
Case Report
Austin J Obstet Gynecol. 2016; 3(1): 1054.
Uterine Sarcoma Morcellation, Impact on Survival
Cusidó Gimferrer M* and Blancafort González CC
Department of Obstetrics, Gynecology and Reproduction, Hospital Universitari Quirón-Dexeus, Sabino Arana, Barcelona, Spain
*Corresponding author: Cusidó Gimferrer M, Department of Obstetrics, Gynecology and Reproduction, Hospital Universitari Quirón-Dexeus, Sabino Arana, Barcelona, Spain
Received: January 18, 2016; Accepted: May 13, 2016; Published: May 17, 2016
Abstract
Lately, power morcellation during benign gynecologic surgery has come under scrutiny because of risk of abdominal dissemination of unsuspected malignant tissue. Although incidence of uterine sarcomas is low (0, 5 a 3, 3% /100.000 women every year) and the benefits of minimal invasive surgery versus laparotomy are widely accentuated, the morcellation of an undiagnosed cancer may be strongly adverse for the patient’s prognosis. The current study pretends to illustrate the case of a morcellated low grade endometrial stromal sarcoma with much decreased overall survival than appreciated currently and present evidence published up to the date.
Keywords: Morcellation; Prognosis; Recurrence; Uterine sarcoma; Surgery
Case Report
A 33-year old woman came to our institution asking for a second opinion after diagnosis and follow up of a subserosal myoma that had presented growth up to 10 cm. The patient complaint of dysmenorrheal, metrorrhagia and vesicle tenesmus and presented no medical nor surgical history of interest.
The bimanual exploration revealed a 10cm. uterine mass in the anterior uterine surface.
The patient was evaluated by the Gynecological Diagnosis Imaging Unit in our institution and she underwent an ultrasound exploration. An image compatible with a 99x56 mm subserosal myoma was discovered. It presented anechogenic areas suggestive of necrosis and diffuse calcifications with Score 2 vascularization (Figure 1).
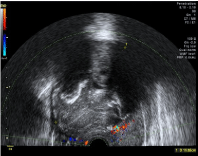
Figure 1: 99x56 mm subserosal myoma with anechogenic areas suggestive
of necrosis and diffuse calcifications. Vascularization Score 2.
Under indication of myoma growth, surgical removal by laparoscopy was offered and laparoscopic myomectomy was performed. The specimen was initially morcellated intraabdominally, but the calcified portion of the myoma had to be pulled out vaginally through a colostomy. The rest of the abdominal cavity was normal.
The immediate post-operative course was uneventful and the patient was discharged home on the 4th post-operative day.
Immunohistochemically, the tumor cells of the surgically resected specimens were finally diagnosed as Low-Grade Endometrial Stromal Sarcoma (LGESS), with more than half myometrial invasion. Progesterone receptors where negative.
According to the International Federation of Gynecology and Obstetrics (FIGO) [1], the uterine sarcoma was staged as an IC endometrial stromal sarcoma.
A toracoabdominal scanner was performed that was negative for malignancy.
A total hysterectomy with bilateral salpingo-oophorectomy where performed 27 days after the myomectomy. During the surgery macroscopic implant in hepatic angle was discovered and completely resected, confirmed as an implant from the low grade endometrial sarcoma by the histopathological study.
The multidisciplinary committee in oncology decided to submit the patient to 6 cycles of combined chemotherapy with Gemcitabine and Taxotere.
The control TC-scan, four months after the initial diagnose, showed diffuse abdominal relapse. The patient complaint of pelvic pain and the physical examination demonstrated a 6cm mass in vaginal cupula.
A second wide cytoreductive surgery had to be performed, resecting implants from diaphragmatic cupula, liver surface, peritoneum and omentum and Resecting two pelvic masses which affected a part of left colon and sigma. Once the patient was discharged, hormonotherapy with aromatasa inhibitors (letrozole) was administrated.
A second line of 6 cycles of chemotherapy with trabectedin and doxorubicin was administrated.
Only a month later the patient suffered a new relapse, this time affecting not only the abdominal cavity, but the abdominal wall (Figure 2) and the vagina.
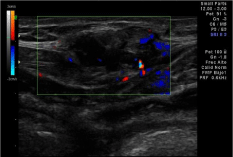
Figure 2: Abdominal wall implant.
Thereafter several chemotherapic and hormonal treatments and radiotherapy were tested including uromitexan and tronoxal, but the control of the disease wasn’t achieved. Finally, palliative treatment was introduced.
The patient died 24 months after the diagnose, having undergone 2 cytoreductive surgeries and 4 different lines of chemotherapy. The disease free survival time was less than 4 months.
Discussion
Uterine sarcoma is a poor prognosis tumor due to its aggressive nature. It represents less than a 1% of gynecologic malignancies with an incidence rate of 0, 5 a 3, 3% /100.000 women every year [2].
The overall survival rate hardly reaches the 50%, even when the diagnose is made in early stages, although there are differences according to histological types. This is the case of low grade endometrial stromal sarcoma and adenosarcomas, noted for their better prognosis [2].
The clinical presentation of uterine sarcomas is unspecific, nearly identical to benign uterine pathology. Metrorrhagia is the main symptom in 70-80% of the cases (50% in our series), closely followed by asymptomatic patients who present myoma growth without ultrasound alarm signs (40% in our series). Very few of the diagnosed sarcomas presented Sonographic features suggestive of malignancy, such as necrosis or increased vascularization (only an 18% in our series), leading to most of the cases being diagnosed due to the postsurgical histopathological study.
Among all histopathological patterns of uterine sarcoma, Endometrial Stromal Sarcoma (ESS) generally occurs in postmenopausal women who present with abnormal bleeding or pelvic pain [3]. According to the latest WHO Classification, ESS can be classified into low-grade ESS and undifferentiated endometrial sarcoma. LGESS are low malignant tumors with a low mitotic rate and tends to grow slowly, developing in the stroma. It is described that endometrial stromal sarcoma constitutes only 0.2% of all the uterine malignancies and usually has an indolent behavior, with 5 and 10-year survival rates of 90% and higher and a low frequency of late recurrence and distant metastasis [4,5]. In the case we reported the OS only reached 24months.
Local therapy consists of total hysterectomy. Given the hormonal responsiveness of most tumors, salpingo-oophorectomy is generally recommended even in the absence of randomized data. Few studies have evaluated the impact of the different surgical techniques, which seems to be directly affecting the outcome of these patients. The introduction of new laparoscopic technologies such as morcellation or the different surgical approaches have forced the scientific community to really evaluate their specific impact and even reconsider some techniques. The main lines of debate include:
- Surgical approach: Laparotomy vs. laparoscopy vs. vaginal access.
- Procedure: Myomectomy versus hysterectomy.
- Extraction methods: Morcellation versus integral extraction versus vaginal fragmentation.
- Adjuvant therapy: Clinical control, Chemotherapy, Radiotherapy and Hormonal therapy.
The extraction method is where lately the discussion has focused: several studies demonstrate that incidental morcellation of uterine sarcomas increases the risk of diffuse abdominal relapse. In that sense, a Cusido M et al. performed a case control study in a total of 37 sarcomas [6] which detected an increased Disease-Free Survival (DFS) in the laparotomy group compared with the laparoscopy group (median, 70.3 months vs. 10.4 months). According to the type of surgery median DFS was 6.3 months in the morcellation cases, 11.9 months in vaginal fragmentation cases and 149.9 months in nonmorcellated cases. No statistically significant differences were found in prognosis related to myomectomy versus hysterectomy.
The sub analysis of the DFS according to the histological subtype in the morcellation group showed significant difference only in the LGESS: DFS was 4.63 months in the morcellation group and 149.9 months in the nonmorcellation group.
Distant recurrence was seen in 60% in the nonmorcellation group, compared to the 12.5% of cases in the morcellation group. In contrast, disseminated abdominal recurrence was seen un 87% of cases in the morcellation group, compared with the 45, 4% of the non morcellation group.
Conclusions
A negative impact of morcellation in uterine sarcomas has been widely described, accepted as a independent factor that increases the risk of early relapse. The use of this technique should be reconsidered specially in myoma with atypical clinical presentation or symptomatology.
The risk of disseminating malignant cells to the abdominal cavity this technique seems to carry, has made some communities recommend avoiding morcellation during benign gynecologic surgeries, in spite of the low incidence of uterine sarcomas.
Morcellation may have a dramatic effect on prognosis especially in patients with low grade ESS.
Taking into account these evidences, patients must be at least informed about the possibility of a no identified sarcoma and the possible impact on prognosis resulting from its morcellation.
References
- FIGO committee on gynecologic Oncology. FIGO staging for uterine Sarcomas. Int J Gyn Obst. 2009; 104:179. And Corrigendum to “FIGO staging for uterine Sarcomas”. Int J GynObst. 2009; 106: 277
- Oncoguía SEGO: Sarcoma Uterino 2014. Guías de práctica clínica en cáncer ginecológico y mamario. Publicaciones SEGO, octubre. 2014.
- Amant F, Vergote I, Moerman P. The classification of a uterine sarcoma as 'high-grade endometrial stromal sarcoma' should be abandoned. Gynecol Oncol. 2004; 95: 412-413.
- Koss LG, spiro RH, brunschwig A. Endometrial stromal sarcoma. Surg Gynecol Obstet. 1965; 121: 531-537.
- Yamazaki H, Todo Y, Mitsube K, Hareyama H, Shimada C, Kato H. Long-term survival of patients with recurrent endometrial stromal sarcoma: a multicenter, observational study. J Gynecol Oncol. 2015; 26: 214-221.
- Cusidó M, Fargas F, Baulies S, Plana A, Rodríguez I, Tresserra F. Impact of Surgery on the Evolution of Uterine Sarcomas. J Minim Invasive Gynecol. 2015; 22: 1068-1074.