
Research Article
Austin J Obstet Gynecol. 2016; 3(1): 1052.
Temporal Relationship between Endometriosis and Overactive: A Nationwide Population-Based Cohort Study in Taiwan
Yi-Chen Tsai1,2, Yen-Mei Hsu2,3, Hui-Mei Peng³, Wei-Pin Chang4*, Chi-Mu Chuang1,2*
¹Faculty of Medicine, School of Medicine, National Yang- Ming University, Taipei, Taiwan
²Department of Obstetrics and Gynecology, Taipei Veterans General Hospital, Taipei, Taiwan
³Department of Nursing, Taipei Veterans General Hospital, Taipei, Taiwan
4School of Health Care Administration, Taipei Medical University, Taipei , Taiwan
*Corresponding author: Wei-Pin Chang, School of Health Care Administration, Taipei Medical University, Taipei , Taiwan, Wuxing Street, Taipei, Taiwan
Chi-Mu Chuang, Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan, No155, Sec 2, Linong Street, Taipei, Taiwan
Received: January 18, 2016; Accepted: February 25, 2016; Published: February 29, 2016
Abstract
Purpose: Both Endometriosis and Over Active Bladder (OAB) are linked with chronic inflammation. We hypothesized that these two diseases may have a temporal relationship due to shared features of chronic inflammation.
Methods: A 1:5 matched case-control study using a National Health Insurance Research Databases (NHIRDs) in Taiwan was conducted to investigation whether endometriosis and overactive bladder is positively associated. Two cohorts were constructed (patients with endometriosis (case cohort, n = 5,421) and those without endometriosis (control cohort, n = 27,105) was compared with respect to development of overactive bladder.
Results: The Kaplan-Meier survival curves demonstrate significantly higher occurrence of OAB in the case cohort than in the control cohort (p = 0.001, logrank test). Moreover, the incidence density was also higher in the case cohort (2.58 per 1,000 patient-years) than in the control cohort (0.83 per 1,000 patientyears). At the end of one-year follow-up, the results show that the case cohort harbors the highest hazard ratio with 8.82-fold [95% Confidence Interval (CI): 4.10-18.99] higher than the control cohort. The hazard ratios decreased with longer period of follow-up, with 3.97 (95% CI: 2.66-5.93) at the end of thirdyear follow-up, and 2.82 (95% CI: 2.09-3.82) at the end of five-year follow-up, respectively.
Conclusions: In conclusion, our data reveal that endometriosis confers higher risk of developing OAB, and this temporal relationship is potentially linked through shared features of chronic inflammation. Chronic inflammation has been demonstrated to play a critical role in the manipulation of a diverse spectrum of disorders, from chronic inflammatory disease to cancer.
Keywords: Endometriosis; Chronic inflammation; Nerve growth factor; Reactive oxygen species; Overactive bladder
Abbreviations
LHID: Longitudinal Health Insurance Database LHID; OAB: Over Active Bladder; NHIRD: National Health Insurance Research Databases; NGF: Nerve Growth Factor; VEGF: Vascular Endothelial Growth Factor
Introduction
Endometriosis is basically an estrogen-dependent gynecological disease characterized by endometrial-like tissue growing ectopically outside the uterine cavity, typically in the ovaries, the recto-vaginal septum, and on the surface of peritoneum [1].
Accumulating evidence has suggested that estrogen may play a critical role in the pathogenesis of chronic inflammatory diseases because the menstruation, pregnancy, and menopausal status are important influencing factors [2,3]. In line with this, endometriosis in both animal model and in human share many structural and molecular features [4]. Further, endometriosis contain or evoke abnormal production in surrounding tissues of many pro-inflammatory substances, including cytokines such as IL-1, IL-6, IL- 8, and IL-10, Tumor Necrosis Factor (TNF), and growth factors, such as Vascular Endothelial Growth Factor (VEGF) and Nerve Growth Factor (NGF) [5-8].
Over Active Bladder disease (OAB) is defined by the Standardization Subcommittee of the International Continence Society (ICS) as urinary urgency, with or without urinary incontinence, usually with frequency and nocturia, with no proven infection or other obvious pathology [9]. Recent epidemiological studies have shown that the overall prevalence of OAB in women is 16.9% [10]. Till now, there is accumulating evidence that chronic inflammation plays a major role in the pathophysiology of OAB [11- 13].
From the above descriptions, it seems that endometriosis and OAB share common etiological and pathophysiological mechanisms of chronic inflammation. As such, there is a putative association between these two disorders. Up to now, there is only one institutionbased study addressing this issue in the literature [14].
In this work, we tested the hypothesis that endometriosis may increase the risk for developing OA. A population-based national health registry database in Taiwan was used to explore the relationship between endometriosis and risk for subsequent development of OAB.
Materials and Methods
Data sources
Dataset were sourced from the National Health Insurance program, which was established since March 1, 1995, by the Bureau of National Health Insurance in Taiwan. The National Health Research Institutes was commissioned to National Health Insurance Research Databases (NHIRDs) for research proposals. The identification codes of beneficiaries were scrambled by a computer [15].
In the current study, we used the Longitudinal Health Insurance Database (LHID), a sub-dataset of NHIRDs, which contains 1 million beneficiaries randomly selected from those enrolled in the nation-wide insurance program. This sub-dataset contained insurant information, outpatient and inpatient visits, and medical treatment records between January 1, 2001, and December 31, 2005.
Ethical committee in the index has approved the current work and informed consent was obtained from individuals participated in this work.
Patient selection and ascertainment of diagnosis
The design of the current work was a matched retrospective casecontrol study. We selected patients with the diagnosis of endometriosis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 617.X) from January 1, 2001, to December 31, 2005, forming the case cohort. For each participant in the case cohort, based on age and index year, was matched to five randomly selected beneficiaries without endometriosis to build the control cohort. In order to maximally reduce the risk “reverse causation” [16], subjects with diagnosis of overactive bladder before the diagnosis of endometriosis were excluded.
Further, selection criteria of endometriosis required that all case ICD-9 codes by assigned by an expert and each participant in the case cohort must be coded with diagnosis of endometriosis for at least two times in the same year in outpatient clinic record. For the selection criteria for overactive bladder [ICD-9-CM] code 596.5X) were assigned by a urologic specialist. We only selected overactive bladder cases in this study if they received equal to or greater than two times of overactive bladder diagnoses for ambulatory care. All study subjects were followed from the baseline date to the first event, which was arbitrarily defined as occurrence of overactive bladder up to the end of 2010.
Patients diagnosed with overactive bladder before or after the study period were excluded from both cohorts. Relevant comorbidities, including hypertension (ICD-9-CM 401.X-405.X), diabetes mellitus (ICD-9-CM 250.X), and hyperlipidemia (ICD-9- CM 272.X) were also captured in the database retrieved.
Identification of level of urbanization
For the investigation of levels of urbanization, all 365 townships in Taiwan were stratified into seven levels according to the standards established by the Taiwanese NHRI based on a cluster analysis of the 2,000 Taiwan census data, with 1 to 7 scales that “1” referring to the most urbanized area and “7” referring to the least urbanized. The criteria on which these strata were determined included the population density (persons/km2), the number of physicians per 100,000 people, the percentage of people with a college education, the percentage of people over 65 years of age, and the percentage of agricultural workers. Because levels 4, 5, 6, and 7 contained very few endometriosis cases, they were combined into a single group, and were re-coded as level 4.
Statistical analysis
Mann-Whitney test was used to compare differences in geographic location, monthly income, and urbanization level of patients’ residences between the case and control cohorts. Event occurrence (defined as occurrence of overactive bladder) was analyzed using the time-to-event analysis method. The elapsed time period was defined as from diagnosis of endometriosis until the occurrence of event (overactive bladder), or the end of the study period (December 31, 2010), whichever came first.
Coding was set at 1, if event occurred, or 0, if event did not occur. No attempt of analysis of competing-risk analysis was made. After adjusting for urbanization level, monthly income, resident region, and comorbidities as potential confounders, we performed a Cox proportional-hazards analysis stratified by age at first diagnosis of endometriosis, to investigate the adjusted risk of developing overactive bladder during the 10-year follow-up period.
All data processing and statistical analyses were performed with SPSS 20 (SPSS, Chicago, IL, USA) and SAS 8.2 (SAS System for Windows, SAS Institute, Cary, NC, USA). The results of comparisons with a two-sided p value of < 0.05 were considered to represent statistically significant differences.
Ethical approval
Insurance reimbursement claims adopted in this study were from Taiwan’s NHIRDs, which is available for research purposes. This study was conducted in accordance with the Helsinki Declaration. This study was also evaluated and approved by the Institutional Review Board of Taipei Veterans General Hospital.
Results and Discussion
The case cohort contained 5,421 patients diagnosed with endometriosis, while 27,105 patients diagnosed without endometriosis were included in the control cohort. Comparison of baseline demographic characteristics between case cohort and control cohort is shown in Table 1. Hypertension (p < 0.001), hyperlipidemia (p < 0.001), diabetes mellitus (p < 0.001), depression (p < 0.001), and obesity (p < 0.001) were more prevalent in the case cohort than in the control cohort. The case cohort also harbored a greater tendency to earn a higher monthly income (p < 0.001), reside in the southern area of Taiwan, and reside in higher levels of urbanization communities (p = 0.024) compared to the control cohort.
Case cohorta
Control cohortb
P value
(n = 5,421)
(n = 27,105)
n
%
n
%
AGE(Years)c
20-29
1,164
21.5
5,820
21.5
30-39
1,928
35.6
9,640
35.6
40-49
2,329
43
11,645
43
0.99
Urbanization level
1 (most urbanized)
2,105
38.8
10,275
37.9
2
1,597
29.5
7,671
28.3
3
819
15.1
4,445
16.4
4 (least urbanized)
900
16.6
4,714
17.4
0.038
Monthly income
0
1,001
18.5
5,895
21.7
NT$ 1-15840
569
10.5
3,091
11.4
NT$ 15841-25000
2,322
42.8
11,338
41.8
≧ 25001
1,529
28.2
6,781
25
0.012
Geographic region
North
2,813
51.9
13,967
51.5
Central
1,106
20.4
6,336
23.4
South
1,305
24.1
5,687
21
Eastern
197
3.6
1,115
4.1
< 0.001
Hypertension
Yes
979
18.1
4,331
16
No
4,442
81.9
22,774
84
< 0.001
Hyperlipidemia
Yes
1,274
23.5
4,917
18.1
No
4,147
76.5
22,188
81.9
< 0.001
Diabetes mellitus
Yes
677
12.5
2,637
9.7
No
4,744
87.5
24,468
90.3
< 0.001
Depression
Yes
734
13.5
2,316
8.5
No
4,687
86.5
24,789
91.5
< 0.001
Obesityc
Yes
188
3.5
638
2.4
No
5233
96.5
26467
97.6
< 0.001
aCase cohort denotes patients diagnosed with endometriosis.
bControl cohort denotes patients diagnosed without endometriosis.
cAge distribution were perfectly matched between case cohort and control cohort with 1:5 algorithm.
dObesity was defined as body mass index greater than 30.0.
Table 1: Comparison of demographic characteristics between case cohort and control cohort from 2001 to 2005.
In total, at the end of five-year follow-up period, there were 182 participants who were newly diagnosed with OAB during the fiveyear follow-up, with 70 in the case cohort (1.3%) and 112 in the control cohort (0.4%). The Kaplan-Meier survival curves demonstrate significantly higher occurrence of event (i.e., occurrence of OAB) in the case cohort than in the control cohort (p = 0.001, log-rank test). Moreover, the incidence density was also higher in the case cohort (2.58 per 1,000 patient-years) than in the control cohort (0.83 per 1,000 patient-years) (Figure 1).
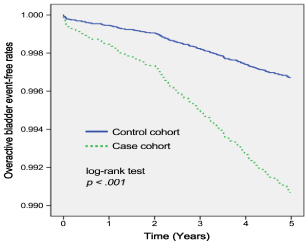
Figure 1: Event-free rate of development of overactive bladder among the
case cohort and the control cohort during the follow-up period.
Next, we evaluated whether there is a different risk for different follow-up period. At the end of one-year follow-up, the results show that the case cohort has the highest hazard ratio with 8.82-fold [95% Confidence Interval (CI): 4.10-18.99] higher than the control cohort. The hazard ratios decreased with longer period of follow-up, with 3.97 (95% CI: 2.66-5.93) at the end of third-year follow-up, and 2.82 (95% CI: 2.09-3.82) at the end of five-year follow-up, respectively (Table 2).
1-year follow-up period
3-year follow-up period
5-year follow-up period
Development of overactive bladder
Case cohort
Control cohort
P value
Case cohort
Control cohort
P value
Case cohort
Control cohort
P value
(n = 5,421)
(n = 27,105)
(n = 5,421)
(n = 27,105)
(n = 5,421)
(n = 27,105)
Yes (%)
20 (0.4)
10 (0.04)
45 (0.8)
53 (0.2)
70 (1.3)
112 (0.4)
No (%)
5,401 (99.6)
27,095 (99.96)
5,376 (99.2)
27,052 (99.8)
5,351 (98.7)
26,993 (99.6)
"Crude HR
(95% CI)"
10.03 (4.70-21.44)***
1
<0.001
4.26 (2.86-6.34)***
1
<0.001
3.15 (2.33-4.24)***
1
<0.001
"Adjusted HR
(95% CI)"
8.82 (4.10-18.99)***
1
<0.001
3.97 (2.66-5.93)***
1
<0.001
2.82 (2.09-3.82)***
1
<0.001
"Total sample number = 32526
aBoth crude and adjusted HRs were calculated by Cox proportional hazard regressions, and stratified by age and sex.
Adjustments were made for patients’ age, urbanization level, geographic region, monthly income, hypertension, hyperlipidemia, diabetes, depression, and obesity.
*Indicates p<0.05; ** Indicates p<0.01; *** Indicates p<0.001"
Table 2: Hazard Ratios (HRs) for development of overactive bladder among at 1-, 3-, and 5-year follow-up period from the index ambulatory visits or inpatient care from 2001 to 2005.
Lastly, we asked whether age at diagnosis of endometriosis conferred any moderator effect on the occurrence of OAB. Age at diagnosis of endometriosis was divided into three groups (20-29 years vs. 30-39 years vs. 40-49 years). Of note, the adjusted hazard ratio shows statistically higher risk of development of OAB in the case cohort than in the control cohort, irrespective of age group, with 3.83 (95% CI: 1.41-10.42) for age group 20-29 years, 2.17 (95% CI: 1.18- 3.98) for age group of 30-39 years, and 2.96 (95% CI: 2.04-4.31) for age group 40-49 years, respectively (Table 3).
Age Group (yrs)
Development of overactive bladder
20-29
30-39
40-49
Case cohort
Control cohort
Case cohort
Control cohort
Case cohort
Control cohort
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
Yes
8 (0.7)
8 (0.1)
16 (0.8)
33 (0.3)
46 (2.0)
71 (0.6)
Crude HR (95% CI)
5.01 (1.88-13.36)**
1
2.43 (1.34-4.42)**
1
3.28 (2.26-4.75)***
1
Adjusted HR (95%CI)
3.83 (1.41-10.42)**
1
2.17 (1.18-3.98)*
1
2.96 (2.04-4.31)***
1
Adjustments were made for patients’ age, urbanization level, geographic region, monthly income, hypertension, hyperlipidemia, diabetes, depression, and obesity.
* Indicates p<0.05; ** Indicates p<0.01; *** Indicates p<0.001.
Table 3: Hazard ratios for development of overactive bladder among case cohort and control cohort stratified by age group.
Conclusion
Based on a nation-wide population based health care database, the results of our work suggest that patients diagnosed with endometriosis harbor higher risk of developing OAB than those without endometriosis, irrespective of length of follow-up or age at diagnosis of endometriosis. This novel finding implicates that, for those who have poorly controlled OAB, should be explored for the possibility of co-existent endometriosis, which may strengthen the quality of care for OAB.
Our hypothesis that a positive association between endometriosis and endometriosis is linked through shared features of chronic inflammation can be corroborated with the following evidence. First, elevation of reactive oxygen species (ROS) has been reported in both endometriosis and OAB [17,18]. ROS are a family of proinflammatory mediators that regulate proliferation of endometriotic cells, making these cells shares some common features with cancer, such as tendency to invasion, unrestrained cell growth, tumor neoangiogenesis, and distant metastases [19]. Second, elevation of Nerve Growth Factor (NGF) has been reported in both endometriosis and OAB [20,21]. NGF has been identified as a critical molecule in chronic inflammation with levels increased in various systemic pro-inflammatory disorders [22-25], and the administration of NGF in laboratory animals has been shown to trigger accumulation of neutrophils at the site of injection [26]. Third, up-regulation of Vascular Endothelial Growth Factor (VEGF) also has been reported in both endometriosis and OAB [27,28]. VEGF plays a dominant role in induction of angiogenesis in endometriosis and mechanical overload-induced signaling cascade of OAB [29].
Chronic inflammation plays a critical role in orchestrating host defenses to microbial infection and mediates tissue repair and regeneration, which may occur due to infectious or non-infectious tissue damage [30]. Signals from chronic inflammation recruit a cascade of tissue responses such as recruitment of leukocytes, cellular proliferation, remodeling of extracellular matrix, and tumor angiogenesis. While diseases such as systemic lupus erythematosus, rheumatoid arthritis, cirrhosis, atherosclerosis, or interstitial lung disease may manifest in very different ways, the same fundamental mechanisms and mediators drive the disease process [31,32]. Further, epidemiological studies have revealed that chronic inflammation predisposes to different forms of cancer. Two pathways have been elucidated, with the intrinsic pathway involves genetic events causing neoplastic cells initiate the expression of pro-inflammatory programs that guide the formation of an inflammatory infrastructure, while the extrinsic pathway involves inflammation-facilitated tumorigenesis, including infections (e.g. Helicobacter pylori for gastric cancer, and human papilloma virus for cervical cancer), autoimmune diseases (e.g. inflammatory bowel disease for colon cancer) and inflammatory conditions of uncertain origin (e.g. prostatitis for prostate cancer) [33]. Collectively, our data and published literature show that chronic inflammation not only drives a positive link between endometriosis and OAB but also triggers a diverse spectrum of disease.
The merits of the current study lie in its analysis of a nationwide population based database, and thus the internal validity is enhanced. However, several limitations still encompassed. First, study participants were recruited only through ICD code, and as such, potential bias including patient selection and diagnosis criteria might be present. Second, the NHIRDs data set does not capture information regarding parity, hormonal use, and age at menopause, all of which may be potential risk factors of OAB. These unmeasured variables may introduce a confounding bias if it is associated with the studied exposure and disease simultaneously [34]. Third, the evidence generated from the current work is essentially a retrospective cohort study, which generally harboring lower quality than that from randomized trials. To this end, further prospective cohort study with adequate sample size is needed to verify the temporal association between endometriosis and OAB.
In conclusion, our data reveal that endometriosis confers higher risk of developing OAB, and this temporal relationship is potentially linked through shared features of chronic inflammation. Chronic inflammation has been demonstrated to play a critical role in the manipulation of a diverse spectrum of disorders, including chronic inflammatory diseases and cancer. Our novel finding may help improve the understanding of pathophysiology, diagnostic accuracy, and treatment efficacy of overactive bladder in the future.
References
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010; 362: 2389-2398.
- Marriott LK, Hauss-Wegrzyniak B, Benton RS, Vraniak PD, Wenk GL. Long-term estrogen therapy worsens the behavioral and neuropathological consequences of chronic brain inflammation. Behav Neurosci. 2002; 116: 902-911.
- Steffan RJ, Matelan E, Ashwell MA, Moore WJ, Solvibile WR, Trybulski E. Control of chronic inflammation with pathway selective estrogen receptor ligands. Curr Top Med Chem. 2006; 6: 103-111.
- Sharpe-Timms KL. Endometrial anomalies in women with endometriosis. Ann N Y Acad Sci. 2001; 943: 131-147.
- Anaf V, Simon P, El Nakadi I, Fayt I, Simonart T, Buxant F, et al. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002; 17: 1895-1900.
- Bourlev V, Volkov N, Pavlovitch S, Lets N, Larsson A, Olovsson M. The relationship between microvessel density, proliferative activity and expression of vascular endothelial growth factor-A and its receptors in eutopic endometrium and endometriotic lesions. Reproduction. 2006; 132: 501-509.
- Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002; 123: 217-226.
- Kyama CM, Mihalyi A, Simsa P, Falconer H, Fulop V, Mwenda JM. Role of cytokines in the endometrial-peritoneal cross-talk and development of endometriosis. Front Biosci (Elite Ed). 2009; 1: 444-454.
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003; 61: 37-49.
- Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R . Prevalence and burden of overactive bladder in the United States. World J Urol. 2003; 20: 327-336.
- Dupont MC, Spitsbergen JM, Kim KB, Tuttle JB, Steers WD. Histological and neurotrophic changes triggered by varying models of bladder inflammation. J Urol. 2001; 166: 1111-1118.
- Tanner R, Chambers P, Khadra MH, Gillespie JI. The production of nerve growth factor by human bladder smooth muscle cells in vivo and in vitro. BJU Int. 2000; 85: 1115-1119.
- Galvin DJ, Watson RW, Gillespie JI, Brady H, Fitzpatrick JM. Mechanical stretch regulates cell survival in human bladder smooth muscle cells in vitro. Am J Physiol Renal Physiol. 2002; 283: F1192-1199.
- But I, PakiÅ M, RakiÄ S. Overactive bladder symptoms and uterine adenomyosis--is there any connection? Eur J Obstet Gynecol Reprod Biol. 2011; 156: 109-112.
- National Health Insurance Research Database. 1995.
- Flanders WD, Augestad LB. Adjusting for reverse causality in the relationship between obesity and mortality. Int J Obes (Lond). 2008; 32: 42-46.
- Homan T, Tsuzuki T, Dogishi K, Shirakawa H, Oyama T, Nakagawa T, et al. Novel mouse model of chronic inflammatory and overactive bladder by a single intravesical injection of hydrogen peroxide. J Pharmacol Sci. 2013; 121: 327-337.
- Shanti A, Santanam N, Morales AJ, Parthasarathy S, Murphy AA. Autoantibodies to markers of oxidative stress are elevated in women with endometriosis. Fertil Steril. 1999; 71: 1115-1118.
- Swiersz LM. Role of endometriosis in cancer and tumor development. Ann N Y Acad Sci. 2002; 955: 281-292.
- Anaf V, Simon P, E Nakadi I, Fayt I, Simonart T, Buxant F. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002; 17: 1895-1900.
- Liu HT, Chancellor MB, Kuo HC. Urinary nerve growth factor levels are elevated in patients with detrusor overactivity and decreased in responders to detrusor botulinum toxin-A injection. Eur Urol. 2009; 56: 700-706.
- Foster PA, Costa SK, Poston R, Hoult JR, Brain SD. Endothelial cells play an essential role in the thermal hyperalgesia induced by nerve growth factor. FASEB J. 2003; 17: 1703-1705.
- Raychaudhuri SP, Jiang WY, Smoller BR, Farber EM. Nerve growth factor and its receptor system in psoriasis. Br J Dermatol. 2000; 143: 198-200.
- Barada KA, Mourad FH, Sawah SI, Khoury C, Safieh-Garabedian B, Nassar CF. Up-regulation of nerve growth factor and interleukin-10 in inflamed and non-inflamed intestinal segments in rats with experimental colitis. Cytokine. 2007; 37: 236-245.
- Raychaudhuri SK, Raychaudhuri SP. Functional significance of nerve growth factor and its receptor (TrkA) in inflammatory arthritis. Arthritis Res Ther. 2010; 12: 404.
- Bennett G, al-Rashed S, Hoult JR, Brain SD. Nerve growth factor induced hyperalgesia in the rat hind paw is dependent on circulating neutrophils. Pain. 1998; 77: 315-322.
- Takehara M, Ueda M, Yamashita Y, Terai Y, Hung YC, Ueki M. Vascular endothelial growth factor A and C gene expression in endometriosis. Hum Pathol. 2004; 35: 1369-1375.
- Christiaansen CE, Sun Y, Hsu YC, Chai TC. Alterations in expression of HIF-1alpha, HIF-2alpha, and VEGF by idiopathic overactive bladder urothelial cells during stretch suggest role for hypoxia. Urology. 2011; 77: 1266.
- Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005; 308: 1587-1589.
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001; 357: 539-545.
- Yang R, Amir J, Liu H, Chaqour B. Mechanical strain activates a program of genes functionally involved in paracrine signaling of angiogenesis. Physiol Genomics. 2008; 36: 1-14.
- Pasceri V, Yeh ET. A tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation. 1999; 100: 2124-2126.
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009; 30: 1073-1081.
- Lee WC. Bounding the bias of unmeasured factors with confounding and effect-modifying potentials. Stat Med. 2011; 30: 1007-1017.