. We found 3 liters of ascites and a 15-cm mass, twisted and necrotic, dependent from the left ovary. A similar tumor of 10 cm was found on the right ovary, resting in the pouch of Douglas. The uterus was hypoplastic. The peritoneum was thickened, with a 3-cm implant where it was in contact with the left adnexal mass. The omentum was retracted and hard, suggestive of tumor infiltration.
Despite the highly suspicious appearance, histology revealed acellular ascites. (Figure 3) and 4 show the macroscopic appearance of the neoplasms. In (Figure 4), it can be seen that the peripheral zone of the twisted mass was hemorrhagic, with a central soft, pink, somewhat gelatinous area. The lobed outer surface was fairly well defined. Histology of the ovarian sections showed a very lax, edematous cellular substrate with a few dense areas of spindle cells containing elongated nuclei and very few mitoses (Figure 5). In (Figure 6), the same spindle cells are seen to form a storiform pattern. The omentum had a less dense cellular pattern, with large hyalinized areas infiltrating both adipose and muscle tissue (Figure 7). A polymorphous inflammatory component and some granulomatous foci were also noted. Immunohistochemistry of the neoplasms was moderately positive for smooth muscle actin, of a cytoplasmic pattern with discrete perivacuolar reinforcement. It also highlighted the marked stromal luteinization, highly positive for inhibin.
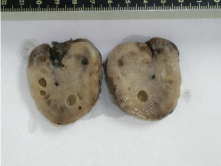
Figure 3: Macroscopic appearance of the right tumor.
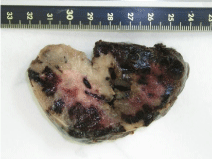
Figure 4: Macroscopic appearance of a fragment of the twisted tumor.
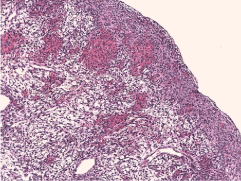
Figure 5: Ovarian histology demonstrating dense masses of spindle cells
with elongated nuclei and very few mitoses.
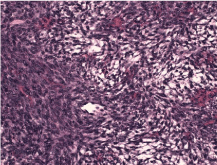
Figure 6: Ovarian section showing spindle cells with a storiform pattern.
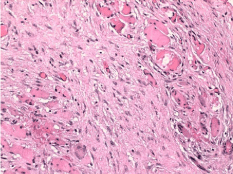
Figure 7: Omental section showing a less dense cellular population infiltrating
both adipose and muscle tissue.
The patient’s course was unfavorable, with peristalsis absent from the immediate postoperative period. She quickly developed recurrent ascites, intestinal obstruction, and renal failure. Repeat computed tomography showed intestine tightly enclosed in a ‘cocoon’ of thickened peritoneum (Figure 8). Because of the intense peritoneal involvement, we started treatment with high-dose dexamethasone. Unfortunately, this was unsuccessful. Five weeks after the onset of symptoms, the patient died of multiple organ failure. She had pulmonary sepsis and abdominal origin septic shock, complicated by acute respiratory failure, intestinal obstruction, and acute exacerbation of renal failure in the context of secondary sclerosing peritonitis.
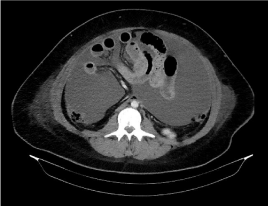
Figure 8: Computed tomography showing a significant bowel encapsulation
(“cocoon”).
Discussion
Thecomas are stromally derived tumors that account for approximately 1% of ovarian neoplasms. These tumors are usually classified into typical, or conventional, thecomas and luteinized thecomas. They usually occur in postmenopausal women, but up to 30% of luteinized thecomas appear in women under 30 years of age. Approximately half of luteinized thecomas secrete estrogen, with 40% non-functioning. In contrast, most conventional thecomas secrete estrogen. [3,20,21]
Often, thecomas are unilateral yellowish-white solid tumors, with cystic areas alternating with areas of calcification. They demonstrate strands of oval/spindle cells with vacuolated cytoplasm, rich in lipids. They also have oval or fusiform nuclei without atypia or mitosis, with a fibroblastic appearance. Luteinized thecomas have areas of single or grouped polygonal or oval cells, with abundant eosinophilic cytoplasm and prominent round central nuclei.
The coexistence of luteinized thecomas and sclerosing peritonitis is rare. Several case series have described this association as more common in premenopausal women. While thecomas themselves are usually unilateral, most that are associated with sclerosing peritonitis are bilateral lesions [3,11]. When coupled with sclerosing peritonitis, these tumors are composed of spindle cells with diffuse edema in the tumor stroma. They have microcystic changes, and very often ascites is present, as it was in our patient. [3,6]. In these cases, the luteinized areas are typically less defined, with smaller luteinized cells or only partially luteinized groups of cells. Cellular atypia is usually not seen, but mitoses (more than 40 per 10 high-power fields) can be observed without implying malignancy. However, when luteinized thecomas are not associated with sclerosing peritonitis, the cells show high mitotic indices with nuclear atypia and are considered malignant [3,22].
The relationship between luteinized thecoma and sclerosing peritonitis has not been fully clarified. There are different hypotheses that attempt to explain the stimulation that causes proliferation of submesothelial fibroblasts. Steroid stimulus is a logical possibility, either caused by decidualization of pregnancy or by the disseminated peritoneal leiomyomatosis seen with exogenous steroids. But this hypothesis does not explain why the stimulation is not observed in most luteinized thecomas or in other hormone-producing ovarian tumors (e.g., granulosa cell tumors). Another proliferative stimulus proposed for this condition is transforming growth factor beta (TGF-β). This cytokine has been shown to stimulate proliferation in peritoneal fibrosis, carcinoid tumors, or in conjunction with chemotherapy for ovarian cancer [3].
Sclerosing encapsulating peritonitis is a fibrotic reaction of the peritoneum, which affects the entire serosa. The most common site of presentation is the small intestine. This peritonitis causes acute intestinal obstruction, which may present with chronic widespread pain, a feeling of bloating, and nausea and vomiting. Ascites is frequently found on examination and, in some cases, pelvic tumors. Several hypotheses have been proposed to explain why this fibrous reaction. In young women without significant prior medical history, a proposed mechanism is retrograde peritonitis through the fallopian tubes [23]. Some authors have also implicated infectious agents: bacteria, [17] mycobacteria, [19] and in some isolated cases, fungal spores, specifically Claviceps fusiformis [24]. Other possible culprits are carcinoid syndrome, asbestosis, hemoperitoneum associated with blunt abdominal trauma, and Mediterranean fever [25]. Sometimes, the etiological agent is easy to identify. This is the case in patients undergoing continuous ambulatory peritoneal dialysis, beta-blocker treatment, and ventriculoperitoneal shunts for hydrocephalus. [19].
Histopathological examination should eliminate any condition that may be associated with sclerosing peritonitis, such as carcinoid syndrome, [27] peritoneal sarcoidosis [26, 27] asbestosis, [27] and other granulomatous conditions of the peritoneum. The typical microscopic image is submesothelial fibrosis or even loss of mesothelium, with or without nonspecific granulomas. Electron microscopy suggests that the fibroblasts and other cells present may be reactive submesothelial mesenchymal cells [27]. Often, the results of histopathology reveal inflammation with abundant fibrosis, hyalinization, and mild lymphocytic infiltration [14,16,17,20,27,28].
Surgery is the most commonly accepted treatment for luteinized thecomas when they involve sclerosing peritonitis and their complications. Other treatments, such as high-doses corticosteroids or antiestrogen drugs, have not been standardized because of the low incidence of the condition, although there are case reports describing favorable results [23,24]. Given the intense peritoneal fibrous reaction present in our patient, we decided to try high-dose dexamethasone. Unfortunately, this treatment was ineffective, and the rapid evolution of her condition did not allow for treatment with antiestrogens.
Surgical management is very difficult, because massive adhesions are usually present that affect the entire small intestine, with areas of large distension that ultimately cause acute intestinal obstruction. Usually, the initial treatment involves bilateral oophorectomy. The initial impression is often malignancy, due to the presence of ascites and the appearance of the peritoneum and omentum. However, the histology and subsequent progression to intestinal obstruction suggest sclerosing peritonitis. After the initial surgery, repeat treatment is often necessary for adhesive complications. The operative morbidity and mortality is estimated to be as high as 50% [29]. Depending on the etiology, this can vary from 6% in young patients with idiopathic presentation to 28% in adults, and up to 80% in patients undergoing peritoneal dialysis [14].
Given the infrequent association between sclerosing peritonitis and thecomas, the diagnosis can only be made if physicians are aware of this possibility. Clinicians should suspect this entity in patients with bilateral ovarian tumors who have large amounts of ascites and severe peritoneal involvement, but in whom the peritoneal histology reveals no malignancy.
References
- Bjorkholm E, Silfversward C. Theca-cell tumors. Clinical features and prognosis. Acta Radiol. 1980; 19: 241-244.
- Clement PB, Young RH, Hanna W, and Scully RE. Sclerosing peritonitis associated with luteinized thecomas of the ovary. A clinicopathological analysis of six cases. Am J SurgPathol. 1994; 18: 1–13.
- Werness BA. Luteinized thecoma with sclerosing peritonitis. Arch Pathol Lab Med. 1996; 120: 303–306
- Iwasa Y, Minamiguchi S, Konishi I, Onodera H, Zhou J, Yamabe H. Sclerosing peritonitis associated with luteinized thecoma of the ovary. Pathol Int. 1996; 46: 510–514.
- Spiegel GW, and Swiger FK. Luteinized thecoma with sclerosing peritonitis presenting as an acute abdomen.Gynecol Oncol. 1996; 61: 275–281.
- Bianco R, de Rosa G, Staibano S, Somma P, Bianco AR. Ovarian luteinized thecoma with sclerosing peritonitis in an adult woman treated with leuprolide and toremifene in complete remission at 5 years. Gynecol Oncol. 2005; 96: 846–849.
- Roth LM. and Sternberg WH. Partly luteinized theca cell tumor of the ovary. Cancer. 1983; 51: 1697–1704.
- Dudzinski M, Cohen M, Ducatman B. Ovarian malignant luteinized thecoma an unusual tumor in an adolescent. Gynecol Oncol. 1989; 35: 104–109.
- Nishida T, Ushijima K, Watanabe J, Kage M, and Nagaoka S. Sclerosing peritonitis associated with luteinized thecoma of the ovary.Gynecol Oncol.1999; 73: 167–169.
- Staats PN, McCluggage WG, Clement PB,Young RH. Luteinized thecomas (thecomatosis) of the type typically associated with sclerosing peritonitis: a clinical, histopathologic, and immunohistochemical analysis of 27 cases. Am J SurgPathol. 2008; 32: 1273–1290.
- Zhang J, Young RH, Arseneau J, Scully RE. Ovarianstromaltumorscontainingluteinor Leydig Cells (luteinized thecomas and stromal Leydig celltumors)- a clinicopathological analysis of fifty cases. Int J GynecolPathol 1982;1:270-285.
- Roth LM. Recent advances in the pathology and classification of ovarian sex cord—stream tumors. Int J Gyneco Pathl. 2006; 25:199-214.
- Black W, Nelson D, Walker W. Multifocal subperitoneal sclerosis. Surgery 1968; 63:706-710.
- Cohen O, Abrahamson J, Ben-Ari J, Frajewicky V, Eldar S. Sclerosing encapsulating peritonitis. J Clin Gastroenterol. 1996; 22: 54-57.
- Jackson BT. Surgical treatment of sclerosing peritonitis caused by practolol. Br J Surg. 1977; 64: 255-257.
- Gandhi VC, Humayun HM, et al. Sclerotic thickening of the peritoneal membrane in maintenance peritoneal dialysis patients. Arch Intern Med. 1980; 140: 1201-1203.
- Leport J, Devars Du Mayne JF, Hay JM. Chylous ascites and encapsulating peritonitis: unusual complication of spontaneous bacterial peritonitis. Am J Gastroenterol. 1987; 82: 463-466.
- Greenlee HB, Stanley MM, Reinhardt GF. Small bowel obstruction (SBO) from compression and kinking of intestine by thickened peritoneum in cirrhotics with ascites treated with Leveen shunt. Gastroenterology. 1979; 76: 1282.
- Ngô Y, Messing B, Marteau P. Peritoneal sarcoidosis. An unrecognized cause of sclerosing peritonitis. Dig Dis Sci. 1992; 37:1776-17780.
- Prat J. Pathlology of the ovary. Philadelphia Ed. Saunders; 2004: 208-212.
- Kurman RJ, Blaunstein´s. Pathology of the female tract, 5th edn. New York: Springer Verlag. 2001:921-923.
- Del Peso G, Bajo A, Gil F, Aguilera A, Ros S, Costero O, et al. Clinical experience with luteinized thecoma of the ovary. Gynecol Oncol. 1999; 73: 167-172.
- Dehn TCB, Lucas MG, Wood RFM. Idiopathic sclerosing peritonitis. Postgrad Med. J 1985; 61: 841-842.
- Narayanan R, Bhargava BN, Kabra SG, Sangal BC. Idiopathic sclerosing encapsulating peritonitis. Lancet. 1989; 2: 127-129.
- Randall GL. Sclerosing peritonitis. Dig Dis Sci. 1989; 34: 1473-1476.
- Sayfan J, Yehuda GA. Peritoneal Encapsulation in childhood. Case report, embryologic analisis, and review of literature. Am J Surg. 1979; 138:725-727.
- Marinho A, Adelusi B. The abdominal Cocoon. Case Report. Br J ObsGyn. 1987; 87249-250.
- Laferla G, McColl KE, Crean GP. CSF Induced sclerosing peritonitis: a new entity? Br J Surg. 1986; 73: 7.
- Krestin GP, Kacl G, Hauser M, Keusch G. Imaging diagnosis of sclerosing peritonitis and relation of radiologic signs to the extent of the disease. Abdom Imaging. 1995; 20: 414-420.