
Case Report
Austin J Obstet Gynecol. 2017; 4(1): 1070.
Askin Tumor in Pregnant Women
Lamine FZ¹*, Belaazri S¹, Lamine S², Kharbach A¹ and Baidada A¹
¹Department of Gynecology-Obstetric and Endoscopic Gynecology, Maternity Souissi, Rabat, Morocco
²Medical Service at the Royal Institute of Territorial Administration, Kenitra, Morocco
*Corresponding author: Lamine FZ, Department of Gynecology-Obstetric and Endoscopic Gynecology, Souissi Maternity, Mohammed V University, Rabat, Morocco
Received: June 05, 2017 Accepted: June 19, 2017; Published: June 26, 2017
Abstract
This is a case report of Askin’s tumor in a 22-year-old woman who is 14 weeks pregnant, and was treated with surgical resection without complementary radio-chemotherapy emphasizing the clinical, radiological and histological aspects of this tumor which remains of very poor prognosis.
Keywords: Askin’s tumor; Surgery; Pregnancy
Introduction
Askin’s tumor is a highly malignant rare tumor that develops from the soft parts of the chest wall, and occurs in young subjects. Our observations outline the peculiarities of this tumor, as well as issues involving diagnosis and therapy.
Case Presentation
A pregnant 22 year old female patient, with no history of pathology, with 14 weeks of amenorrhoea, had been suffering from chest pain for 3 months and dyspnea. On clinical examination, the patient was in good general condition, weighing 63kg with a height of 1.65m. The pleuro-pulmonary examination revealed a left basal mass. The peripheral lymph nodes did not objectify any adenopathy. There was no hepatomegaly or splenomegaly.
An enormous endothoracic opacity of the left base was found on the thoracic radiograph (Figure 1). The thoracic Computed Tomography (CT) revealed the nature of this opacity (Figure 2). Abdominal ultrasound eliminates diaphragmatic involvement, no liver damage, adrenals are free.
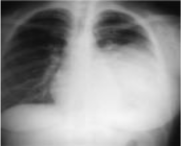
Figure 1: Thoracic radiograph: Enormous endothoracic left opacity.
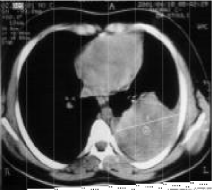
Figure 2: Thoracic CT scan: Left thoracic tumor process.
A left thoracotomy was performed, revealing a voluminous encephaloid mass at the level of the posterior cul-de-sac that completely adhered to the left lower lobe. Monobloc resection of this 8 x 8 x 5 cm tumor was performed. The pathology exam study (Figure 3) showed proliferating small cells with a minima eosinophilic cytoplasm and vesicular nuclei. Chromatin was dispersed with perinuclear reinforcement.
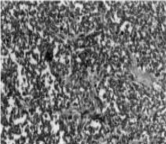
Figure 3: Ranges of tumor cells separated by a thin fibro-vascular stroma.
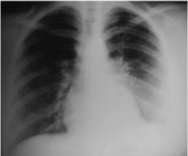
Figure 4: Post operatory thoracic radiograph.
Nuclei were sometimes nucleolated. These cells were arranged in diffuse areas furrowed by capillaries. Certain cells were arranged round these capillaries forming pseudorosettes. This aspect was very suggestive of a neuro-ectodermic tumor (Askin’s tumor). In the immunohistochemical study, the tumor cells strongly expressed synaptophysin, which is a neuron specific enolase. Askin’s tumors are known to express focal chromogranin, and low molecular weight cytokeratins.
The clinical course was simple since the thoracic radiography of control was normal (Figure 4). We considered it advisable to avoid any adjuvant treatment based on chemotherapy and radiotherapy, since this 22-year-old woman wanted a successful first pregnancy.
Discussion
Askin’s tumor was first described by Askin in 1979 [1], a rare, as highly malignant tumor originating from the soft parts of the chest wall and belonging to the neuro tumor group (PNET: Primitive Neuro-Ectodermal Tumor). It is seen in children, adolescents and young adults, with about 75% of cases occurring in women [2,3]. The histogenicity of this tumor remains uncertain [4,5], but it is most likely the result of migration of cells from the neural crest during embryogenesis. The detection of a translocation t (11,22) q (21,12), by Aurias and Turc-Carel in Ewing’s sarcoma and the same translocation in Askin’s tumor, neuro-epithelioma as well as in extra-skeletal Ewing’s sarcoma prompted the authors to include these tumors without the same denominator of Peripheral Neuro- Ectodermal Tumors (PNET) [6]. A genetic basis for the oncogenesis of these tumors is suspected because of the existence of multiple tumors of this type in members of the same family and the existence of several tumors affecting the same person [7,8].
Clinical and radiological aspects
The tumor is most often manifested as a thoracopulmonary mass, rarely in a respiratory symptomatology of chest pain, dyspnea in relation to a pleural effusion that is most often haemorrhagic. Sometimes, Claude-Bernard-Horner’s syndrome, or cervical and / or axillary adenopathies can be observed, especially in the case of mass expansions at the apex [9].
The Askin’s tumor does not have a radiological specificity, so it may take the form of another round cell tumor or metastasis. Imaging allows the dimensions of the tumor, the endo and exo thoracic extension (costal lysis, diaphragmatic invasion) and the existence or absence of a pleural effusion to be assessed. In standard radiography, this tumor is most often takes the form of a parietal opacity of a hemithorax, while in CT it assumes the form of a heterogeneous mass on the chest wall. Magnetic Resonance Imaging (MRI) is of great value in the assessment of mediastinal, pleural, intra-spinal and, especially, brachial plexus involvement in patients with associated pain in the higher limb [4]. CT and MRI are used to exclude parietal benign tumors.
Pathology anatomy
Macroscopically, these tumors assume the appearance of a graywhite mass, often round or ovoid, sometimes multil-obliquely, with a variable diameter of up to 15cm, sometimes containing sections of necrosis and haemorrhage.
Histological study allows diagnosis and shows an undifferentiated sarcomatous tissue with small round cells that must be distinguished from other malignant small cell tumors of the chest wall namely: non-Hodgkin’s malignant lymphomas, Ewing’s sarcoma, rhabdo - alveolar myosarcoma, neuroblastoma, and neuroepithelioma.
Some criteria that are important for the diagnosis of PNET are:
• The presence of Homer-Wright rosette,
• Originating from a peripheral nerve,
• Positivity for NSE or leu-7,
• The existence of cytoplasmic extensions, neurosecretory granules and microtubules,
• The translocation t (11,22) q (24,12),
• Detection of proto-oncogenes (N-myc, C-myb, C-ets-1),
• Activity of the biosynthetic enzymes of the neurotransmitters (thyrosine hydroxylase, dopamine B hydroxylase and acetylcholine transferase).
It takes 2-3 of these criteria to confirm a diagnosis of Askin’s tumor [10].
Treatment
The treatment of Askin’s tumors is not well established. Surgery remains the basis of treatment, as complete as possible, since the quality of the excision limits local recurrences which may involve the tumor, pleura, pericardium, diaphragm and vertebrae in cases of invasion.
Additional treatment based on radiotherapy and chemotherapy can be prescribed. Radiotherapy at variable doses between 3.5 and 5 rad and/or antimitotic chemotherapy may include several protocols based on: Cyclophosphamide, Actinomycin D, Vincristine, Methotrexate, 5Fluoro-Uracyl, Adriamycin, Dacarbazine, Tenoposide, and cisplatinum.
The treatment of bone and pulmonary metastases is based on radiotherapy [8].
Prognosis
Askin’s tumors have a poor prognosis, and thoracic recurrences are frequent. The mean survival is 8 months with little differences between treatments [1,4,11].
Metastases may extend to the lung, liver, adrenals, skeleton and sympathetic chains [9].
Conclusion
Rare malignant tumors, the treatment of Askin tumor remains difficult to codify, the vital prognosis is bleak because of local recurrences, and its metastatic potential despite surgical resection and radio-chemotherapy.
References
- Askin FB, Rosai J, Sibley RK, Dehnerl P, MC Alister WH. Malignant small cell-tumor of the thoraco-pulmonary region in childhood. A distinctive clinicopathologic entity of uncertain histogenesis. Cancer. 1979; 43: 2438-2451.
- Winer-muram HT, kauffman WH, Gronemeyer SA, Gregory JS. Primitive neuro-ectodermal tumors of the chest wall (Askin-tumors). CT and MR finding. AJR. 1993; 145: 265-268.
- Ablin DS, Azouz EM, Jain KA. Large intrathoracic tumors in children imaging finding. AJR. 1995; 165: 925-934.
- Burge H, Novotny D, Schieblesm, Delamy D, MC Karneyw. MRI of Askin tumor. Case report at 1,5 T. Chest. 1990; 97: 1252-1254.
- Coulomb M, Ferretti G, Ranchoup Y, Thonyf, Blanc F. Tumers neurogènes périphériques du thorax chez l’adulte. Cours de perfectionnement postuniversitaire. Journées francophones de radiologie. 1994.
- Mozabraud A. Sarcome d’Ewing, neuro-épithéliome et tumeur d’Askin: Anatomie pathologique osseuse tumorale: expérience personnelle. Springer- Verlag, Paris. 1994.
- Enzinger F, WeisssW. Primitive neuro–ectodermal tumors and related lesions. Soft tissus tumors. Mosby, 3e edition. 1995; 929-965.
- Kabiri H, EL fakiry, Mahassini N, Benamorj, S Alaziz, A Elmaslout, et al. Tumeur maligne thoraco–pulmonaire á petites cellules (tumeur d’Askin). Rev Pneumol Clin. 1999; 55: 21–25.
- Fink IJ, Kurtz DW, Cazenave L, Lieber MR, Miser JS, Chaudra R, et al. Malignant thoraco–pulmonary small-cell (Askin) tumor. AJR. 1985; 145: 517- 520.
- Marina NM, Ectubanas E, Parman DM, bourman C, green A. Peripheral primitive neuro-ectodermal tumor (peripheral neuro–epitheliuma). Children: a review of the Ste Jude experience and controversies in diagnosis and management. Cancer 1989; 64: 1952–1560.
- Barburen C, Haberman JJ, Zarhouni EA. Peripheral primitive neuroectodermal tumors. CT and MRI evaluation. Europ J Radiol. 1996; 21: 225– 232.