
Review Article
Austin J Obstet Gynecol. 2016; 3(2): 1058.
HCG the Master Molecule
Cole LA*
USA hCG Reference Service, 34 Broad moor Way, P.O. Box 950, Angel Fire NM 87710, USA
*Corresponding author: Laurence Anthony Cole, USA HCG Reference Service, 34 Broad moor Way, P.O. Box 950, Angel Fire NM 87710, USA
Received: June 28, 2016; Accepted: July 20, 2016; Published: July 25, 2016
Abstract
HCG is here considered the master molecule. The master molecule because HCG controlled human and the human brain evolution. The master molecule because hCG seemingly controls human pregnancy and whether pregnancy will have a term outcome. The master molecule because hCG drives human cancer, and partly whether a cancer is aggressive an kills a person.
Keywords: HCG; Hypertensive disease; Endocrine receptor; Hyperglycosylated hCG; zygote
Introduction
HCG is called here the master molecule because it controls so many critical events in human life, it even organized the evolution of humans from early primates. Hyperglycosylated hCG a variant of hCG is the guard gate deciding which pregnancy can go term, which pregnancy with lead to hypertensive disease, that drives human cancer and in many ways decides which cancer is terminal.
It is important to understand that hCG is not just one molecule but rather is a group of six independent molecules each sharing the same α-subunit and ß-subunit amino acid sequences or the same genes. A major part of this multi-molecule biochemical oddity is that both subunits of the hCG group of molecules evolved from transforming growth factor-ß2 (TGF-ß2), containing cysteine knot four peptide, and three disulfide bridge structures from TGF-ß2 [1-5].
The hCG group also evolved directly from the pituitary hormone Luteinizing Hormone (LH), making hCG both a hormone and a TGF-ß2 antagonist (Table 1) [3-5]. The six primary hCG molecules are the placental hormone hCG (hCG-1) made by syncytiotrophoblast cells which acts on a placental, uterine and corpus luteal LH/ hCG endocrine receptor (Table 1). The placental autocrine hyper glycosylated hCG (hCG-2) made by cytotrophoblast cells which acts on a cytotrophoblast TGF-ß2 receptor (Table 1). The pituitary hormone sulfated hCG (hCG-3) made by gonadotrope cells which acts on an ovarian theca and granulosa LH/hCG endocrine receptor (Table 1).
Parameter
Hormone hCG
Hyperglycosylated hCG
Sulfated
hCGHyperglycosylated cancer hCG
Hyperglycosylated cancer free ß-subunit
Mutated Fetal
hCGNew name
hCG-1
hCG-2
hCG-3
hCG-4
hCG-4ß
hCG-5
Source cells
Syncytiotrophoblastcells
Cytotrophoblast cells
Pituitary
Gonadotrope cellsTrophoblastic malignancy cells
Non-trophoblastic malignancy cells
Fetal kidney & liver cells
Mode of action
Endocrine
Autocrine
Endocrine
Autocrine
Autocrine
Non-Endocrine
Total MW
36,525
39,149
35,943
40,461
26,271
Variable
Site of action
LH/hCG receptor
TGFß antagonism
LH/hCG receptor
TGFß antagonism
TGFß antagonism
Fetal organ
Amino acids α-subunit
92
92
92
92
-
Variable
Amino acids ß-subunit
145
145
145
145
145
Variable
Peptide MW
25,813
25,813
25,813
25,813
15,543
Variable
O-linked sugar units
4
4
4
4
4
Not determined
Type O-linked sugars sugars
Type 1
Type 2
Type 1 + SO4
Type 2
Type 2
Not determined
N-linked sugar units
4
4
4
4
2
Not determined
Type N-linked sugars
Biantennary
Biantennary
Biantennary + SO
4Triantennary ß
Triantennary
Not determined
Sugar side chain MW
10,712
13,336
10,130
14,648
10,728
Not determined
Percentage sugars
29%
34%
28%
36%
41%
Not determined
Isoelectric point (pI)
3.5
4.5
3.2
3.1
Not determined
Not determined
Clearance ½-life
36 hours
36 hours
18 hours
Not determined
Not determined
Not determined
Table 1: The 6 primary variants of CG. MW is molecular weight, SO4 is sulfated sugars.
Then there is cancer hyper glycosylated hCG (hCG-4), an autocrine produced by all human trophoblastic cancers that binds a TGF-ß2 receptor on the cancer cells (Table 1). Then there are all other cancers, non-trophoblastic cancers. They lack a critical component for hCG α-subunit -ß-subunit combination and produce a cancer hyperglycosylated hCG free ß-subunit (hCG-4ß). This cancer hyper glycosylated hCG free ß-subunit also binds the TGF-ß2 receptor on cancer cells. Finally there is a fetal hCG produced during pregnancy (hCG-5) [6-8]. This drives fetal organ growth and development during pregnancy. While it has been demonstrated that this molecules is produced by fetal liver and kidney, there is insufficient data to determine the structure of this primary form of hCG (Table 1) [6-8].
These six different primary forms of hCG vary in molecular weight from 26,271 (hCG-4ß) to 40,461 (hCG-4), and in proportion sugar from 28% (CG-3) to 41% (hCG-4ß). HCG is considered the most glycosylated glycoprotein and the most acidic glycoprotein in the human genome.
Intriguingly, the six hCG forms seem to fall into two groups, placental hormone hCG (hCG-1), pituitary sulfated hCG (hCG- 3), and seemingly fetal hCG (hCG-5) are all hormones and act on an LH/hCG joint receptor. Secondly is hyper glycosylated hCG (hCG-2), cancer hyper glycosylated hCG (hCG-4) and cancer hyper glycosylated hCG free ß-subunit (hCG-4ß) which are all antagonists binding a TGF-ß2 receptor. The structural difference between placental hormone hCG (hCG-1) and hyper glycosylated hCG (hCG- 2) is very small. They have the exact same α-subunit and ß-subunit amino acid sequences, and only differ by the O-linked sugar side chains. The placental hormone hCG (hCG-1) has four of three or four sugar residue type one sugar side chains. Hyperglycosylated hCG (hCG-2) has four of five or six sugar residue type two sugar side chains. How, I ask, can just two sugars on 4 sugar side chains change a molecule from being a hormone and acting on a hormone receptor, to being an autocrine and acting on an autocrine receptor?
The three dimensional structure of generic hCG has been carefully investigated [9,10]. Unfortunately, to make hCG crystals all eight sugar side chains on hCG had to be removed, as well as the hCG ß-subunit C-terminal peptide. Crystals were made of 50% of the hCG root structure. I call it the root structure in that it cannot be attributed specifically to any one of the six hCG primaries. Unfortunately this tells you little about the differences between the hormone hCG (hCG- 1) and hyper glycosylated hCG (hCG-2). Stephen Butler PhD took this 50% structure and used sophisticated thermodynamic computer modelling software using Raptor (Bioinformatics Solutions Inc.) computer modelling to complete the 3D models of the hormone hCG (hCG-1) and hyper glycosylated hCG (hCG-2) (Figure 1). The three dimensional models of these complete molecules are shown in Figure 2 (Butler SA, structures not published).
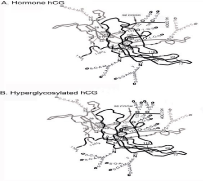
Figure 1: The Stephen Butler models of structure of hormone hCG and
hyperglycosylated hCG.
As illustrated, the principal difference is in the ß-subunit C-terminal peptides, shown in black on the figure from ß-subunit residue (Figure 2, the hormone hCG) 110 to 145. As shown the hormone CG ß-subunit C-terminal, starting at residue 110 first folds over α-subunit loop 40-57, and then over ß-subunit loop 92- 102 coming to ß-subunit sugar attachment site at residue 127. The C-terminal peptide then folds downwards into the center of the molecule folding over ß-subunit loop 40-55, it then fold back on itself and terminates at residue 145.
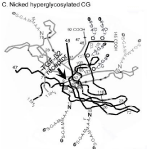
Figure 2: Nicked hyperglycosylated hCG and the TGF-ß2 receptor.
The ß-subunit C-terminal peptide on hyper glycosylated hCG (Figure 2, hyper glycosylated hCG) is a little simpler. Starting again at residue 110 it again first folds over α-subunit loop 40-57, and then over ß-subunit loops 92-102 coming to ß-subunit sugar attachment site at residue 127. The big difference is that does not fold over ß-subunit loop 40-55. This is what makes the big difference, or separates CG (CG-1) and hyper glycosylated hCG. It then terminates at residue 145.
While hyper glycosylated hCG is rapidly nicked by a leukocyte elastase at residue ß47-48 immediately upon secretion [11], the hormone hCG is blocked from nicking by the C-terminal peptide as it fold over ß-subunit loop ß40-55. The end result is that hyper glycosylated hCG is rapidly nicked, and the hormone hCG is very slowly nicked. As shown in Figure 3, nicking of hyper glycosylated hCG exposes the TGF-ß2 cystine knot on hyperglycosylated hCG, a binding site for antagonizing the TGF-ß2 receptor. Nicking also destroys all hCG/LH hormone receptor activity of hyper glycosylated hCG (hCG-2). The end result is that the hormone hCG acts only on the hCG/LH hormone receptor and hyper glycosylated hCG acts only by antagonizing a TGF-ß2 receptor.
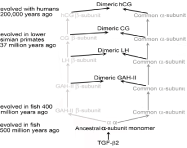
Figure 3: The evolution of hCG and LH from TGF-ß2.
Introducing hCG is not simple. There are two groups of hCG primary molecules. The molecules that are hormones and act on an LH/hCG hormone receptor, and the molecules that TGF-ß2 receptor antagonist. There are six separate hCG primary molecules total. They all use the same α- and ß-subunit amino acid sequence or are seemingly coded for by the same α- and ß-subunit genes. A small structural change in the cancer and pregnancy hyper glycosylated hCG molecules incidentally permits and does not permit nicking of molecules and opening up TGF-ß2 binding sites and closing of hormone binding sites.
HCG Controlled Human Evolution
Amazingly, the evolution of hCG instigated and completely controlled the evolution of humans and the human brain [12-15]. The evolution of hCG started out with the evolution of the primate LH. As illustrated in Figure 3, LH subunits were direct descendants of TGF-ß2 monomer. TGF-ß2 evolved in fish about 500 million years ago to ancestral α-subunit monomer. This then evolved into the α- and ß-subunits of gonadotropin ancestral hormone -I and -II (GAH-I and GAH-II) and Thyroid Stimulating Hormone (TSH) in fish. Over hundreds of millions years the α- and ß-subunits mutated and evolved separately becoming separate molecules. GAH-I was the pituitary ancestral hormone to Follicle Stimulating Hormone (FSH), and GAH-II was the pituitary ancestral hormone to LH [1,2].
All of these evolutionary molecules and subunits each carried a key structural remnant of TGF-ß2 called the cysteine knot structure (Figure 4) [1,2]. This was a four peptide three disulfide bridge structural element, found in LH, FSH, TSH and hCG subunits (the evolutionary descendants of LH) today. These four hormones that evolved together from TGF-ß2 are called the glycoprotein hormones.
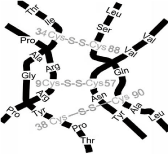
Figure 4: The cysteine knot structure, structure of cysteine knot on hCG
ß-subunit.
More recently, approximately 37 million years ago with the evolution of Aotus, Anthropoid Platyrrhine primates, CG (no h since not human) evolved. A deletion mutation occurred in the ß-subunit of LH (Figure 5) [16,17]. The deletions of a single base cause a frame-shift in subsequent nucleotide triplet codons. This included a termination codon following LH ß-subunit amino acid codon 121. The LH ß-subunit gene was read through the termination codon to residue 145 where a further termination codon was located (Figure 5). This generated the new CG ß-subunit molecule. This combined with the common α-subunit to make CG dimer.

Figure 5: The deletion mutation in LH ß-subunit gene.
CG was primarily expressed by pituitary gonadotrope cell, in response to hypothalamic gonadotropin releasing hormone (GnRH) initiation. GnRH is the initiation signal for LH, hCG and FSH. For unclear reason GnRH is also produced by trophoblast cells during pregnancy [18,19]. This is how cytotrophoblast cells started secondarily producing hyper glycosylated CG, and syncytiotrophoblast cells or fused cytotrophoblast cells started secondarily producing the hormone hCG in Aotus.
Aotus placenta upon producing the hormone CG and the cytotrophoblast autocrine hyper glycosylated CG started feeding the fetus by more efficient hemochorial placentation, and ceased feeding by epitheliochorial placentation the inefficient method used by mammals and earlier primates. As illustrated in Figure 6, the mechanics of hemochorial placentation were built by the hormone hCG and hyper glycosylated hCG. Briefly, hyper glycosylated hCG promoted cytotrophoblast cell growth out from columns in the implanted blastocyst. This grew as a tree shaped pattern forming the villous trophoblast or root of hemochorial placentation. The hormone hCG then promoted fusing or differentiation of the villous cells to form a skin of syncytiotrophoblast which surrounds the villous core [20]. The hormone hCG then promotes the extension of uterine spiral arteries to reach the hemochorial placentation apparatus [21,22]. The hormone hCG also builds an umbilical circulation to link the villous core with the fetal circulation [23,24].
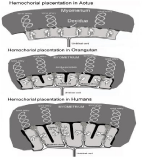
Figure 6: Hemochorial placentation in Aotus, Orangutan and Humans.
Briefly (Figure 6), maternal blood fills the chamber surrounding the villous core. Nutrients, oxygen and glucose filters through the single layer of syncytiotrophoblast cells surrounding the villous core and enter the villous structure, where nutrients enter the fetal umbilical circulation and enter the fetus (Figure 6).
Aotus CG ß-subunit had just three acidic sugar side chains. While human CG ß-subunit had six acidic side chains. Aotus CG ß-subunit C-terminal peptide had to undergo 17 amino acid changes or point mutations in order to be converted to human CG. Aotus CG was a minimal or starting form of CG, Isoelectric point (pI) 6.3 and circulating ½-life 2.4 hours. Human CG was a super-potent molecule, pI = 3.5 and circulating ½-life 36 hours. Aotus CG came about 37 million year ago, and it took the 37 million years to evolve the human molecule. As indicated in Table 2, it took 37 million years and evolution through at least 32 species to generate the super-potent human molecule. We have information on Langur and Baboon Catarrhines, Orangutan Hominidae, and Homo Habilis, Hominin as intermittent steps in order to trace this evolution [17,25].
Species
Evolved ya
Placentation
Sugar side chains
on hCG ß-subunit
Pi / Clearance rate
Depth of implantation
Brain mass
%body weight
Bipedalism
Hand/Tools
Homo sapiens
200,000 ya
Hemochorial
6 sugar side chains
pI=3.5 / 36 h
30%
2.4% (34X)
Homo heidelbergensis, Hominin
400,000 ya





Homo erectus, Hominin
1,000,000 ya
Homo ergaster, Hominin
1,800,000 ya
Homo Habilis, Hominin
2,000,000 ya
Hemochorial
5 sugar side chains
Extinct ?
Extinct ?
1.2% (17X)
Australopihecus garni, Hominin
2,500,000 ya





Australopihecus africus, Hominin
2,700,000 ya
Australopihecus afarensis, Hominin
3,500,000 ya
Australopihecus anamensis, Hominin
4,000,000 ya
Ardipithecus ramidus, Hominin
4,300,000 ya
Orrorin tugensis, Hominin
6,000,000 ya
Sahelanthropus tchadensis, Hominin
7,000,000 ya
Hominidae or great apes
4-12,000,000 ya
Chimpanzee, Hominidae
6,000,000 ya
Orangutan, Hominidae
12,000,000 ya
Hemochorial
4 sugar side chains
pI =4.9 / 6.0 h
10%
0.74%(11X)
Hominidae or great apes
4-12,000,000 ya





Hominidae or great apes
4-12,000,000 ya
Bonobo, Hominidae
4-12,000,000 ya
Hominidae or great apes
4-12,000,000 ya
Hominidae or great apes
4-22,000,000 ya
Gibbon, Hylobatidae
16,800,000 ya
Hominoidea, Africa and Southeast Asia
12-22,000,000 ya
Hominoidea, Africa and Southeast Asia
12-22,000,000 ya
Hominoidea, Africa and Southeast Asia
12-22,000,000 ya
Hominoidea, Africa and Southeast Asia
12-22,000,000 ya
Hominoidea, Africa and Southeast Asia
12-22,000,000 ya
Platyrrhine, new world primates
19-40,000,000 ya





Platyrrhine, new world primates
19-40,000,000 ya
Platyrrhine, new world primates
19-40,000,000 ya
Platyrrhine, new world primates
19-40,000,000 ya
Platyrrhine, new world primates
19-40,000,000 ya
Aotus, Anthropoid primate
37,000,000 ya
Hemochorial
3 sugar side chains
pI=6.3 / 2.4 h
1%
0.17% (2.4X)
Tarsier, Haplorrhine
65-25,000,000 ya





Haplorrhine monkeys
65-25,000,000 ya
Haplorrhine monkeys
65-25,000,000 ya
Haplorrhine monkeys
65-25,000,000 ya
Strepsirrhine monkeys
65-25,000,000 ya
Lemur, Strepsirrhine primate
55,000,000 ya
Epitheliochorial
LH: 1 sugar side chain
LH: pI=8 / 0.33 h
No implantation
0.07%
Table 2: Laboratory values at hospital admission.
Aotus with the first CG had pI = 6.3 and a circulating ½-life of 2.4 hours. This CG ß-subunit had 3 sugar side chains. We know that Langur and Baboon Catarrhines that evolved 20 million year ago produced a more acidic CG with a ß-subunit with four sugar side chains [17,25]. That the four sugar side chains remained on Orangutan that evolved 10,000,000 years ago. We know that Orangutan CG had a pI = 4.9 and a longer circulating ½-life of 6.0 hours, a half way species. We also know that human CG had a pI = 3.5 and a circulating ½-life of 36 hours. We know that over these 37 million years that Aotus implanted in the uterus minimally by 1% of uterine thickness, that Orangutan implanted by 10% of uterine thickness, and that humans implanted by 30% of uterine thickness. That overall efficiency of hemochorial placentation, pictured in Figure 6, advanced from 1X in Aotus to 10X in Orangutan to 30X in humans. Overall, over the 37 million years of evolution CG became increasing more acidic and increasing with a longer circulating ½-live and more and more efficient. Parallel to this increasing potency, hemochorial placentation and implantation, functions promoted by CG, became increasingly more efficient.
The brain in mammals and early primates was generally very small or about 0.10% of total body weight. This was largely due to inefficient epitheliochorial placentation, a fetal feeding system that worked poorly, not efficiently enough to support brain growth. In primates, 7 brain growth genes are present, MCPH1 gene [26], WDR62 gene and CDK5RAP2 gene [27], CEP152 gene [28], ASPM gene [29], CENPJ gene [27] and STIL gene [30]. All, however, were dependent on the efficiency of placentation in order to allow brain growth [31-33]. When placentation was improved by hemochorial placentation in Aotus brain growth advanced. As CG advanced hemochorial placentation from Aotus to human brain growth advanced in parallel. So that prior to Aotus in Lemur brain size was 0.07% of body weight. In Aotus brain size was 0.17% of body weight (2.4X over Lemur), in Langur and Baboon brain size was 0.47% of body weight (6.7X over Lemur), in Orangutan brain size was 0.74% of body weight (11X over Lemur), in Homo habilis brain size was 1.2% of body weight (17X over Lemur), and in humans was 2.4% of body weight (34X over Lemur) (Table 2).
It is through this series of evolutionary pathways that over 37 million years that hCG became a super and super-duper molecule that the human brain developed. Following species with efficient and super-efficient hemochorial placentation bipedalism developed and became super-efficient, hand and use tool developed and became super-efficient or that human evolved. It would be fair to say that with the evolution of CG and super-potent CG, and the resulting efficient and super-efficient hemochorial placentation that the human and human brain completely developed.
I call CG a master molecule in the title of this review. How CG developed super-efficient hemochorial placentation leading to the development of humans can be considered a major part of why it is master molecule, along with CG’s critical roles in pregnancy, the pituitary and cancer as described hereafter.
HCG and Pregnancy
Three primary forms of hCG are critical to pregnancy, the hormone hCG as produced by syncytiotrophoblast cells, the autocrine hyper glycosylated hCG, and fetal hCG will all be dealt with here. Pregnancy is welcomed by the hormone hCG and the autocrine hyper glycosylated hCG (Figure 7).
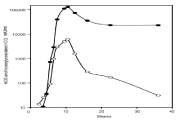
Figure 7: Production of the hormone hCG (•) and hyperglycosylated hCG (°)
during pregnancy.
As shown, uterine pregnancy starts with implantation at three weeks gestation and the production of hyper glycosylated hCG. Pregnancy commences with the production of the hormone hCG one or two days later [34]. On the day of implantation (first day of hCG detection) the median hyper glycosylated hCG in urine is 0.30 ng/ml, the median hormone hCG is 0.12ng/ml or hyper glycosylated hCG is 79% of total hCG. After 7 days, the median hyper glycosylated hCG is 21ng/ml, the median hormone hCG is 12ng/ml or hyper glycosylated hCG is 61% of total hCG. After 14 days, the median hyper glycosylated hCG 169ng/ml, the median hormone hCG is 161ng/ml or hyper glycosylated hCG is 57% of total hCG. After 21 days, the median hyper glycosylated hCG is 616 ng/ml, the median hormone hCG 1,552ng/ ml or hyper glycosylated hCG is 31% of total hCG. The concentration of the hormone hCG exceeds the concentration of hyper glycosylated hCG in all weeks of pregnancy thereafter (Figure 7) [34].
Pregnancy starts at two weeks gestation with fertilization of the ovum. The zygote quickly divides to two cells, four cell, eight cells and the sixteen cells it then starts to differentiate into placenta cells and fetal cell forming a morula. The morula keeps differentiating as a 32 cell blastocyst is formed. The blastocyst is shelled losing the zona pellucida outer coating. Syncytiotrophoblast microvilli on the blastocyst then link to the glycalyx or the uterine epithelium cytotrophoblast cells, tying the structure together, then blastocyst cytotrophoblast cells secrete hyper glycosylated hCG and the invasion process of implantation starts. Hyperglycosylated hCG acts on the cytotrophoblast cell TGF-ß2 receptor antagonizing it [3-5], and the cytotrophoblast cells secrete metalloproteinases and collagenases [35], which burn an implantation hole in the uterus (Figure 8). The blastocyst firmly buries itself into the uterine stroma [34].
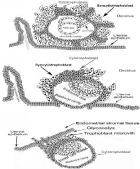
Figure 8: The stages of implantation.
In the second stage of implantation which occurs at the end of the first trimester of pregnancy, hyper glycosylated hCG deeply implants the placenta in the uterus [12]. In human this is at 30% of uterine thickness. One again cytotrophoblast cell hyper glycosylated hCG antagonizes TGF-ß2 receptor leading to metalloproteinases and collagenase production. Interestingly, poor hyper glycosylated hCG production at pregnancy implantation leads to pregnancy failure, spontaneous abortion, biochemical pregnancy and ectopic pregnancy. Poor hyper glycosylated hCG production at the end of the first trimester and inappropriate deep implantation leads to gestational hypertension and preeclampsia.
Norwitz et al. [36] Semprini and Simon [37] and Wilcox et al. [38] all demonstrated that most pregnancy failures were due to inappropriate implantation at three week gestation. As shown my laboratory [39-41], deficiency of hyper glycosylated hCG can large be shown to be responsible (Figure 9). As shown in Figure 9, 191 of 191 term delivery pregnancies produced >40% proportion hyper glycosylated hCG (hyper glycosylated hCG as a percentage of total hCG) on the day of implantation of pregnancy [41]. It is though that >40% may be an essential criterion for normal outcome pregnancy. In contrast, only 13 of 49 spontaneous abortion pregnancies produced >40% hyper glycosylated hCG on the day of implantation, only 11 of 61 biochemical pregnancies produced >40% hyper glycosylated hCG, and none of 18 ectopic pregnancies produce >40% hyper glycosylated hCG on the day of implantation (Figure 9) [41]. The 13 spontaneous abortion and 11 biochemical pregnancies that produced >40% hyper glycosylated hCG were all gross genetic abnormality pregnancies. This figure shows that <40% hyper glycosylated hCG is an absolute marker of failing pregnancy. Supplementation with hyper glycosylated hCG injection on the day of implantation may prevent failing pregnancies.
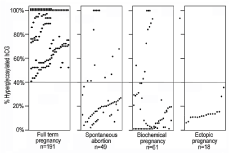
Figure 9: Hyperglycosylated hCG and pregnancy failures.
The story does not end with hyper glycosylated hCG on the day of implantation. Examining 3,391 pregnancies during the three trimesters of pregnancy measuring hyper glycosylated hCG 141 cases of preeclampsia/gestational hypertension were identified [12]. As shown, preeclampsia/gestational hypertension can be predicted from 10 weeks to 18 week of gestation, preeclampsia/ gestational hypertension cases present with hyper glycosylated hCG concentrations (ng/mg creatinine) less that the third percentile, <3% (Figure 10). Seemingly, in these cases insufficient hyper glycosylated hCG is produced for deep implantation of the placenta at 30% of the thickness of the uterus. This leads to inefficient hemochorial placentation and to the fetus having to raise the mother’s blood pressure [12,42-45].
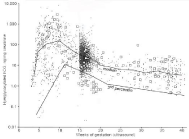
Figure 10: Hyperglycosylated hCG through the length of pregnancy, and in
preeclampsia cases (large squares).
It appears that hyper glycosylated hCG is that finicky valve produced during pregnancy, that decided which pregnancies fail and which make it to term, and which pregnancies develop deadly preeclampsia or gestational hypertension.
The hormone hCG during pregnancy has multiple critical functions. The primary function appears to be building and maintaining hemochorial placentation, the highly efficient fetal feeding system established by the placenta. Each placenta has 5-7 hemochorial placentation chambers.
Hyperglycosylated hCG promotes cytotrophoblast cell growth out from columns in the implanted blastocyst. This grows a tree shaped pattern of cytotrophoblast cells forming the villous trophoblast or root of hemochorial placentation (Figure 6). The hormone hCG then promotes fusing or differentiation of the villous cells to form a skin of syncytiotrophoblast which surrounds the villous core [20]. This syncytiotrophoblast skin forms the single cell barrier between maternal and fetal circulations (Figure 6). The hormone hCG then promotes the extension of uterine spiral arteries to reach and bleed into the hemochorial placentation chamber [21,22]. The hormone hCG also builds an umbilical circulation to link the villous core with the fetal circulation [23,24].
Briefly (Figure 6), maternal blood fills the chamber surrounded the tree-shaped villous core. Nutrients, oxygen and glucose filters through the single layer of syncytiotrophoblast cells and enters the villous structure, where nutrients enter the fetal umbilical circulation and enter the fetus (Figure 6).
Other critical function of the hormone hCG includes promotion of progesterone production by maternal ovarian corpus luteal cells, from five weeks to eight weeks of gestation [46]. Starting at around 8 weeks of gestation, the placental syncytiotrophoblast cells take over pregnancy progesterone production. The hormone hCG also has clear protective functions. The hormone hCG protect the uterine myometrial muscle from having contractions during the course of pregnancy [47,48]. It is thought that at term hCG levels have to decline significantly for the contraction of labor and delivery to proceed [47,48]. The hormone hCG also acts on maternal macrophages to prevent attack of placenta and fetal tissues as foreign tissues [49,50].
Fetal hCG made by fetal liver and kidney has been shown to promote growth and differentiation of fetal organs during fetal development during pregnancy [6-8]. Fetal hCG has been shown to cease at pregnancy parturition [6-8].
Hyperglycosylated hCG promotes blastocyst implantation in pregnancy [34,39-41]. Hyperglycosylated hCG is also seemingly responsible for deep implantation at the end of the first trimester pregnancy [12]. Among other functions, hyper glycosylated hCG seems to be responsible for placental cell growth during the course of pregnancy [51]. As shown in Figure 7, hyper glycosylated hCG is produced continuously during the course of pregnancy.
I call hCG the master group of molecules at the end of the section on hCG evolution. I also call it again the master molecule when considering the key role played by hCG primary molecules in pregnancy. It runs pregnancies, not only by driving hemochorial placentation but protecting pregnancies in multiple different ways. It decides which pregnancies are successful and makes it to term, and which pregnancies may become terror cases afflicted with preeclampsia. As it happens hCG is seemingly responsible for fetal development and generation of new human organs.
HCG and Pituitary
HCG evolved from LH ß-subunit. Like LH it is primarily a pituitary gland gonadotrope cell hormone and secondarily a placental hormone. We researched 276 sequential menstrual cycles from 111 women who attempted over six menstrual cycles to achieve pregnancy, and eventually became pregnant [52]. Daily hCG and LH was measured was measured over multiple menstrual cycles. In every case and every menstrual cycle the LH mid-cycle peak appeared on the same day as the hCG mid cycle peak. The median LH midcycle peak in the urines was 119mIU/ml, and the median hCG midcycle peak was 1.1mIU/ml, including the 24% hCG peaks that were not detected (<1.0mIU/ml) [52]. LH and hCG biological activities were determined and it was demonstrated that pituitary hCG is the principal luteotrophic activity produced by the pituitary [52]. Pituitary hCG seemingly drives ovulation and seemingly luteogenesis, folliculargenesis and luteotrophic steroidogenesis. While it is now proven that pituitary hCG has the principal ovulation activity, further evidence is needed to confirm that hCG, and not LH has principal luteogenesis, folliculargenesis, steroidogenesis and male activities.
Other publications show that pituitary hCG is sulfated, like LH, it is pulsatile, is controlled by GnRH, and very much mimics LH [53-56]. Pituitary sulfated hCG is the hormone that along with FSH controls the menstrual cycle. It seemingly promotes the conversion of cholesterol to androstenedione in theca cells, the first stage of estrogen synthesis in the follicular phase of the menstrual cycle. It seemingly promotes follicular growth, promotes follicular ovulation, seemingly promotes luteogenesis, and seemingly promotes progesterone synthesis by the corpus luteum in the luteal phase of the menstrual cycle.
In addition 56 women were found unable to get pregnant after 6 menstrual cycles of trying. Investigation of the cases showed that 30 of 56 went through 6 menstrual cycles with no detectable midcycle hCG peak. It was inferred because of this big difference (0 of 6 hCG cycles vs. 76% of all cycle showing a detectable peak) that these women had hCG problems, choriogonadotropinism, this may be a major cause of infertility not previously recognized.
HCG and Human Cancer
Cancer hyper glycosylated hCG and its free ß-subunit drives all or most human cancers, driving cancer cell growth and cancer cell invasion of adjacent cells or malignancy. Trophoblastic cancers, i.e. Choriocarcinoma, testicular germ cell malignancy and ovarian germ cell malignancy produce cancer hyperglycosylated hCG, while all other cancers cannot combine the α-subunit and ß-subunit due to an absence of placental disulfide isomerase critical for the ß-subunit, so produce a cancer hyperglycosylated hCG free ß-subunit [57].
Both cancer hyperglycosylated hCG and cancer hyperglycosylated hCG free ß-subunit are unique forms of the hyperglycosylated hCG molecule in that they have triantennary N-linked oligosaccharides attached to the ß-subunit differing themselves from the pregnancy hyperglycosylated hCG molecules [58-60].
In the cancer model, cancer hyperglycosylated hCG and hyperglycosylated hCG free ß-subunit are secreted as autocrines and feedback as an antagonist to a TGF-ß2 receptor [3-5]. In response the receptor blocks apoptosis in the cancer cell, promotes growth of the cancer and promotes production of collagenases metalloproteinases invasive enzymes [35,57].
From 1984 to 1992 I carried out extensive tumor marker studies testing 959 serum samples and 1495 urine samples for hCG ß-subunit tumor markers (Table 3) [61-69]. Serum was tested for hCG ß-subunit using an hCG ß immunoassay and urine was tested for ß-core fragment the urine terminal degradation product. Testing a wide variety of cancers of different histologies, in serum I could detect 38% of malignancies (range 17%-100%) and in urine I could detect 52% of malignancies (range 28%-100%) (Table 3) [61-69]. I asked myself why do only 52% of cancers and not 100% of cancers produce this marker?
Malignancy
Serum hCGß
Urine hCG ß-core fragment
Sensitivity (3 fmol/ml)
Sensitivity (3 fmol/ml)
# Cases
Sensitivity
# Cases
Sensitivity
Bladder cancer
170
35%
113
48%
Breast cancer
42
36%
37
57%
Cervical cancer
60
37%
410
48%
Choriocarcinoma
63
100%
63
100%
Colorectal cancer
136
17%
80
36%
Endometrial cancer
55
33%
233
44%
Gastric cancer
83
57%
Hepatic cancer
14
50%
Lung cancer
143
18%
90
28%
Intestinal cancer
17
47%
Lymphoma
4
50%
Ovarian germ cell cancer
30
100%
11
64%
Ovarian other cancers
150
38%
207
70%
Pancreatic cancer
29
33%
29
55%
Renal cancer
15
67%
Testicular germ cell cancer
17
100%
17
100%
Uterine cancer
63
41%
Vulvar cancer
Table 3: Laboratory values at hospital admission.
In 2000 it was first shown that cancer hyperglycosylated hCG and cancer hyperglycosylated hCG free ß-subunit both functioned through binding a TGF-ß2 receptor [3,4,57]. Two types of autocrines bind TGF-ß2, simple autocrines and complex autocrines act on a TGF-ß2 receptor [70,71]. Complex autocrines are produced at higher concentration, these autocrine circulate the body and return binding the cancer cell TGF-ß2 receptor. Simple autocrines are produced at low concentrations, they only minimally circulate and mostly just act directly after secretion on the TGF-ß2 receptor [70,71].
As shown in Table 3, urine ß-core fragment detects 70% of ovarian cancers. I investigated 24 new cases of ovarian cancer pretherapy. As shown in Table 4, 17 of 24 cases were detected in the ß-core fragment assay with concentration of 3.2 to 58.6 fmol/ml or within the >3.0 fmol.ml cut-off. Seven cases were negative in the assay, cut-off 3 fmol/ml, but contained 0.40 – 2.0 fmol/ml ß-core fragment. It was concluded that 17 cases produced a complex autocrine and were detected by the assay. That the remaining seven cases, while producing a tiny concentration of ß-core fragment, were probably a simple autocrine, yet still produced ß-core fragment. It was concluded that all cancers seemingly produce the tumor markers, but that a significant proportion were simple autocrine cancers producing just tiny barely detectable concentrations.
Pathologic Diagnosis
Stage
Status
hCG ß-core fragment
fmol/mlCA-125
U/ml)Ovarian Serous cystadenocarcinoma
IV
Recurrent
0.40
60
Ovarian Serous cystadenocarcinoma
IIIc
New
0.70
271
Ovarian Brenner tumor
Ia
New
0.88
2.0
Ovarian Serous cystadenocarcinoma
IIIc
Recurrent
1.20
237
Ovarian Mucinous cystadenocarcinoma
Ia
New
2.0
4.0
Ovarian Endometrioid carcinoma
I
New
2.0
5.0
Ovarian Serous cystadenocarcinoma
II
New
2.0
7.0
Ovarian Clear cell carcinoma
IIIc
Recurrent
3.20
33
Ovarian Serous cystadenocarcinoma
IIIc
New
3.30
1253
Ovarian Mixed epithelial tumor
IIIc
New
3.51
4.0
Ovarian Serous cystadenocarcinoma
IIIc
New
3.70
531
Ovarian Serous cystadenocarcinoma
IIIc
New
5.10
1010
Ovarian Mixed epithelial tumor
IIc
New
5.26
54
Ovarian Serous cystadenocarcinoma
IV
Recurrent
6.30
4537
Ovarian Serous cystadenocarcinoma
IIIc
Recurrent
6.90
1520
Ovarian Endometrioid carcinoma
IIc
New
7.60
880
Mixed mesodermal
III
Recurrent
11.30
156
Ovarian Serous cystadenocarcinoma
IV
Recurrent
11.90
1090
Ovarian Serous cystadenocarcinoma
IIIc
New
12.05
4834
Ovarian Mixed epithelial tumor
IIIc
Recurrent
17.90
1600
Ovarian Serous cystadenocarcinoma
IV
New
20.40
2420
Serous cystadenocarcinoma
IIb
New
28.00
109
Clear cell carcinoma
III
Persistent
48.78
78
Squamous carcinoma in teratoma
II
New
58.60
22
Table 4: Laboratory values at hospital admission.
In 2006 I showed that trophoblastic cancers responded to the cancer hyperglycosylated hCG which they produced. It stimulated the cancers to grow and to invade [72,73]. Ray Iles PhD and Stephen Butler PhD in England had demonstrated that non-trophoblastic cancers are similarly stimulated by cancer hyperglycosylated hCG free ß-subunit and hCG free ß-subunit [3,74]. As of today, 8 independent or isolated groups in India, England, Poland, Brazil and the U.S.A. have each proven that hyperglycosylated hCG, hyperglycosylated hCG free ß-subunit or hCG free ß-subunit each promote cancer growth and cancer invasion [3,72,75-80]. When 8 separate groups all show the same thing it must be accepted as fact.
Myself and my laboratory staff showed using JAR and JEG-3 Choriocarcinoma cell lines, NTERA testicular germ cell malignancy cell line, SCaBER and T24 Bladder cancer cell lines, and KLE and Hec- 1-a endometrial cancer cell lines that in 7 of 7 cell lines cancer growth and cancer invasion was controlled by cancer hyperglycosylated hCG and cancer hyperglycosylated hCG free ß-subunit [57]. It has now been shown by the 8 competing groups that hyperglycosylated hCG and hyperglycosylated hCG free ß-subunit controls 30 of 30 different human cancers. No cancer have been found to date that do not respond indicating that all human cancers function through this mechanism.
It was also shown by my laboratory using B152 monoclonal antibody to hyperglycosylated hCG and hyperglycosylated hCG free ß-subunit, that the antibody distinctly blocked and suppressed cancer cell growth and cancer cell invasion [81]. This antibody may be useful in human cancer treatment or as a human cancer cure [81].
The HCG Assay
The era of the hCGß radioimmunoassay was the era when the total hCGß test shined and starred. The total hCGß radioimmunoassay was the hCG test at its best. The hCGß radioimmunoassay detected all pertinent hCGß molecules. In serum is a mixture of hCG, hyperglycosylated hCG, nicked hCG (nicked at ß47-48), nicked hCG missing the ß-subunit C-terminal peptide, nicked hCG ß-subunit and nicked hCG ß-subunit missing the ß-subunit C-terminal peptide and nicked ß-subunit of hyperglycosylated hCG. The same plus ß-core fragment is detected in urine samples. The hCGß radioimmunoassay using a single polyclonal serum to the hCG ß-subunit three dimensional core detected all of these variables [82,83].
Today is the era of automated immunometric assays, assays that run on specified machines. There are 13 automated total hCG tests sold today throughout the world, these are the tests that are run today by all clinical laboratories: Abbott Architect, Abbott AxSYM, Beckman Access 2, Beckman DxI 800, Ortho Vitros ECiQ, Perkin- Elmer DELFIA, Roche Elecsys hCG+ß, Siemens ACS180, Siemens Centaur, Siemens Dimension, Siemens Immulite, Siemens Stratus and Tosoh A1A. All thirteen immunometric assay require two antibodies to two distant sites on hCG. When a monoclonal is made from hCG ß-subunit, an antibody to ß-subunit three dimensional core and antibody to hCG ß-subunit C-terminal peptide are the most common antibodies generated. The problem is today that 12 of 13 commercial assays (all except Siemens Immulite) use an antibody to ß-subunit C-terminal peptide.
The problem with an assay using an antibody to the C-terminal peptide is that this peptide is mostly sugar by molecular weight. Problems come with detection of hyperglycosylated hCG and other sugar variation of hCG. Specificity studies show that assay using these antibodies can poorly detect nicked hCG and nicked hyperglycosylated hCG, and do not detect nicked hCG missing the ß-subunit C-terminal peptide, or 3 critical degradation products present in serum and urine [84,85].
One automated hCG assay sold today, the Siemens Immulite hCG, uses two separate antibodies to the core of ß-subunit. This assay has similar specificity to the hCGß radioimmunoassay detecting all serum forms of hCG. We measured the Siemens Immulite hCG and a mixture of all other assay in 45 pregnancy serum samples (Table 5). As found, the median result was 65mIU/ml using the Siemens Immulite test and 38mIU/ml using the other 12 total hCG test. This is simply unacceptable, giving physicians an hCG result that measures only 58% of what it should.
Case
Total hCG, Immulite 2000 assay mIU/ml
Total hCG, Other assay mIU/ml
Other assay,
% Immulite resultOther assay used
1
441,624
292,136
66%
Siemens Dimension
2
16,621
9,358
56%
Siemens Dimension
3
7,370
5,743
78%
Siemens Dimension
4
6,464
6,717
104%
Siemens Dimension
5
444
103
23%
Siemens Centaur
6
275
263
96%
Siemens Dimension
7
235
48
20%
Abbott Architect
8
206
87
42%
Siemens Dimension
9
168
50
30%
Siemens Dimension
10
156
103
66%
Siemens Centaur
11
148
38
26%
Siemens Centaur
12
148
38
26%
Siemens Dimension
13
140
66
47%
Ortho Vitros Eci
14
140
66
47%
Siemens Centaur
15
138
120
87%
Siemens Dimension
16
137
93
68%
Tosoh A1A
17
127
97
76%
Siemens Dimension
18
118
70
59%
Abbott AxSym
19
118
66
56%
Beckman Access 2
20
110
74
67%
Siemens Centaur
21
106
111
105%
Siemens Dimension
22
88
61
69%
Siemens Centaur
23
82
66
80%
Siemens Centaur
24
47
38
81%
Siemens Dimension
25
46
38
83%
Siemens Dimension
26
32
14
44%
Siemens Dimension
27
32
17
53%
Abbott Axsym
28
32
7
22%
Siemens Centaur
29
31
28
90%
Siemens Dimension
30
19
14
74%
Siemens Dimension
31
18
4.5
25%
Roche Elecsys hCG+ß
32
17
<2.0
<12%
Siemens Centaur
33
16
8.0
50%
Siemens Centaur
34
15
9.0
60%
Siemens Dimension
35
13
9.0
69%
Siemens Centaur
36
12
10
83%
Beckman DxI
37
11
11
100%
Siemens Dimension
38
9.2
7.1
77%
Roche Elecsys hCG+ß
39
8.0
8.0
100%
Siemens Centaur
40
6.0
5.0
83%
Siemens Dimension
41
5.9
4.6
78%
Siemens ACS180
43
3.0
<1.0
<33%
Siemens Dimension
44
2.8
<1.0
<36%
Siemens Dimension
45
2.4
<2.0
<83%
Beckman Access 2
46
2.0
1.0
50%
Siemens Dimension
Median 65
Median 38
Table 5: Laboratory values at hospital admission.
As it happens the Siemens Immulite is one of the least used total hCG test. As the director of the hCG Reference Service and as the author who has published over 50% of publication on hCG, I wrote to all manufacturers complaining that this is unacceptable, and that the hCG tests has gone way downhill since the era of the hCG radioimmunoassay. All manufacturers wrote back to me claiming that the test sell big time and that is what matters to them. I presented this data to a conference of Obstetrics and Gynecology physicians. The general impression was that they ordered total hCG test and expected a full and complete result. I then wrote to clinical laboratories complaining. The general impression is that this is what is sold and what is very popular.
In desperation I wrote multiple manuscripts describing these unacceptable problems. I first sent the manuscript to Clinical Chemistry the principal and chief journal of laboratory medicine. The editors rejected the paper outright without sending it to reviewers. I then sent the multiple manuscripts to Acta Clinica Chemistry, and found that the editors similarly rejected the papers. Finally I sent the papers to Journal of Reproductive Immunology. Once again the editors rejected the paper as inappropriate. I spoke with an editor at Journal of Reproductive Endocrinology who once and for all explained the journal problem. As he said: “We cannot publish a paper that hurts all the manufacturers that support them.” I include the data from this paper in this review (Table 5), even though it is not published.
Basically, the hCG assay has gone way downhill since the era of the hCGß radioimmunoassay (1970-1990). I know that today Quest Diagnostics, the largest clinical laboratory in the U.S.A. uses the Siemens Centaur hCG assay. One of the assay yielding the poorest hCG values (Table 5), and the most popular hCG assay used in the U.S.A.
There are specific hCG assay available for free α-subunit, for free ß-subunit, for ß-subunit core fragment, and now for hyperglycosylated hCG (B152 assay). The B152 hyperglycosylated hCG assay was prepared using my hyperglycosylated hCG preparation C5 [58]. My hyperglycosylated hCG preparation C5 is unique in that is has 100% type two O-linked oligosaccharides at four different site [58]. I have purified 6 hyperglycosylated hCG preparations from Choriocarcinoma patient urines and only this one is 100% type two glycosylated. Others have reported that 100% type two glycosylation does not exist [59]. Intriguingly, antibody B152 was made without problem from C5 hCG [86]. I have since made 15 attempts at producing a hyperglycosylated hCG-specific antibody. All failed. I used hyperglycosylated hCG, hyperglycosylated hCG ß-subunit and isolated hyperglycosylated hCG ß-subunit C-terminal peptide. All efforts used hyperglycosylated hCG variants with 50- 75% type two glycosylation. Considering that B152 is the only know antibody specific for hyperglycosylated hCG it is assumed that hyperglycosylated hCG with 100% type two glycosylation was critical to antibody creation.
Conclusion
HCG must biochemically be a master molecule. Existing in six primary forms and binding two receptors, the LH/hCG hormone receptor and a TGF-ß2 autocrine receptor makes it a master. hCG forms control human pregnancy and effective manages super-efficient hemochorial placentation. Hyperglycosylated hCG production effectively decides which pregnancy will make it to term and which one will fail. Finally, hyperglycosylated hCG and its free ß-subunit physically drives human cancers, deciding which cancers are highly malignant and kill people, and which ones are easily cured. I call hCG a master molecule.
References
- Isaacs NW. Cysteine knots. Curr Opinion Struc Biol. 1995; 5: 391-395.
- Sun PD, Davies DR. The Cystine-Knot Growth-Factor Superfamily. Ann Revi Biophys Biomolec Structure. 1995; 24: 269-272.
- Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of hCG is a result of an anti-apoptosis effect and not cell proliferation. Brit J Cancer. 2000: 82; 1553-1556.
- Ahmud F, Ghosh S, Sinha S, Joshi SD, Mehta VS, Sen E. TGF-ß-induced hCG-ß regulates redox homeostasis in glioma cells. Molec. Cellul. Biochem. 2015; 399: 105-112.
- Berndt S, Blacher S, Munuat C, Detilleux J, Evain-Brion D, Noel A, et al. Hyperglycosylated human chorionic gonadotropin stimulates angiogenesis through TGF-ß receptor activation. FASEB J. 2013; 27: 1309-1321.
- McGregor WG, Raymoure WJ, Kuhn RW, Jaffe RB. Fetal tissues can synthesize a placental hormone Evidence for chorionic gonadotropin β-subunit synthesis by human fetal kidney. J Clin Invest. 1981; 68: 306-309.
- Goldsmith PC, McGregor WG, Raymoure WJ, Kuhn RW, Jaffe RB, Cellular localization of chorionic gonadotropin in human fetal kidney and liver. J Clin Endocrinol Metab. 1983; 57: 54-61.
- Abdallah MA, Lei ZM, Li X, Greenwold N, Nakajima, ST, Jauniaux E, et al. Human Fetal nongonadal tissues contain human chorionic gonadotropin/ luteinizing hormone receptors. J Clin Endocrinol Metab. 2004; 89: 952-956.
- Lapthorn AJ, Harris DC, Littlejohn A, Lustbader JW, Canfield RE, Machin KJ, et al. Crystal structure of hCG. Nature. 1994; 369: 455-461.
- Wu H, Lustbader JW, Liu Y, Canfield RE, Hendrickson WA. Structure of human chorionic gonadotropin at 2.6 å resolution from MAD analysis of the selenomethionyl protein. Structure. 1994; 2: 545-558.
- Cole LA, Kardana A, Andrade-Gordon P, Gawinowicz MA, Morris JC, Bergert ER, et al. The Heterogeneity of hCG: III. The occurrence, biological and immunological activities of nicked hCG. Endocrinology. 1991; 129: 1559-1567.
- Cole L.A. Hyperglycosylated hCG Screening for Preeclampsia / Gestational Hypertension (Supplement: Explains How to Start Screening Patients for Preeclampsia Immediately). Prenatal Diagn. In press. 2016.
- Cole L.A. HCG and hyperglycosylated hCG in the establishment and evolution of hemochorial placentation. J. Reprod. Immunol. 2009; 82: 111-117.
- Cole L.A. The evolution of the primate, hominid and human brain. Primatology. 2015.
- Cole L.A, Khanlian S.A, Kohorn, E.I. Evolution of the Human Brain, Chorionic Gonadotropin and Hemochorial Implantation of the Placenta: Origins of Pregnancy Failures, Preeclampsia and Choriocarcinoma. J. Reprod. Med. 2008; 53: 449-557.
- Fiddes J C, Goodman H M. The cDNA of the ß-subunit of human chorionic gonadotropin suggests evolution of a gene by read through into the 3’ untranslated region. 1979; 351-356.
- Maston GA, Ruvolo M. Chorionic gonadotropin has a recent origin within primates an evolutionary history of selection. Mol. Bio. Evol. 2002; 19: 320-335.
- Wong BC, Oehninger S, Gibbons WE, Dong K/ Estrogen down-regulates GnRH gene expression in human placental cytotrophoblast cells. Molec Cellul Endocrinol. 2004; 213: 199-210.
- Luo G, Snegovskikh V, Guller S, Rahman M, Funai E, Ma Y, Thung S, Norwitz E. Effect of thrombin on GnRH-I and GnRH receptor (GnRH) gene expression in human cytotrophoblast cells. Am J Obstet Gynecol. 2007; 192: 123.
- Shi QJ, Lei ZM, Rao CV, Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinol. 1993; 132: 387-395.
- Toth P, Li, X, Rao CV, Lincoln SR, Sanfillipino JS, Spinnato JA, Yussman MA, Expression of functional human chorionic gonadotropin/human luteinizing hormone receptor gene in human uterine arteries. J Clin Endocrinol Metab. 1994; 79: 307-315.
- Zygmunt M, Herr F, Keller-Schoenwetter S, Kunzi-Rapp K, Munstedt K, Rao CV, Lang U, Preissner KT. Characterization of human chorionic gonadotropin as a novel angiogenic factor, J Clin Endocrinol Metab. 2002; 87: 5290-5296.
- Rao CV, Li, X, Toth P, Lei ZM, Cok VD, Novel expression of functional human chorionic gonadotropin/luteinizing hormone receptor in human umbilical cords. J Clin Endocrinol Metab. 1993; 77: 1706-1714.
- Rao CV, Li, X, Toth P, Lei ZM, Expression of epidermal growth factor transforming growth factor-alpha and their common receptor genes in human umbilical cords. J Clin Endocrinol Metab. 1995; 80:1012-1020.
- Crawford RJ, Tegear GW, Niall HD. The nucleotide sequence of baboon chorionic gonadotropin ß-subunit genes have diverged from the human. Gene. 1986; 46: 161-169.
- Shi L, Ming L, Lin Q, Xuebin Q, Bing S. Functional divergence of the brain-size regulating gene MCPH1 during primate evolution and the origins of humans. BMC Biol. 2013; 62: 1-11.
- Bond J, Roberts E, Springell K, Lizarraga SB, Scott S, et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet. 2005; 37: 353-355.
- Guernsey DL, Jiang H, Hussin J, Arnold M, Bouyakdan K, Perry S, et al. Mutations in centrosomal protein CEP152 in primary microcephaly families linked to MCPH4. Am J Hum Genet. 2005; 87: 40-51.
- Rouprina N, Pavlicek A, Mochida GH, Solomon G, Gersch W, Yoon YH, et al. Accelerated evolution of the ASPM gene controlling brain size begins prior to human brain expansion. PLoS Biol. 2004; 2: 653-664.
- Kumar A, Girimaji SC, Duvvari MR, Blanton SH. Mutation in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly Am J Hum Genet. 2009; 84: 286-290.
- Martin RD. Evolution of placentation in primates: Implications of mammalian phylogeny. Evolutionary Biol. 2008; 35: 125-145.
- Martin RD. Relative brain size and basal metabolic rate in terrestrial vertebrates, Nature 293. 1981; 57-60.
- Capellini I, Venditti C, Barton RA. Placentation and Maternal Investment in Mammals. Am Natur. 2011; 177.
- Cole LA. The Mechanisms of Human Pregnancy Implantation, Endocrine in press. 2016.
- Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, Heath JK. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. Euro Molec Biol Org J. 1987; 6: 1899-1904.
- Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy, New Engl J Med. 2001; 345: 1400-1408.
- Semprini AE, Simon G. Not so efficient reproduction. Lancet. 2000; 356: 257-258.
- Wilcox AJ, Baird DD, Weinberg CR.Time of implantation of .the conceptus and loss of pregnancy. New Engl. J. Med. 1999; 340: 1796-1799.
- Sasaki Y, Ladner DG, Cole LA. Hyperglycosylated hCG the source of pregnancy failures. Fertil Steril. 2008; 89: 1871-1786.
- Cole LA. Hyperglycosylated hCG and Pregnancy Failures. J Reprod Immunol. 2012; 93: 119-122.
- Cole LA. Preventing Pregnancy Failures: Most Failures Caused by hyperglycosylated hCG deficiency. J Assist Reprod Genetics 2016, in press.
- Irmgard-Finger I, Jastrow N, Irion O. Preeclampsia: A danger growing in disguise. Intl J Biochem Cell Biol. 2006; 40: 1979-1983.
- Robillard RY, Hulsey TC, Dekkar GA, Chaouat G. Preeclampsia and human reproduction. An essay of a long term reflection. J Reprod Immunol. 2003; 59: 93-107.
- Burton GJ. Placental oxidative stress from miscarriage to preeclampsia, J Soc Gynecol Invest. 2004; 11: 342-352.
- Pennington KA, Schitt JM, Jackson DL, Schultz LC, Schust DJ. Preeclampsia multiple approaches for a multifactorial disease. Die Model Mech. 2012; 5: 9-18.5.
- Niswender GD. Molecular control of luteal secretion of progesterone. Reprod. 2002; 123: 333-339.
- Eta E, Ambrus G, Rao V, Direct regulation of human myometrial contractions by human chorionic gonadotropin. J Clin Endocrinol Metab. 1994; 79: 1582-1586.
- Doheny HC, Houlihan DD, Ravikumar N, Smith TJ, Morrison JJ, Human chorionic gonadotrophin relaxation of human pregnant myometrium and activation of the BKCa channel. J Clin Endocrinol Metab. 2003; 88: 4310-4315.
- Akoum A, Metz CN, Morin M, Marked increase in macrophage migration inhibitory factor synthesis and secretion in human endometrial cells in response to human chorionic gonadotropin hormone. J Clin Endocrinol Metab. 2005; 90: 2904-2910.
- Wan H, Marjan A, Cheung VW, Leenen PJM, Khan NA, Benner R, Kiekens RCM, Chorionic gonadotropin can enhance innate immunity by stimulating macrophage function. J Leukocyte Biol. 2007; 82: 926-933.
- Brennan MC, Wolfe MD, Murray-Krezan CM, Cole LA, Rayburn WF. First Trimester Hyperglycosylated Human Chorionic Gonadotropin and Development of Hypertension. Prenat Diagn. 2013; 33: 1075-1079.
- Cole LA, Butler SA. Pituitary Sulfated hCG Drives Ovulation, its Shortage is a Major Cause of Infertility. Endocrinol 2016, in press.
- Birken S, Maydelman Y, Gawinowicz MA, Pound A, Liu Y, Stockell Hartree A. Isolation and Characterization of Human Pituitary Chorionic Gonadotropin. Endocrinol; 1996; 137: 1402-1411.
- Stenman UH, Alfthan H, Ranta T, Vartiainen E, Jalkanen J, Sepppala M. Serum levels of human chorionic gonadotropin in nonpregnant women and men are modulated by gonadotropin-releasing hormone and sex steroids. J Clin Endocrinol Metab. 1987; 64: 730-736.
- Odell WD, Griffin J. Pulsatile secretion of human chorionic gonadotropin in normal adults. N Engl J Med 1987; 317: 1688-1691.
- Cole LA, Butler SA. Hyperglycosylated hCG, hCGß and Hyperglycosylated hCGß: Interchangeable Cancer Promoters. Molec Cellul Endcrinol. 2012; 349: 232-238.
- Elliott MM, Kardana A, Lustbader J, Cole LA. Carbohydrate and Peptide structure of the - and -subunits of human chorionic gonadotropin from normal and aberrant pregnancy and choriocarcinoma. Endocrine. 1997; 7: 15-32.
- Valmu L, Alfthan H, Hotakainen K, Birken S, Stenman UH, Site-specific glycan analysis of human chorionic gonadotropin beta-subunit from malignancies and pregnancy by liquid chromatography - electrospray mass spectrometry. Glycobiol. 2006; 16: 1207-1218.
- Cole LA. HCG and Hyperglycosylated hCG Carbohydrate Structures Corrected. J Glycobiol. 2014.
- Cole LA, Hussa RO, Rao CV. Discordant synthesis and secretion of human chorionic gonadotropin and subunits by cervical cancer cells. Cancer Res. 1981; 41: 1615-1619.
- Cole LA. O-Glycosylation of proteins in the normal and neoplastic trophoblast. Troph Res. 1987; 2: 139-148.
- Cole LA, Wong Y, Latif M, Chambers JT, Chambers SK, Schwartz PE, et al. Urinary human chorionic gonadotropin free ß-subunit and ß-fragment: new markers of gynecologic cancers. Cancer Res. 1988; 48: 1356-1360.
- Cole LA, Schwartz PE, Wong Y. Urinary Gonadotropin Fragments (UGF) in cancers of the female reproductive system: I. Sensitivity and specificity, comparison with other markers. Gyn Onc. 1988; 31: 82-90.
- Cole LA, and Wang Y. Use of intact human chorionic gonadotropin and its free alpha- and ß-subunit in the detection and management of gynecologic cancers. Ch. J. Medicine. 1988; 68: 101-103.
- Nam JH, Cole LA, Chambers JT, Schwartz PE. Urinary gonadotropin fragment, a new tumor marker: I. Assay development and cancer-specificity. Gynecol Oncol. 1990; 36: 383-390.
- D'Agostino RS, Cole LA, Ponn RB, Stern H, Schwartz PE. Urinary gonadotropin fragment in patients with lung and esophageal disease. J Surg Oncol. 1992; 49: 147-150.
- Cole LA, Nam J-H, Park S-Y, Koh MW, Tanaka A. Urinary Beta Core Fragment: 7 years later. J Tumor Marker Onc. 1994; 9: 53-60.
- Yoshimura M, Pekary AE, Pang X-P, Berg L, Cole LA, Kardana A, Hershman JM. Effect of peptide nicking in the human chorionic gonadotropin beta-subunit on stimulation of recombinant human TSH receptors. Eur J Endocrinology. 1994; 130: 92-96.
- Fairbank, St-Pierre P, Nabi IR. The complex biology of autocrine motility factor/phosphoglucose isomerase (AMF/PGI) and its receptor, the gp78/AMFR E3 ubiquitin ligase. Mol BioSyst. 2009; 5: 793-801.
- Offermann MK, Faller DV. Autocrine induction of major histocompatibility complex class I antigen expression results from induction of beta interferon in oncogene-transformed BALB/c-3T3 cells. Mol Cell Biol. 1989; 9: 1969–1977.
- Cole LA, Dai D, Butler SA, Leslie KK, Kohorn EI. Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG-regulated neoplasia. Gynecol Oncol. 2006; 102: 144-149.
- Cole LA, Khanlian SA, Riley JM, Butler SA. Hyperglycosylated hCG (hCG-H) in Gestational Implantation, and in Choriocarcinoma and Testicular Germ Cell Malignancy Tumorigenesis. J Reprod Med. 2006; 51: 919-929.
- Iles RK. Ectopic hCGß expression by epithelial cancer: Malignant behavior metastasis and inhibition of tumor cell apoptosis. Molec Cellul Endocrinol. 2007; 260; 264-270.
- Jankowska A, Gunderson SI, Warchol JB. Reduction of hCGß subunit expression by modified U1 snRNA caused apoptosis in cervical cancer cells. Molec Cancer. 2008; 7: 26-29.
- Li D, Wen X, Ghali L, Al-Shalabi F, Purkis P, et al.. hCG β expression by cervical squamous carcinoma-in vivo histological association with tumour invasion and apoptosis. Histopathol. 2008; 53: 147-155.
- Gillott DJ, Iles RK, Chard T. The effects of beta-human chorionic gonadotrophin on the in vitro growth of bladder cancer cell lines. Br J Cancer. 1996; 73: 323-326.
- Guo X, Liu G, Schauer IG, Yang G, Mercado-Uribe I, Yang F, Zhang S, He Y, Liu J. Overexpression of the β Subunit of Human Chorionic Gonadotropin Promotes the Transformation of Human Ovarian Epithelial Cells and Ovarian Tumorigenesis. American Journal Pathology. 2011; 179: 1385–1393.
- Neto IB, Ribeiro de Aparecida SM, Correa de Noronha SAA, Wolgien MCG, Barros AJ, Nakaie CR, et al. Angiotensim (1-7) and human chorionic gonadotropin (hCG) modulate nuclear transcription factors or nuclear receptors genes in the tumorigenic undifferentiated breast cancer cell line SKBR3. J Cancer Ther. 2013; 4: 70-74.
- Khare P, Bose A, Singh P, Javed S, Jain SK, Singh O, Pal R. Gonadotropin and tumorigenesis :Direct and indirect effects on inflammatory and immunosuppressive mediators and invasion. Molec Carcinogen. 2016.
- Cole LA, Butler SA. B152 anti-hyperglycosylated human chorionic gonadotropin free β-Subunit. A new, possible treatment for cancer. J Reprod Med. 2015; 60: 13-20.
- JL Vaitukaitis, GD Braunstein, G Ross. A radioimmunoassay which specifically measures human chorionic gonadotropin in the presence of human luteinizing hormone. Amer. J. Obstet. Gynecol. 1972; 113: 751-758.
- Cole LA. Chapter 29. Antibodies and hCG tests. In: HCG (hCG), second edition. Ed: Cole LA, Butler SA. Elsevier, Burlington MA, 2014.
- Cole LA, Sutton JM, Higgins TN, Cembrowski GS. Between-Method Variation in hCG Test Results, Clin Chem. 2004; 50: 874-882.
- Cole LA, DuToit S, Higgins TN. Total hCG tests. Clin Chim Acta. 2011.
- Cole LA, DuToit S, Higgins TN. Total hCG tests. Clin Chim Acta. 2011.
- Birken S, Krichevsky A, O’Connor J, Schlatterer J, Cole LA, Kardana A, et al. Development and Characterisation of antibodies to a nicked and hyperglycosylated form of hCG from a choriocarcinoma patient. Endocrine J. 1999; 10: 137-144.