
Special Article - Endometriosis
Austin J Obstet Gynecol. 2016; 3(3): 1062.
Deep Infiltrating Endometriosis of the Sigmoid Colon Masquerading as Colon Cancer
Sood N¹, Dhanani M², Santoni C², Landmann RG³, Geiger XJ4 and Dinh TA2*
¹Department of Obstetrics and Gynecology, Flushing Hospital Medical Center, Flushing, New York, USA
²Department of Medical and Surgical Gynecology, Mayo Clinic, Jacksonville, Florida, USA
³Department of Surgery, Mayo Clinic, Jacksonville, Florida, USA
4Department of Pathology, Mayo Clinic, Jacksonville, Florida, USA
*Corresponding author: Dinh TA, Department of Medical and Surgical Gynecology, Mayo Clinic, Jacksonville, Florida, USA
Received: September 30, 2016; Accepted: November 17, 2016; Published: November 19, 2016
Abstract
Superficial endometriosis involving the serosa of the colon is not an uncommon presentation. However, Deep Infiltrating Endometriosis (DIE) involving more than 5mm of the peritoneal surface reaching up to the muscularis propria and submucosa is rare. Further DIE causing luminal stricture and colonic obstruction occurs in less than 1.7% of the cases. We report a case of a forty six year old woman who initially presented for evaluation of cyclical abdominal pain. Radiologic and endoscopic investigations were suggestive of a malignancy. She underwent surgery including laparoscopic low anterior resection of sigmoid colon with side-to-end coloproctostomy and bilateral salpingo-oopherectomy. The histopathology revealed multiple endometriotic implants involving the submucosa, muscularis propria and serosa of the sigmoid colon. A left ovarian endometrioma was also identified. Our case highlights the diagnostic challenge in establishing an accurate pre-operative diagnosis and differentiates DIE from colon cancer. A multidisciplinary team approach with a combination of medical and surgical interventions can achieve effective therapy.
Keywords: Deep infiltrating endometriosis; Colon cancer
Case Presentation
A forty six year old gravida two Caucasian female with medical history of essential hypertension presented to our clinic for the management of abdominal pain for five months. She underwent a laparoscopic assisted vaginal hysterectomy, for pain and irregular bleeding, eight months prior to initial presentation. The pathology from the hysterectomy specimen showed benign endometrium with adenomyosis. Postoperatively, she experienced cyclical pain every 28 days, moderate in intensity and localized to the left lower quadrant of the abdomen. There was no history of dyspareunia, dyschezia, hematochezia or hematuria. She denied any weight loss or fever. Her physical examination was unremarkable except for a BMI of 31.7kg/ m2. A left sided pelvic mass was seen on CT abdomen and pelvis. CT colonography revealed an apple core lesion measuring 3.3cm in the mid-sigmoid region with severe stenosis of the lumen, suggestive of malignancy (Figure 1). Colonoscopy demonstrated a severe stenosis 25cm above the anal verge. Biopsies were inconclusive with nonspecific reactive changes of the colonic mucosa. Her serum CEA level was 0.6.
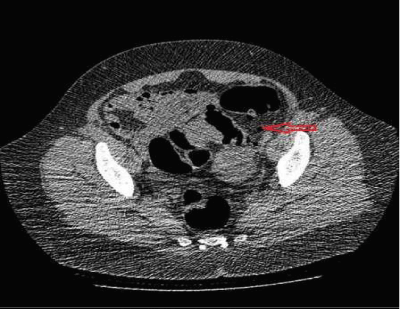
Figure 1: Pre-operative CT colonography shows “apple core” lesion
within mid-sigmoid, measuring 3.3cm with severe stenosis of the lumen.
Diverticulosis of the sigmoid seen. No extra colonic findings.
The presumptive clinical diagnosis was colonic malignancy. However, due to symptoms of cyclical pain a differential diagnosis of colonic endometriosis was also considered. The patient underwent bilateral ureteral stenting with laparoscopic low anterior resection of the sigmoid colon with side-to-end coloproctostomy at 12cm above the anal verge. Bilateral salpingo-oophorectomy was also performed. Intraoperatively, numerous endometriotic deposits and adhesions were noted in the pelvis. The left ovary was markedly enlarged and filled with chocolate colored fluid, consistent with an endometrioma. The right tube and ovary appeared normal. A purplish mass was seen on the sigmoid colon suggestive of endometriosis.
On gross examination, a 3.5cm x 2.5cm dark red puckered, indurated luminal stricture measuring 4.0cm in circumference was noted in the resected sigmoid colon specimen. The wall of the stricture area measured 1.5cm in thickness (Figure 2). The histopathology revealed multiple endometriotic implants involving the submucosa, muscularis propria and serosa. There was no evidence of malignancy (Figure 3a, 3b). Left ovary measured 3.4cm x 3.0cm x 1.7cm with a 2.4cm unilocular ovarian endometrioma. Left fallopian tube, right ovary and fallopian tube were unremarkable. A final diagnosis of deep infiltrating sigmoid endometriosis was made. Postoperatively, the patient recovered well and has been symptom free for 18 months. She was started on estrogen replacement therapy for postmenopausal symptom relief without complications.
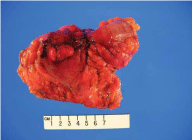
Figure 2: Resected sigmoid colon with central maroon puckered
indurated area, hemorrhagic serosal implants and serosal adhesions.
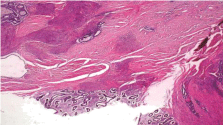
Figure 3a: Serosal endometriotic glands and stroma implants (bottom
center) with multiple deep muscularis propria endometriosis foci. There is
surrounding hyperplastic smooth muscle and serosal fibrosis (H&E, original
magnification X20).
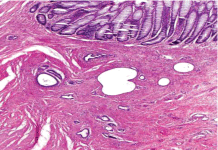
Figure 3b: Unremarkable colonic mucosa (top) overlying endometriotic foci
within the submucosa and muscularis with resultant submucosal expansion
and muscularis hypertrophy (H&E, original magnification x 100).
Discussion
Endometriosis is a progressive and benign estrogen-dependent disease defined by the presence of endometrial tissue (glands and stroma) outside the uterine cavity. First described in 1860 by Von Rokitansky, it is present in up to 10% of women in reproductive age group [1]. Extra pelvic endometriosis is seen in 3.8% to 37% of all cases [2]. In 5.3% to 12% of these women, endometriosis involves the bowel [3].
The recto-sigmoidal junction is the most common site involved (65.7%) followed by sigmoid colon (17.4%). Cecum with the ileocecal junction (4.1%), appendix (6.4%), small bowel (4.7%) and omentum (1.7%) are the other sites described [4].
Various theories proposed in the pathogenesis of endometriosis indicate that the etiology is complex and multifactorial, involving retrograde menstruation, lymphatic dissemination, immune dysfunction and coelomic metaplasia. The endometrium and peritoneal mesothelium being derivatives of the same coelomic wall epithelium explains the latter theory. Iatrogenic deposition of endometrial tissue following gynecologic procedures and caesarean sections may also explain occurrence of colonic endometriosis. In the current case, the patient underwent laparoscopic hysterectomy eleven months prior to the diagnosis of DIE [5].
Colonic involvement could either be superficial or deep (DIE). The latter, also known as adenomyosis externa, is defined as endometriosis located more than 5mm beneath the peritoneal surface [6]. Several classification systems have been attempted to explain the phenotypes of DIE based on the depth of invasion as well as anatomical site of the deposits [7-9].
The symptoms of intestinal endometriosis vary according to the site of involvement. The common symptoms are rectal bleeding and pain, altered bowel habits, abdominal bloating and cramping, most of which tend to get worse during menstruation [10]. However, DIE presenting as luminal stenosis and colonic obstruction is rare (1.7%) [11]. The lack of pathognomonic signs makes the diagnosis difficult. This often leads to a pre-operative diagnostic challenge to differentiate intestinal endometriosis from malignant tumors, inflammatory bowel disease or ischemic colitis.
The optimal manner for identification of intestinal endometriosis is by direct visualization of the implants at surgery. Radiologic and endoscopic examinations, though not diagnostic, are essential preoperative evaluations. Computerized Tomography (CT) colonography and Magnetic Resonance Imaging (MRI) are highly sensitive tests for the detection of DIE, the former having a higher overall diagnostic accuracy (area under the curve, 0.786 vs 0.691; P < 0.001). Changes in lumen caliber with bowel wall thickening and mass formation have been shown to significantly correlate with morphologic changes in rectosigmoid endometriosis [12]. Colonoscopy usually reveals an eccentric wall thickening, luminal stricture with or without surface nodularity. A biopsy of a suspicious lesion may be done. However histologic diagnostic yield could be as low as 47% since DIE is usually limited to the serosa or muscularis propria and the biopsy specimen usually consists of mucosal tissue [13]. Recto-sigmoid endoscopic-ultrasound has recently been shown to have comparable sensitivity to MRI for evaluation of rectal and sigmoid infiltrates and depth of infiltration [14,15].
To achieve the best therapeutic result, a multidisciplinary treatment team consisting of a gynecologist, colorectal surgeon and urologist, is ideal. The choice of the surgical intervention depends on the site and depth of bowel infiltration. It can range from shaving (superficial peeling of serosa with laser or diathermy), to superficial excision (selective removal of implants without entering the bowel mucosa). More deep involvement of muscularis propria, submucosa and mucosa are treated by full thickness disc excision (excision of the implants with opening, followed by closure of the bowel wall) or bowel resection and anastomosis [16]. Minimally invasive treatment options are favored over open surgery, enhancing recovery and patient outcome.
Adjuvant therapy has been proven to decrease mean pain scores and lesion size with improved quality of life [17]. Combination regimen with progestin is more accepted and better tolerated than GnRH analogues. Women who underwent radical surgical treatment for severe endometriosis had significantly increased median time to symptom recurrence with a combination of aromatase inhibitors with either a progestin or gonadotropin-releasing hormone (GnRH) analogue [18].
Conclusion
Deep infiltrating endometriosis of the colon benefits from a multidisciplinary treatment approach. Aggressive surgical and medical management is recommended for optimal resolution of symptoms. DIE should be considered in the differential diagnosis of presumed malignant colonic strictures in a premenopausal woman with cyclical gastrointestinal symptoms.
References
- Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 2004; 18: 177-200.
- Remorgida V, Ferrero S, Fulcheri E, Ragni N, Martin DC. Bowel endometriosis: presentation, diagnosis, and treatment. Obstet Gynecol Surv. 2007; 62: 461-470.
- Zanetti-Dallenbach R, Bartley J, Muller C, Achim Schneider, Christhardt Köhler. Combined vaginal-laparoscopic-abdominal approach for the surgical treatment of rectovaginal endometriosis with bowel resection: a comparison of this new technique with various established approaches by laparoscopy and laparotomy. Surg Endosc. 2008; 22: 995-1001.
- Chapron C, Chopin N, Borghese B, Foulot H, Dousset B, Vacher-Lavenu MC, et al. Deeply infiltrating endometriosis: pathogenetic implications of the anatomical distribution. Hum Reprod. 2006; 21: 1839-1845.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014; 2014: 179515.
- Cornillie FJ, Oosterlynck D, Lauweryns JM, Koninckx PR. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil Steril. 1990; 53: 978-983.
- Koninckx PR, Martin D. Treatment of deeply infiltrating endometriosis. Curr Opin Obstet Gynecol. 1994; 6: 231-241.
- Donnez J, Nisolle M. Advanced laparoscopic surgery for the removal of rectovaginal septum endometriotic or adenomyotic nodules. Baillieres Clin Obstet Gynaecol. 1995; 9: 769-774.
- Chapron C, Fauconnier A, Vieira M, Barakat H, Dousset B, Pansini V, et al. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod. 2003; 18: 157-161.
- Machairiotis N, Stylianaki A, Dryllis G, Zarogoulidis P, Kouroutou P, Tsiamis N, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol. 2013; 8: 194.
- Ruffo G, Crippa S, Sartori A, Partelli S, Minelli L, Falconi M. Management of rectosigmoid obstruction due to severe bowel endometriosis. Updates Surg. 2014; 66: 59-64.
- Jeong SY, Chung DJ, Myung Yeo D, Lim YT, Hahn ST, Lee JM. The usefulness of computed tomographic colonography for evaluation of deep infiltrating endometriosis: comparison with magnetic resonance imaging. J Comput Assist Tomogr. 2013; 37: 809-814.
- Kim KJ, Jung SS, Yang SK, Yoon SM, Yang DH, Ye BD, et al. Colonoscopic findings and histologic diagnostic yield of colorectal endometriosis. J Clin Gastroenterol. 2011; 45: 536-541.
- Bazot M, Bornier C, Dubernard G, Roseau G, Cortez A, Daraï E. Accuracy of magnetic resonance imaging and rectal endoscopic sonography for the prediction of location of deep pelvic endometriosis. Hum Reprod. 2007; 22: 1457-1463.
- Philip CA, Bisch C, Coulon A, de Saint-Hilaire P, Rudigoz RC, Dubernard G. Correlation between three-dimensional rectosonography and magnetic resonance imaging in the diagnosis of rectosigmoid endometriosis: a preliminary study on the first fifty cases. Eur J Obstet Gynecol Reprod Biol. 2015; 187: 35-40.
- Meuleman C, Tomassetti C, D'Hoore A, Van Cleynenbreugel B, Penninckx F, Vergote I, et al. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update. 2011; 17: 311-326.
- Nawathe A, Patwardhan S, Yates D, Harrison GR, Khan KS. Systematic review of the effects of aromatase inhibitors on pain associated with endometriosis. BJOG 2008; 115: 818-822.
- Ferrero S, Venturini PL, Gillott DJ, Remorgida V. Letrozole and norethisterone acetate versus letrozole and triptorelin in the treatment of endometriosis related pain symptoms: a randomized controlled trial. Reprod Biol Endocrinol. 2011; 9: 88.