
Research Article
Austin J Obstet Gynecol. 2016; 3(2): 1055.
Surgical Experience of Giant Hepatoblastoma Involving the Inferior Vena Cava in Children: Report of 21 Cases of a Single Center
Ai-Jun Li, Wei-Ping Zhou, Xiao-Yu Yang, Yin Lei, Bin Wu, Long-Jiu Cui and Meng-Chao Wu*
Eastern Hepato Biliary Surgery Hospital, The Second Military Medical University, Shanghai, China
*Corresponding author: Meng-Chao Wu, Eastern Hepato Biliary Surgery Hospital, The Second Military Medical University, Shanghai, China
Received: March 03, 2016; Accepted: May 25, 2016; Published: May 27, 2016
Abstract
Background: Hepatic resection is the main treatment modality for giant Hepato Blastoma (HB), but operative treatment for giant Hepato Blastoma (HB) invading inferior vena cava is regarded as challenging because of its deep location in the liver and possibly worse prognosis especially in children. With the development of diagnostic technique, preoperative preparation, surgical technique, and postoperative management, some similar cases had a better prognosis after operation. The objective of our study is to report our experience in hepatectomy for giant Hepato Blastoma (HB) Invading Inferior Vena Cava (IVC) in children and to review the clinical features and survival rates retrospectively.
Methods: A retrospective review of 21 patients who underwent operation for HB in children between January 1998 and December 2012 was performed. Clinical features were analyzed such as age, gender, symptoms, laboratory investigations, postoperative pathological results, operation technique, and intra-operative and postoperative complications etc. Survival and recurrence outcomes were analysed using Cox hazard models and the Kaplan-Meier method
Results: They included 14 boys and 7 girls ranging in age from 4 months to 168 months with a mean of 46±42.44months, the median duration of survival after resection was 36 months. Only one boy had abdominal pain and distension, and 20 cases without symptoms were determinated by chance. Most of the blood tests are normal excluding those for a–fetoprotein 773.84±400.05 (normal vs. abnormal, 3 vs. 18), platelet ranging from 201×109/L to 910×109/L (mean, 773.82±180.06×109/L). Liver function was classified as Child-Pugh A for all patients. Four patients were positive for hepatitis B surface antigen. The tumor diameter ranged from 5.0cm to 20.0cm (mean, 12.38±3.99 cm). All the patients performed surgery successfully and two patients had received 3-7 cycles of routine chemotherapy before surgery. Right hemihepatectomy (n=11), left hemihepatectomy (n=2), left trisegmentectomy (n=1), and right trisegmentectomy (n=5) and middle hepatectomy (n=2) were performed. Partial resection of the involved IVC together with the tumor was performed in 5 cases, in which, the IVC wall defect was repaired with a traumatic suture in 4 cases, the IVC wall was end-to-end anatomized in one case. Push-away and dissection of the IVC was performed in 16 cases. No death occurred in this series of patients. The intraoperative hemodynamics was stable in all patients. Patients were observed in the intensive care unit for 3.4±0.3 days. Postoperative complications were in 3 children: in which, one baby had sepsis, fever, jaundice, intra-abdominal abscess, and sub-diaphragmatic abscess with pleural effusion. All the patients were confirmed by pathological examination (fetal type, 13 cases; embryonic type, 4 cases; undifferentiated type, 4 cases). Overall and disease-free 5-year survival rates of the 21 patients with solitary HCC invading inferior vena cava were 18% and 18%, respectively.
Conclusion Giant HB attached to the IVC wall in children can be completely removed with a safe operation and can achieve a good prognosis. Careful separation of the liver and IVC is a key point for minimizing the size of the resected IVC and to avoid unnecessary IVC resection.
Keywords: liver tumor; Hepatectomy; Hepato blastoma; Inferior vena cava
Background
Hepato Blastoma (HB) is the most common primary liver malignant tumor of young pediatric patients, accounting for about 79% of all liver malignant tumors [1]. The overall incidence of HB is 0.5∼1.5/1,000,000 (0.5∼1.5/million), with a male/female ratio of 1.2∼3.6:1 and the highest incidence in pediatric patients aged 6 months to 3 years after birth [2]. Only about 5% HB was diagnosed after 4 years of age [3]. It is considered that surgery for HB in children is the most effective treatment. But in most cases, tumors were not thought to be resettable because of its advanced stage or difficult liver resection. With technical advances in hepatectomy, the resection rate of liver tumors and postoperative outcome has been improved substantially. However, resection of liver tumors involving the Inferior Vena Cava (IVC) remains a technical challenge in clinical practice.
This article reports our experience with the surgical treatment of 21 HB patients who were confirmed by postoperative pathology between January 1998 and December 2012. The clinical features and survival rates of patient with HB invading inferior vena cava were also retrospectively reviewed and analyzed.
Clinical Data
Materials and methods
From January 1998 to December 2012, 21 children underwent surgery for HB at the Eastern Hepato biliary Surgery Hospital in Shanghai, China. All of the clinical features for these patients were retrospectively reviewed (Table 1). All pre-, peri- and post-operative factors were recorded including age, patients’ demographics, diagnosis, intra-operative blood loss, blood chemistry, morbidity (intra- and postoperative), hospital stay, histology, local recurrence, disease-free and overall survival. All the results are reported as median. Cumulative survival rates were generated using the Kaplan- Meier method. Statistical significance was defined as a P value less than 0.05.
Patient characteristics
Data
Age in months , mean (range)
46±42.44, 36(4-168)
Gender, female/male
7/14
HBsAg, positive, n (%)
4/21(19.1%)
Child-Pugh classification(Grade A)
21
AFP(µg/L),normal/abnormal
773.84±400.05, 3/18
PLT(109/L), mean(range)
773.82±180.06, 450(201-910)
Tumor size(cm),mean(range)
12.38±3.99, 10(5-20)
Type of operation , n
21
Right hemihepatectomy
11
Left hemihepatectomy
2
Left trisegmentectomy
1
Right trisegmentectomy
5
Middle hepatectomy
2
PTC Operative time, min, median (range)
17.95±6.98, 19(5-30)
Blood loss, ml, (range)
196.43±286.11(20-1200)
Postoperative complications , n
3
Sepsis
1
Fever
3
Jaundice
2
Intra-abdominal abscess
2
Sub-diaphragmatic abscess with pleural effusion
1
Type of pathological examination, n
21
Fetal type
13
Embryonic type
4
Undifferentiated type
4
HBsAg: Hepatitis B Antigen; PLT: Platelet; AFP: Alpha-Fetoprotein.
Table 1: Background characteristics of 21 patients with HB.
Surgical procedures
The first procedure was the assessment of resectability. After endotracheal induction of general anesthesia, right or bilateral subcostal incisions were made and extended superiorly in the midline to the xiphoid. The location and size of the tumor were explored. The liver was then mobilized by dividing the falciform, right and left triangular ligaments, and coronary ligaments. An intraoperative ultrasound was performed to assess the number and the size of the lesions as well as to assess the relationship of the tumor to major vascular structures. The hepatoduodenal ligament was surrounded with a vascular tape to expedite a subsequent Pringle’s maneuver. The supra-hepatic and infra-hepatic IVC was exposed, vascular occlusion bands were placed around the IVC with modified Total Hepatic Vascular Exclusion (THVE) if necessary. The parenchyma of the liver was divided to expose the IVC. The parenchymal transaction was performed with inflow occlusion (Pringle maneuver). The hepatic tissue was clamped and cut off by the clamp-crushing method. From easy to difficult and from superficial to deep, the exposed vessels along the course were cut and ligated with silk thread, until the outer sheath of ICV was reached. This resulted in a clear demarcation of the hepatic vein and the IVC.
(1) The outer sheath of IVC was separated gently from the tumor capsule. Because most giant HB had “capsule”, the separation was relatively easy in cases where there was no adhesion or invasion. The short hepatic vein was identified, cut and ligated. Push-away and dissection of the IVC was performed in 16 cases.
(2) If it was still impossible to separate the IVC wall below the outer sheath, it indicated that the tumor had directly invaded the IVC wall, followed by occlusion of the suprahepatic and infrahepatic IVC with vascular tape or Satinsky clamp were used to clamp the normal IVC wall near the tumor and remove the tumor together with the involved IVC wall (specimen and IVC removed en bloc). After removing the tumor, the IVC wall defect was repaired with a traumatic suture. In 4 patients, direct suturing was performed to repair the IVC, the vessel wall being involved for a mean length of 1.4cm (range 0.3 to 0.5m in diameter).
(3) The hepatic parenchyma was divided back to the IVC, if the involvement was relatively wide, clamping was done at both ends of the normal IVC wall near the involvement to completely isolate the involvement and the IVC, and then the tumor was removed together with the involved IVC wall. The IVC wall was then end-to-end anastomosis in one case.
In cases where end-to-end anastomosis was difficult, the detected IVC wall was replaced by the great saphenous vein, jugular vein, or G-Tex; there are not any patients in used graft.
The cut surface of the liver was mainly compressed by alignment sutures, suture fixation with a pedicle of the greater omentum, or complete exposure of the cut surface of the liver sprinkled with fibrin glue after complete homeostasis. Before closing the abdomen, an abdominal double cannula was routinely placed below the diaphragm and continuous drainage was applied post-operatively. The tube was removed when the amount of discharge decreased to < 10mL per day.
Results
The 21 cases of HB in this series included 14 boys and 7 girls aged from 4 months to 168 months with a mean of 46±42.44 months (Figure 1). The tumor diameter ranged from 5.0cm to 20.0cm (mean, 12.38±3.99 cm). Two patients received routine preoperative chemotherapy for 3∼7 cycles. The giant HB tumors were resected by as close as feasible to the lines of segmental anatomy. 3 patients with HB had undergone a previous attempt at resection in other hospitals that was aborted because of IVC involvement. Only one boy had abdominal pain and distension, and 20 cases without symptoms were determinated by chance. Most of the blood tests are normal excluding those for a –fetoprotein (normal vs. abnormal, 3 vs. 18), platelet ranging from 201x109/L to 910x109/L (mean, 773.82±180.06×109/L). Liver function was classified as Child-Pugh A for all patients. Four patients were positive for hepatitis B surface antigen. B-ultrasonography suggested tumors in the hepatic parenchyma with uneven distribution of enhanced or reduced echoes and clear margins in 16 cases, and unclear margins in 5 cases (Figure 2). CT scan showed smooth rounded tumors in 16 cases; multi-nodularly fused tumors in 5 cases; calcified tumors in 2 cases; well-capsulized tumors in 14 cases; incompletely capsulized tumors in 5 cases; and non-capsulized tumors in 2 cases. Plain CT scan showed that the intensity was lower than that of the normal hepatic parenchyma without enhancement in 5 cases, and with uneven enhancement in the remaining 16 cases, all of which involved the IVC with close attachment to the IVC. MRI showed that there was a clear margin between the tumor and the normal liver tissue with low signal on T1W1 and high signal on T2W1 in 15 cases, and with mixed high signal in the remaining 6 cases. The capsule was shown complete as a circular low-intensity signal on T2W1 in 5 cases; necrotic as an even higher signal on T2W1 in 3 cases; and hemorrhagic as a high signal in the low signal on T1W1 and mixed high signal on T2W1 in one case. Liver resection was successfully performed in all 21 cases, including right hemihepatectomy (n=11), left hemihepatectomy (n=2), left trisegmentectomy (n=1), and right trisegmentectomy (n=5) and middle hepatectomy (n=2). Partial resection of the involved IVC together with the tumor was performed in 5 cases, in which, the IVC wall defect was repaired with a traumatic suture in 4 cases, the IVC wall was end-to-end anastomosis in one case; Push-away and dissection of the IVC was performed in 16 cases. No death occurred in this series of patients. The time of intraoperative blood occlusion was 17.95±6.98 min. Hemodynamics was stable during operation in all cases. There was no perioperative death. Patients were observed in the intensive care unit for 3.4±0.3 days. Postoperative pathology showed the histologic fetal type in 13 cases, embryonic type in 4 cases, and mixed type in 4 cases.
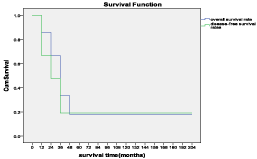
Figure 1: Cumulative overall survival rate and disease-free survival rates of
21 patients with HB.

Figure 2: 18 Months girl with HB and 15 years old after operation.
Postoperative complications
Three children had postoperative complications, included bile leakage (n=3), sepsis (n=1), fever (n=3), jaundice (n=2), intraabdominal abscess (n=1) sub-diaphragmatic abscess with pleural effusion (n=1). A 2-year old boy who presented with postoperative intra-abdominal bleeding performed re-operation and discharged 18 days after surgery. A 4-month old boy who suffered from sepsis, fever, jaundice, intra-abdominal abscess after operation was intraabdominal abscesses drained, and developed a bile leak that was successfully treated by percutaneous trans-hepatic biliary drainage and he remains alive at the end of follow-up. A 12-year old girl who also presented with postoperative obstructive jaundice was treated by percutaneous trans-hepatic biliary drainage after right trisegmentectomies, followed by reconstruction of using a Roux-en-Y hepaticojejunostomy 6 months after surgery (Figure 3).
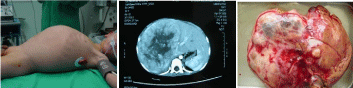
Figure 3: 4-yr-old baby with HB involving the inferior vena cava. The tumor
was removed together with the involved IVC wall (the specimen and IVC
removed en bloc), the IVC wall was then end-to-end anastomosed.
Follow-up
Follow-up observations lasting 12 months to 14 years showed tumor-free survival in 4 patients, including 2 patients who have survived more than 5 years. There was a patient who developed liver, brain and lung metastases 5 months after surgery and died 9 months after surgery. Overall and disease-free 1-year survival rates of the 21 patients are 67% and 48%, respectively. The 1-, 3- and 5-year survival was 67%, 18% and 18% respectively. Multivariate analysis revealed that only satellite lesions status was an independent prognostic factor of disease-specific survival.
Discussion
Hepatoblastoma staging
There are two different strategies regarding the treatment of paediatric HB. North American staging of hepatoblastoma in children is a postoperative system according to tumor resectability and the presence or absence of distance metastasis. The North American groups support immediate surgery for localised tumors [4,5], whereas Europe favors pre-operative chemotherapy in all cases, followed by surgery [6-8]. In 1990, a new different staging system, the PRE Treatment Extent of disease scoring system, was made by the International Society of Pediatric Oncology on Childhood Liver Tumor (SIOPEL) which was based on the imaging findings to determine possible treatment protocols before and during therapy [9,10].
Pathogenesis of hepatoblastoma
The pathogenesis of HB is mainly associated with abnormal proliferation and differentiation of hepatocytes during fetal development, and abnormal progressive proliferation of the immature fetal tissue reaming in the liver after births, forming immature tissue blocks. This pathologic process can occur in late fetal stages or as an onset in adulthood.
Possible factors contributing to HB include:
Heredity: (1).Chromosomal abnormality is one of the hereditary factors. About 5% HB cases are associated with some hereditary syndromes such as Familial Adenomatous Polyposis (FAP) and Beckwith-Wiedemann syndrome (BWS) [11-14]. (2).Abnormal transduction of the Wnt/b-catenin gene and its molecular targets play a role in the HB tumor genesis [13]. (3).Risk factors for HB development include BWS, familial adenomatous polyposis syndrome, Li Fraumeni, trisomy 21 or 18 and Fanconi anemia predispose to HB development [3]. (4).The loss of the heterogeneity in chromosome 11p in BWS associated with the production of insulin-like growth factor 2 may be a mechanism for the development of HB [15].
The occurrence of HB is increased in underweight newborns whose birth weight is below 1500g [16, 17].
The influence of pregnancy on the fetus: The occurrence of HB is also associated with the use of contraceptives, gonadotropins and alcohol drinking of pregnant women [18-20]. Occupations, closecontact environments, long-term exposure to petroleum or paint increase the risk of developing HB in their children [21]. A study also indicated that smoking of the parents increases the risk of developing HB in their children [22].
Diagnosis
Clinically, HB presents as a painless abdominal mass, although abdominal pain is noticed in some pediatric patients. HB children usually present with normal growth and development unless they are complicated with other hereditary syndromes such as BWS. Sexual precocity can be seen in children whose HB tumors secrete HCG.
Liver function is normal and there is no jaundice in most HB patients. AFP in normal infants reaches the level (<25ng/ml) of normal adults about 6 months after birth, while AFP is elevated significantly in more than 90% HB children, and AFP level is parallel with the course of HB. AFP level declines to the normal range after complete resection of the HB tumor, and elevates again when recurrence occurs. Anemia is present in about 70% HB patients. Thrombocythemia is present in about 50% HB patients, of whom 30% patients have a platelet count > 8000×109/L. Thrombopoietin (THPO) is the main factor causing thrombocythemia. In normal persons, THPO is mainly expressed in the kidneys, liver, spleen and bone marrow. The high expression of THPO and its receptors suggests that HB could cause thrombocythemia through abnormal expression of THPO. For this reason, special attention should be paid to platelet evaluation in infants.
Children with liver tumors should undergo B-ultrasound, CT or MRI examination. Calcification may be observed in a small proportion of HB tumors. It is necessary to perform chest CT scan, because the lung is the place where malignant tumor metastasis most frequently occurs.
Treatment
(1) Assessment on the residual liver: Surgeons should make a judgment on the possibility of complete resection of the tumor on the basis of imaging data. In cases with normal liver function, negative HBsAg and no cirrhosis, the extent of hepatectomy can be as large as 80% or 85%. Patients can often recover quickly with vitality, and the residual liver can compensate three weeks after surgery with a good prognosis. For this reason, the indications of surgery for liver tumors in children should be expanded.
(2) Perioperative management: Special instruments for children such as the gastric tube, urinary catheter, and phlegm suction tube and hepatic portal occlusion tube should be made available before surgery. Considering the traits of pediatric patients such as relatively weak vitality and quick change of the vital signs, monitoring should be intensified, including the amount and rate of fluid replacement and drug dosage. Intra- and postoperative fluid replacement and management of high temperature: As the temperature regulatory center of children is immature, special attention should be paid to the control of intraoperative blood loss. Postoperative pulmonary complications: Respiratory tract infection, atelectasis and inhalation pneumonia are more likely to occur in pediatric patients. Therefore, frequent dorsiflexion, burping, massage, phlegm suction, oxygen inhalation, warm keeping, and use of broad-spectrum antibiotics are needed after surgery.
(3) Characteristics of surgery for tumors involving the IVC: Complete tumor resection, effective control of hemorrhage and prevention of air embolism are the crux for the success of surgical treatment for tumors involving the IVC. The tumor is closely attached to the IVC and hepatic veins, especially for tumors in segment IV, VIII, and caudate lobe (IX), where the tumor is strangulated between the two hepatic veins and the IVC, which are often squeezed and distorted by the tumor, causing displacement of the anatomic structure. The conventional resection method is likely to cause tumor rupture, or damage the hepatic veins and IVC, resulting in uncontrollable hemorrhage and air embolism.
The abdominal wall of children is very thin and lacks the subcutaneous tissue. Positive surgery is suggested in cases where the tumor occupies the whole upper abdomen and adheres with the surrounding tissue; there is no ascites; the liver is sclerotic; and the tumor is on one side and the margin is clear. As most HB tumors have capsules, the relationship between the tumor and the IVC is mainly a neighbor relation, and therefore it is not very difficult to separate or dissect the tumor from the IVC wall. In some cases where the tumor invades the IVC, it is necessary to resect part of the IVC wall and repair it. Sometimes, the IVC is squeezed flat by the tumor and the wall becomes very thin. In such cases, special attention should be paid lest the IVC is resected by taking mistaken for the “tumor capsule”.
If the circumference of the IVC to be resected is <75%, cavoplasty can be performed. If the amount of IVC to be resected is >75%, complete resection and reconstruction is required [6]. In our case, the surgical management of partial resections of the IVC invading <30% circumference of the vena cava, reconstruction of the IVC is not required.
In addition, as the ligaments of children are relatively loose and flexible and contain little fat, they are relatively free and not easily fixed. For this reason, the IVC is easy to be pulled into an angle, which may damage the IVC wall, or even tempt to resect the whole segment of IVC completely by mistake. Special attention should be paid to protecting the porta hepatis and avoiding damaging the bile ducts when the tumor is large and grows across the hepatic portal. In one case of our series with a giant tumor in the right liver, we first isolated and ligated the right hepatic portal extrahepatically, and then resected the tumor. The patient developed obstructive jaundice after surgery, for which PTCD drainage was indwelled, followed by biliary intestinal anastomosis in six months. The patient has survived four years without recurrence. Postoperative bile leakage (50-100ml) occurred in three cases, which was managed by local drainage, and the patients were discharged without significant adverse events.
The pediatric liver is easy to regenerate after surgery as long as there is no cirrhosis and the residual liver is not distorted or compressed. There was a 4-month-old infant in our series that had a giant HB in the middle liver lobe. His resected liver accounted for about 80% of the whole liver. After anastomosis of the residual liver wound surface, the outlet was obstructed due to compression of the hepatic veins, causing continuous blood oozing from the wound surface. The suture was removed and the wound surface was compressed with gauze pads, which were pulled out gradually after surgery. Transient jaundice occurred after surgery in this patient, and subsided naturally two weeks after surgery. The postoperative biliary fistula was improved after local drainage. The residual liver proliferated remarkably after surgery.
Prognosis
The diagnosis of HB is mainly based on histology. In 1967, Ishak and Glunz [23] proposed two HB subtypes: (1) the epithelial type, which is further classified as the fetal, embryonic and anaplastic subtypes; and (2) the mixed type, in which the main structure of epithelia is mixed with some mesenchymal components, mature bone, cartilage, osteoid tissue, and sarcomatoid spindle cells. Based on the statistical data of our cases, the epithelial type accounts more than 90%, which is including the fetal subtype in 13 cases and the embryonic type in 4 cases. The remaining four cases were the mixed type. Survival rate is low due to giant size of tumors and vascular invasion.
Conclusions
In summary, considering the traits of pediatric patients, every effort should be made to resect the tumor as much as possible no matter how large it is. In cases where the tumor invades the IVC, it is not very difficult to dissect the tumor from the IVC, because most HB tumors have a capsule, so HB with IVC mostly is neighbor relations. Most hepatic malignancies attached to the IVC wall can be completely removed without IVC resection. Unlike as adults, pediatric IVC wall is relatively small, thin. Careful separation of the liver and IVC is a key point for minimizing the size of the resected IVC and to avoid unnecessary IVC resection. The platelet count is elevated in most HB children, the mechanism of which needs to be further investigated. After accurate selection on the basis of strict criteria and a good functional hepatic reserve, liver resection still plays a key role in the treatment HCC with involvement of the IVC.
Acknowledgment
Special thanks to Professor Michael G. Sarrh for their contribution to this article.References
- Perilongo G, Malogolowkin M, Feusner J. Hepatoblastoma clinical research: lessons learned and future challenges. Pediatr Blood Cancer. 2012; 59: 818-821.
- Tiao GM, Bobey N, Allen S, Nieves N, Alonso M, Bucuvalas J, et al. The current management of hepatoblastoma: a combination of chemotherapy, conventional resection, and liver transplantation. The Journal of pediatrics. 2005; 146: 204-211.
- Reynolds P, Urayama KY, Von Behren J, Feusner J. Birth characteristics and hepatoblastoma risk in young children. Cancer. 2004; 100: 1070-1076.
- Litten JB, Tomlinson GE. Liver tumors in children. Oncologist. 2008; 13: 812-820.
- Finegold MJ. Chemotherapy for suspected hepatoblastoma without efforts at surgical resection is a bad practice. Med Pediatr Oncol. 2002; 39: 484-486.
- Meyers RL, Czauderna P, Otte JB. Surgical treatment of hepatoblastoma. Pediatr Blood Cancer. 2012; 59: 800-808.
- Tsay PK, Lai JY, Yang CP, Hung IJ, Hsueh C, Tsai MH. Treatment outcomes for hepatoblastoma: experience of 35 cases at a single institution. J Formos Med Assoc. 2011; 110: 322-325.
- Perilongo G, Shafford E, Maibach R, Aronson D, Brugières L, Brock P, et al. Risk-adapted treatment for childhood hepatoblastoma: Final report of the second study of the International Society of Paediatric Oncology—SIOPEL 2. European Journal of Cancer. 2004; 40: 411-421.
- Czauderna P, Otte JB, Aronson DC, Gauthier F, Mackinlay G, Roebuck D. Guidelines for surgical treatment of hepatoblastoma in the modern era--recommendations from the Childhood Liver Tumour Strategy Group of the International Society of Paediatric Oncology (SIOPEL). Eur J Cancer. 2005; 41: 1031-1036.
- Aronson DC, Schnater JM, Staalman CR, Weverling GJ, Plaschkes J, Perilongo G, et al. Predictive value of the pretreatment extent of disease system in hepatoblastoma: results from the International Society of Pediatric Oncology Liver Tumor Study Group SIOPEL-1 study. Journal of clinical oncology. 2005; 23: 1245-1252.
- Clericuzio CL, Chen E, McNeil DE, O'Connor T, Zackai EH, Medne L. Serum alpha-fetoprotein screening for hepatoblastoma in children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia. J Pediatr. 2003; 143: 270-272.
- Hamada Y, Takada K, Fukunaga S, Hioki K. Hepatoblastoma associated with Beckwith-Wiedemann syndrome and hemihypertrophy. Pediatric surgery international. 2003; 19: 112-114.
- Armengol C, Cairo S, Fabre M, Buendia MA. Wnt signaling and hepatocarcinogenesis: the hepatoblastoma model. Int J Biochem Cell Biol. 2011; 43: 265-270.
- Feka P, Gauthier F, Wildhaber BE, Beckwith Wiedemann Syndrome with Hepatoblastoma and Infantile Hepatic Hemangioma: A Patient-Specific Management. Journal of Pediatric Surgery Case Reports. 2013.
- Malogolowkin MH, Katzenstein HM, Meyers RL, Krailo MD, Rowland JM, Haas J. Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children's Oncology Group. J Clin Oncol. 2011; 29: 3301-3306.
- Stocker JT. Hepatic tumors in children. Clin Liver Dis. 2001; 5: 259-281.
- Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system. IARC press Lyon. 2000; 184-189.
- Tanimura M, Matsui I, Abe J, Ikeda H, Kobayashi N, Ohira M. Increased risk of hepatoblastoma among immature children with a lower birth weight. Cancer Res. 1998; 58: 3032-3035.
- Meyer P, LiVolsi V, Cornog JL. Letter: Hepatoblastoma associated with an oral contraceptive. Lancet. 1974; 2: 1387.
- Braunstein GD, Bridson WE, Andrew Glass, Hull EW, and Robert Mcintire K. In vivo and in vitro production of human chorionic gonadotropin and alpha-fetoprotein by a virilizing hepatoblastoma. Journal of Clinical Endocrinology & Metabolism. 1972; 35: 857-862.
- O'Leary LM, Hicks AM, Peters JM, London S. Parental occupational exposures and risk of childhood cancer: a review. Am J Ind Med. 1991; 20: 17-35.
- Sorahan T, Lancashire RJ. Parental cigarette smoking and childhood risks of hepatoblastoma: OSCC data. Br J Cancer. 2004; 90: 1016-1018.
- Ishak KG, Glunz PR. Hepatoblastoma and hepatocarcinoma in infancy and childhood. Report of 47 cases. Cancer. 1967; 20: 396-422.
- Tannuri U, Santos MM, Tannuri AC, Gibelli NE, Moreira A, Carnevale FC, et al. Which is the best technique for hepatic venous reconstruction in pediatric living-donor liver transplantation? Experience from a single center. J of Pediatric Surgery. 2011; 1379–1384.