
Special Article – In Vitro Fertilisation
Austin J Obstet Gynecol. 2016; 3(3): 1063.
What is Implantation Failure after In Vitro Fertilization and Embryo Transfer?
Coulam CB*, Goodman C, Chapman C and Rinehart JS
Reproductive Medicine Institute, USA
*Corresponding author: Coulam CB, Reproductive Medicine Institute, Chicago, IL, USA
Received: October 10, 2016; Accepted: November 23, 2016; Published: November 25, 2016
Abstract
Problem: Recurrent Implantation Failure (RIF) is a major cause of failure to achieve pregnancy after In Vitro Fertilization (IVF) and embryo transfer treatment cycles. However, no formal criteria defining the number of failed cycles or the total number of embryos transferred in these IVF attempts exist.
Methods: From January 1, 2014, and June 30, 2015, 644 women underwent IVF or embryo transfer procedures involving 2093 embryos. 427 women had positive HCG tests 7 days after transfer of 1364 embryos, resulting in 381 Embryonic Cardiac Activity (ECA) demonstrated by 6 weeks gestation. Of the 381 positive ECA, 55 had Preimplantation Genetic Screening (PGS) and 326 had no PGS. The percentage of pregnancies with demonstrable ECA was determined for each number of embryos transferred, and cumulative pregnancy rates for those undergoing PGS and not undergoing PGS were computed. In addition, the cumulative pregnancy rates were stratified by maternal age.
Results: The total number of embryos transferred to achieve 80% of pregnancies with FHT is 4. The rate of ECA after 1 embryo transferred double from 13% to 29% with PGS. Whereas, after a cumulative of 4 embryos transferred the rate of ECA was 75% amongst PGS and 82% with no PGS. When stratified by maternal age, older women benefited most by PGS.
Conclusion: The total number of embryos transferred to achieve 80% of pregnancies with ECA is 4. After a cumulative of 4 embryos transferred, PGS does not increase probability of successful pregnancy except in women over age 40 years.
Keywords: Recurrent implantation failure; Definition; Cumulative number of embryos transferred
Introduction
Recurrent Implantation Failure (RIF) occurs when transferred embryos fail to implant following a number of In Vitro Fertilization (IVF) treatment cycles [1,2]. However, there are no formal criteria defining the number of failed cycles or the total number of embryos transferred in these IVF attempts. As a result different fertility centers practicing IVF use different definitions for RIF [1-4] making it difficult to identify individuals who would be expected to continue to experience failure of implantation. Since the processes of implantation are complex, assessing causes of RIF can involve substantial resources. Thus, identifying individuals most likely to be benefited from such investigations would be desirable.
Successful implantation is a complex process involving two main players- a functional embryo at the blastocyst stage and a receptive endometrium [5]. Synchronized cross-talk between embryo and endometrium is necessary for apposition, attachment and invasion of embryos leading to successful implantation [6]. The mechanisms involved in the cross-talk require many mediators originating in the embryo, as well as in the endometrium [6-9]. Chromosomal abnormalities occurring within the embryo have been shown to account for up to 60% of RIF [10]. To answer the question “What is implantation failure in our practice?” outcomes of unselected consecutive IVF procedures performed between January 1, 2014 and June 30, 2015 were analyzed to determine the cumulative number of embryos needed to achieve 80% probability of pregnancy with demonstrated Embryonic Cardiac Activity (ECA). IVF outcomes after transfer of embryos that had undergone Preimplantation Genetic Screening (PGS) were compared with those not having PGS.
Materials and Methods
From January 1, 2014, and June 30, 2015, 644 women underwent IVF or embryo transfer procedures involving 2093 embryos. 427 (66%) women had positive HCG tests 7 days after transfer of 1364 embryos, resulting in 381 (59% of patients, 89% of positive HCG) ECA demonstrated by 6 weeks gestation. Of the 381 positive ECA, 55 had Preimplantation Genetic Screening (PGS) and 326 had no PGS. The percentage of pregnancies with demonstrable ECA was determined for each number of embryos transferred, and cumulative pregnancy rates were computed. Cumulative pregnancy rates were compared between those embryos undergoing PGS and those not screened with PGS. In addition, cumulative pregnancy rates were stratified by maternal age. Differences were compared using ANOVA one way analysis of variance. Significance was defined as p<0.05.
Results
Cumulative rates to achieve fetal cardiac activity compared with total number of embryos transferred are shown in (Figure 1). The rate of ECA after 1 embryo transferred double from 13% to 29% with PGS. Whereas, after a cumulative of 4 embryos transferred the rate of ECA was 75% amongst PGS and 82% with no PGS. These differences in rate of positive ECA are significant at P=0.005. After a cumulative of 4 embryos transferred, PGS does not increase probability of successful pregnancy.
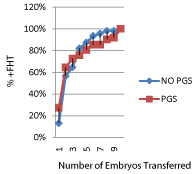
Figure 1: Cumulative rates (%) to achieve fetal cardiac activity (FHT)
compared with total number of embryos transferred.
Figure 2 shows pregnancy rates occurring in the total groups of 644 women stratified by maternal age. Pregnancy rates decreased with increasing maternal age (P=0.0009). When only those women who achieved pregnancy associated with demonstrable embryonic cardiac activity after receiving non PGS embryos were stratified according to age, the differences in pregnancy rates per number of embryos transferred was lost after 4 embryos were transferred (Figure 3). After a cumulative of 4 embryos was transferred, no significant differences in pregnancy rates were observed among the different age groups that received non PGS embryos (Figure 3a). When women who achieved pregnancy after PGS were stratified by age, only those over the age of 40 years had a significant increase in pregnancy rates after a cumulative of 4 embryos were transferred (P= 0.03) (Figure 3b).
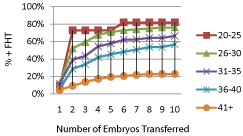
Figure 2: Pregnancy rates (%) to achieve fetal cardiac activity (FHT)
compared with total number of embryos transferred stratified by maternal
among the total of 644 patients involving 2093 embryos.
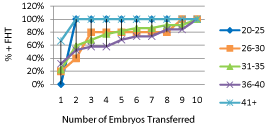
Figure 3a: The percentage of pregnancies with demonstrable FHT compared
for each number of embryos transferred, and cumulative pregnancy rates for
those undergoing PGS.
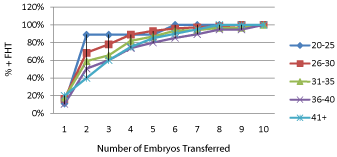
Figure 3b: The percentage of pregnancies with demonstrable FHT compared
for each number of embryos transferred, and cumulative pregnancy rates for
those undergoing not undergoing PGS.
Discussion
Recurrent implantation failure is a diagnosis generated by IVF. Knowing that an embryo was transferred to the uterus and not getting a rise in hCG has been referred to as implantation failure [1,2]. Since the process of implantation is complex [11], a number of steps in this process could lead to failure. There is no way to assure that the embryo actually was placed in the uterine cavity but the occurrence of aberrant placement is presumed to be rare. The process from embryo transfer to rising hCG and establishment of embryonic cardiac activity can be divided into embryo or uterine factors [5]. The most common reason an embryo would fail to implant is the high frequency of aneuploid embryos in morphologically normal embryos [10,12]. The development of PGS should reduce the incidence of implantation failure but even with morphologically normal euploid embryos the implantation rates remain between 50 - 70% per embryo [13-15]. At this point the problem could be simply a single event or one that is persistent. A single event that prevented implantation should be overcome by repeated embryo transfers. However for some individuals, the problem is persistent. The individual with persistent implantation failure will need to seek alternative treatments. The options include donor oocytes if the cause of the failure seems to be related to the embryo and gestational hosts for those whose problem seems to be uterine in nature. The issue for the clinician is to determine when it seems that the individual has persistent implantation failure. One approach is to define implantation failure by the number of embryos that have been transferred. The hypothesis is that at some point further embryo transfer will not result in implantation. The debate is what that number might be or should it be defined by number of cycles of IVF regardless of the number of embryos transferred. The current culture techniques permit more uniform quality of embryos to the blastocyst stage. Therefore, the total number of embryos transferred seems to be preferred as a way to identify persistent implantation failure. The addition of PGS further argues for using the number of embryos transferred as a means of identifying the patient with persistent implantation failure because PGS eliminates the major cause of recurrent implantation failure (aneuploidy) and thus the embryos are more equivalent in their pregnancy potential. Failure then calls into question the endometrium as a causative factor. As with many biological events, the frequency distribution of cumulative pregnancy rates vs number of embryos transferred follows a Poisson distribution with the tail to the right. This creates the problem of where to draw the line as to when the diagnosis of persistent implantation failure becomes highly probable. The limit for diagnosis could be set at almost any percentage and by convention most would chose the 95% cumulative implantation rate as the number of embryos transferred to make the diagnosis. But clinically, that may be too stringent of a criterion. For example, what if it became clinically useful to define permanent recurrent implantation failure at the 80% rate so that diagnostic testing could be implemented to identify the patient where further treatment would have a low chance of subsequent success. This would help the patient seek other forms of treatment in their pursuit of the resolution of their infertility issues.
Our data show the total number of embryos transferred to achieve 80% of pregnancies with ECA is 4. After a cumulative of 4 embryos transferred, PGS does not increase probability of successful pregnancy. While PGS helps select embryos for transfer that are most likely to result in live births [10-15], the more embryos that are transferred and pregnancy failure persists, the less likely the reason for failure is the embryo. Although the frequency of abnormal chromosome complement is increased in embryos with increasing maternal age [16], the overall has been reported around 50% [10]. Thus after 4 embryos are transferred, one would expect that statistically at least one of those embryos would have been chromosomally normal.
The odds of having at least one euploid embryo in assisted reproductive cycles are significantly decreased by increasing maternal age [16,17]. As a result, older women would be expected to benefit more from PGS. This fact is confirmed by data presented in Figure 3b which showed that only women over the age of 40 years had a significant increase in pregnancy rates after a cumulative of 4 embryos were transferred. Taken together, the current data suggest PGS should be used to make the diagnosis rather than evaluation of implantation failure. Individuals who have not achieved successful pregnancy after transferring 4 embryos should be evaluated for uterine factor contributing to implantation failure.
References
- Simon A, Laufer N. Assessment and treatment of repeated implantation failure (RIF). J Assist Reprod Genet. 2012; 29: 1227–1239.
- Shufaro Y, Schenker JG. Implantation failure, etiology, diagnosis and treatment. Int J Infertil Fetal Med. 2011; 2: 1–7.
- Tan BK, Vandekerckhove P, Kennedy R, Keay SD. Investigation and current management of recurrent IVF treatment failure in the UK. BJOG. 2005; 112: 773.
- Coughlan C, Ledger W, Wamg Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod BioMed Online. 2014; 28: 11114-11138.
- Psychoyos A. Hormonal control of ovoimplantation. Vitam Horm. 1973; 31: 201-256.
- Simon C, Martin JC, Pellicer A. Paracrine regulators of implantation. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000; 14: 815–826.
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Updat. 2006; 12: 731–746.
- Fukui A, Funamizu A, Yokota M, Yamada K, Nakamua R, Fukuhara R, et al. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol. 2011; 90: 105–110.
- Gonen-Gross T, Goldman-Wohl D, Huppertz B, Lankry D, Greenfield C, Natanson-Yaron S, et al. Inhibitory NK receptor recognition of HLA-G: regulation by contact residues and by cell specific expression at the fetal-maternal interface. PLoS One. 2010; 5: 8941.
- Scott RT, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013; 100: 697-703.
- Paria BC, Song H, Dey SK. Implantation: molecular basis of embryo-uterine dialogues. Int J Dev Biol. 2001; 45: 597-605.
- Lee H-L, McCulloh DH, Adler A, Ampeloquio E, Hodes-Wertz B, Grifo J. Preimplantation genetic screening (PGS) with vitrification reduces miscarriage and improves cumulative live birth compared to unscreened frozen embryos. Fertil Steril. 2014; 102: 173-174.
- Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012; 5: 24.
- Scott RT, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil Steril. 2013; 100: 697-703.
- Forman EJ, Tao X, Ferry KM, Taylor D, Treff NR, Scott RT. Single embryo transfer with comprehensive chromosome screening results in improved ongoing pregnancy rates and decreased miscarriage rates. Hum Reprod. 2012; 27: 1217-1222.
- Liu J, Wang W, Sun X, Liu L, Jin H, Li M, et al. DNA microarray reveals that high proportions of human blastocysts from women of advanced maternal age are aneuploid and mosaic. Biol Reprod. 2012; 87: 148.
- Ata B, Kaplan B, Danzer H, Glassner M, Opsahl M, Tan SL, et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod Biomed Online. 2012; 24: 614-620.