
Research Article
Austin J Obstet Gynecol. 2017; 4(3): 1081.
MCM7 Gene Expression versus Risk of Malignancy Index (RMI) for Prediction of Ovarian Malignancy
Samir L¹, Mansour A¹, Shaban E¹, Saed NEI², Hussein N¹, Yehia M¹ and ElSaed N¹*
¹Department of Medical Biochemistry and Molecular Biology, Ain Shams University, Egypt
²Department of Obstetrics and Gynecology, Ain Shams University, Abbassia, Cairo, Egypt
*Corresponding author: Nashwa ElSaed, Assistant Professor of Obstetric and Gynecology, Ain Sham University, Ain Shams University, Egypt
Received: November 05, 2017; Accepted: November 30, 2017; Published: December 07, 2017
Abstract
Minichromosomal Maintenance protein 7 (MCM7), a member of the MCM family of proteins critical to the DNA replication complex, has recently been shown to improve the sensitivity and specificity of Epithelial Ovarian Cancer (EOC) diagnosis but its function in Carcinogenesis is not clear. We evaluated MCM7 gene expression, RMI and CA125 serum level as diagnostic tools of primary ovarian cancer in Egyptian women. The MCM7 gene expression was evaluated by SYBR green Quantitative Real Time PCR (Q RT-PCR) in ovarian tumor tissues of 50 Egyptian women. 25 malignant and 25 benign tumor tissues were studied. Serum Human cancer antigen 125 (CA 125) was measured in the serum of all participants of the study using immune sorbent assay (ELISA). The MCM7 showed significant difference among ovarian malignant tumors patients compared to the control subjects (p< 0.01). Absolute sensitivity of MCM7 & 96% specificity were found with the best cutoff value at 1.96 .There was a significance correlation between MCM7 with RMI and with CA125 in all patients of the study (p<0.01 for both). The mRNA expression of MCM7 was positively correlated with the progression of the stage & grade of the tumor (p <0.01) for both.
Keywords: Ovarian Cancer; RMI; MCM7; Real time PCR; CA125
Introduction
Ovarian cancer is highly metastatic and is the leading cause of death amongst all gynecological malignancies [1] and it is the fifth most common cause of cancer-related death among women [2]. This is because the majority of women are diagnosed lately. Worldwide, ovarian cancer has been estimated to affect 225 500 women with Global mortality of this cancer remains high, with 140,200 deaths per year [3]. Global mortalityofthis cancer remains high and minimal improvement in mortality has been observed over the past decade [4]. This poor overall survival is mainly due to late diagnosis, disease recurrence and resistance to standard platinum-based chemotherapy [2]. The 5-year survival rate of more than 70% of patients with advanced-stage EOC is only 35% [5].
Different modalities were either tried to predict malignant ovarian masses depending on ultrasound findings of the mass, menopausal state and & some biomarkers such as CA125 either separately or combined [6]. The major limitation of CA125 is that it may be high in benign disease, such as endometriosis, ovarian cysts, and pelvic inflammatory disease [7]. The combination of serum levels of CA125 with menopausal status, other tumor markers, and ultrasound parameters increases the discriminating power of this method between benign and malignant pelvic masses [8].
In 1990, Jacobs and his colleagues originally developed the Risk of Malignancy Index (RMI) [9]. The Risk of Malignancy Index (RMI) is a simple scoring method based on menopausal status, ultrasound findings, and the serum CA125 level. This method has given significantly better results than the use of a single parameter. The RMI is the product of the Ultrasound scores (U), the Menopausal score (M), and the absolute value of the serum CA125RMI = U × M × CA125 [9]. This score has long been used for prediction of malignancy in ovarian masses. Sensitive and specific screening test that could detect ovarian cancer at a curative stage has yet to be developed.
Minichromosomal maintenance (MCM) proteins play an important role in DNA replication, they are related to cell proliferation, and serve as useful markers for cancer screening, surveillance, and prognosis. They are encoded by genes which are parts of the MCM genes from MCM 2-7 which have hexametric structure [10]. MCM proteins are involved in two critical steps in DNA synthesis: the first step is DNA replication initiation mediated by hexametric MCM complexes at replication origins, and the second step is DNA elongation mediated by MCM helicase activities [11]. MCM7 can be used as a prognostic indicator in various cancers, such as endometrial cancer [12], prostate cancer [13], neuroblastoma [14], colorectal cancer [15] and small cell lung cancer [16]. In endometrial, prostate, colon, and lung cancer a high MCM7 labeling index has a worse prognosis in cumulative overall survival [11] thus, MCM7 is a useful marker for predicting disease prognosis.
The aim of this study is to ascertain MCM7 clinical utility to do a comprehensive assessment of MCM7 protein expression in benign and malignant ovarian tissues by real time PCR and its relation to CA125 and RMI that may have predictive and therapeutic impact on patients.
Materials and Methods
Participants
This case-control study was conducted on 50 Egyptian female patients with established diagnosis of ovarian mass who were admitted A in Shams University Maternity Hospital, gynaecologic outpatient clinicfrom October, 2012-February, 2014 and written consent was taken from patients.All women gave their informed consent to participate in the study, which was approved by the Research Ethics Committee of A in Shams University, Faculty of Medicine. All participants in the present study were planned for surgical intervention for removal or exploration of an ovarian mass. None of them was pregnant or has malignant tumor other than ovarian tumor.
Five milliliter Fasting blood samples were collected from all the participants, To obtain and clarify serum, samples were left to stand at room temperature for at least 30min to allow the blood to clot and then centrifuged at 2000rpm for 15min and aliquoted to be analyzed according to manufacturer’s protocols of Abcam Cancer Antigen CA125 Human ELISA Kit (ab108653) supplied from Abcam Incorporation USA, that used for the measurement of human CA125, The minimum detectable concentration of CA125 by this assay is estimated to be 5UmL-1. The samples that exceeded the reading of highest standard were further diluted 2 times; absorbance value was read at 450nm within 15min.
Studied individuals were classified into two main groups
Group A: Twenty five cases with malignant ovarian lesions (mean age 49.12±15.034) 80% were epithelial and 20% were stromal tumors, 32% were low Grade (Gx and G1) and 68% were high grade (G2 and G3), 60% were early stage (I and II) and 40% were late stage (III).
Group B: Twenty five cases with benign ovarian lesions as a control group (mean age 43.36±12.065).
All patients of the study were subjected to complete detailed history taking, general and local examination, radio-diagnostic investigations as pelvic ultrasonography (us) and all patients were subjected to surgery for excision of the tumor mass. Then, tumor samples were sent for pathological staging and grading according to (TNM) classification. Clinical staging of the disease was done according to TNM classification [17].
Tissue samples
Human ovarian tumor tissue samples (both benign and malignant) were obtained directly at the operating theater in a Petri dish on ice. These were selected to be representative of the tumor. Blood was washed by ice cold saline. The fat, necrotic tissue, skin and muscle tissue were rapidly dissected from tissue of interest. The tissue samples were wrapped in aluminum foil and immediately were chilled on ice for further RNA extraction.
RNA extraction
The RNA extraction of all samples was done by TRIzol® Reagent manufactured by Life Technologies Corporation, Carlsbad, California, which was based on a modified salt precipitation procedure in the presence of highly effective RNase inhibitors and was kept at -80°C till its use for q-Real Time PCR of MCM7 and Glyceraldehyde 3P dehydrogenase (GABDH) as a house keeping gene for tissue samples.
RNA quality and quantity in μgμL-1
It was then determined using an Ultraspec 1000, UV/visible spectrophotometer (Amersham Pharmacia Biotech, Cambridge, UK).
Reverse transcription
Reverse transcription was performed using QuantiTect® Reverse Transcription kit manufactured by (QIAGEN, Germany). It was used for cDNA synthesis with integrated removal of genomic DNA contamination, for use in real-time two-step RT-PCR.
Relative quantitative real time PCR (q-real time PCR)
The volume of the first-strand reaction was brought to 20μL with RNAase free water and template cDNA (1μg/reaction) were amplified on an iCycler (Bio-Rad) using 10μL 2x QuantiTect SYBR Green PCR Master Mix and 2μL of the gene-specific oligonucleotide primers. All PCRs were done by initial activation step at 95°C for 15min followed by 45 cycles of 15, 30 and 30 sec at 95, 50 and 72°C, respectively. Bio-Rad software was used to calculate threshold cycle (Ct) values for all target genes and for the reference gene Glyceraldehyde 3P dehydrogenase (GABDH). The expression values for the tumor samples are presented as fold expression in relation to the control sample, the actual values were calculated using the 2-ΔΔCT equation: Then calculation of the relative quantification (RQ) or fold change is done by the following equation:
Statistical analysis
The data was expressed as median and independent samples Mann-Whitney or Kruskall Wallis Test. Spearman’s rho correlation was used to explore the relationship between MCM7, CA 125 and RMI among the groups of the study. The threshold value for optimal sensitivity and specificity of MCM7, CA 125 and RMI were determined by Receiver Operating Characteristics (ROC) curve. The cutoff value that maximized the sum of sensitivity and specificity was chosen for discrimination between benign and malignant groups. All statistical analysis were performed using the software package SPSS for Windows, version 15.0 (SPSS Inc., Chicago, Illinois). Significant p-value considered when it is #0.05.
Results
Concerning the comparison between malignant and benign groups as regards the demographic data and clinical characteristics, there is statistical significant difference between the two groups related to menstrual state and US score (p>0.05). Histopathological findings of the malignant group were analyzed, Epithelial Ovarian Cancer (EOC) were 20 samples (80%) and other types where 5 samples (20%), the low grade EOC samples were 5 (25%) and high grades where 15 (75%) early stages EOC were 10 samples (50%) and late stage 10 (50%) there was no statistical association between the grade and stages of the cancer group data not shown.
Figure 1 shows the Quantitative real time PCR for MCM7, and ROC Curves analyses of the 3 markers. The best cutoff points was calculated by ROC curve to discriminate the malignant and benign cases, the best cut off point was 1.96 for MCM7, 6.05UMl-1 for CA 125 and 9.65 for RMIas shown in (Figure 2). As regards MCM7 positivity rate, MCM7 mRNA was=cutoff value in 100% (25/25) of the malignant group and in 4% (1/25) of the benign group with highly significant difference between the two groups (p< 0.01) as in Table 1. There is no significant difference between the expressions of MCM7 in relation to the different clinicopathological factors in malignant groups of the study as shown in Table 2. The MCM7 expression positivity rate by RT-PCR analysis in both groups of the study in relation to different clinicopathological factors is shown in Table 3. Expression of MCM7 in Ovarian tissue samples from Malignant group as measured by q-real time PCR showed 100% sensitivity, 96% specificity and after its combination with RMI the sensitivity reached to absolute value with 98% accuracy and 96.15% PPV as shown in Table 4. The expression of ovarian MCM7 was positively correlated with CA125 and RMI with high significance (p<0.01) in Table 5.
MCM7 expression
Positive
Negative
c2
(=1.96)
(<1.96)
(P)
Malignant (25)
25
0
46.154
100%
0%
(0.00)**
Benign (25)
1
24
4.00%
96%
**p < 0.01: is highly significant
Table 1: Positivity rate of MCM7 gene expression measured by real time PCR (No of cases = cut off value) in all groups of the study.
Clinicopathological factors
Median
Range
Mean
c2
(no.)
rank
(P)
Parity
NP (No.=10)
96.945
47.67-645.83
10.25
2.331
MP (No.=15)
277.24
44.17-1612.41
14.83
(0.13)
Breast F
Positive (No.=10)
298.96
44.17-1612.41
15.1
1.359
Negative (No.=15)
157.04
47.67-645.83
11.6
(0.24)
M.S
Premenopausal
134.82
47.67-1239.03
11.63
0.807
(No.=12)
(0.37)
Postmenopausal
277.24
1568.24-1612.41
14.27
(No.=13)
F.H
Positive (No.=4)
231.995
81.86-341.32
15.25
0.446
(0.5)
Negative (No.=21)
157.04
44.17-1612.41
12.57
Smoking
Smokers (No.=3)
341.32
283.07-1239.03
20.83
3.931
Nonsmokers (No.=12)
174.855
44.17-645.83
12.29
(0.14)
Passive Smokers (No.=10)
174.25
1612.41-1564.74
11.5
OCT
Past administration (No.=6)
231.995
44.17-320.68
13.42
0.025
Never (No.=19)
157.04
47.67-1612.41
12.87
(0.87)
US
US=1(No.=9)
94.68
47.67-1612.41
12.61
0.039
US=3(2-5) (No.=16)
174.855
44.17-1239.03
13.22
(0.84)
MS: Menopausal State; FH: Family History; OCT: Oral Contraception; US: Ultrasound Score.
Table 2: MCM7 gene expression as measured by real time PCR in relation to clinicopathological factors in malignant group only.
Clinico-
MCM7
c2
pathological factors
(P)
(No.)
Positive
Negative
(=1,96)
(<1,96)
Parity
NP (No.=17)
10(38,5%)
7(29,2%)
3.607
MP (No.=33)
16(61,5%)
17(70,8%)
(0.058)
Breast F
Positive (No.=25)
11(42.3%)
14(58.3%)
1.282
Negative (No.=25)
15(57,7%)
10(41.7%)
(0.258)
M.S
Premenopausal (No.=12)
13(50%)
18(75%)
3.311
Postmenopausal (No.=13)
13(50%)
6(25%)
(0.069)
F.H
Positive (No.=5)
5(19.2%)
9(37.5%)
2.066
Negative (No.=20)
21(80.8%)
15(62.5%)
(0.151)
Smokers (No.=2)
3(11.5%%)
2(8.3%%)
1.241
Smoking
(0.538)
Nonsmokers (No.=13)
13(50.0%)
9(37.5%)
Passive Smokers (No.=10)
10(38.5%)
13(54.2%)
OCT
Past administration (No.=4)
7(26.9%%)
8(33.3%%)
1,131
Never (No.=21)
19(73.1%)
16(66.7%%)
(0.288)
US
US=0(No=20)
1(3.8%)
19(79.2)
33,316
US=1(No.=14)
9(34.6%)
5(20.8%)
(0,00)**
US=3(2-5) (No.=16)
16(61.6%)
0(0%)
Total
26
24
50
M.S: Menopausal State; FH: Family History; OCT: Oral Contraception; US: Ultrasound Score.
**p=0.01 is highly significant.
Table 3: The positivity rates of MCM7 in relation to different clinicopathological factors in all groups.
Parameter
Sensitivity
Specificity
PPV
NPP
Accuracy
MCM7
100%
96%
96.15%
100%
98%
RMI
100%
92%
92.59%
100%
96%
CA125
96%
84%
85.71%
95.45%
90%
Combined MCM7 &RMI
100%
96%
96.15%
100%
98%
Table 4: Sensitivity, specificity, predictive values and accuracy of detection of MCM7 gene by real time PCR in ovarian cancer.
Spearman's rho
CA125 (U/ml)
US Score
RMI
MCM7 real time PCR value
Correlation Coefficient
0.555
0.714
0.64
Significance
0.00**
0.00**
0.00**
no.
50
*: P value < 0.05 is significant; **: P value < 0.01 is highly significant by sperman correlation.
Table 5: Correlation between levels of MCM7 measured by real Time PCR & CA125 serum levels, US score and RMI in all groups of the study.
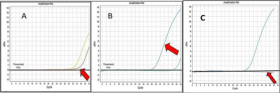
Figure 1: Amplification curves of MCM7 gene in different groups of the study was done by real time PCR.
Collective Amplification Curves of the q-real time PCR products of MCM7 Gene with its housekeeping gene (GABDH) in both Malignant and Benign Groups. The
transverse line indicates the threshold level.
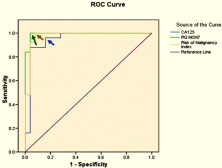
Figure 2: Combined ROC curve analysis for, serum CA125 (U/ml), RMI and
q-RT-PCR for MCM7 gene expression, in ovarian malignant group versus
Ovarian benign group. In CA125 curve the area under the curve was 0.94,
Arrow denotes cut off point at 6.05 U/ml at which CA125 sensitivity was
96% specificity was 84%. In RMI curve the area under the curve was 0.976.
Arrow denotes cut off point at 9.65 at which RMI sensitivity was 100 % and
specificity was 92%. In MCM7 CURVE the Area under the curve was 0.994.
Arrow denotes Cut off point at 1.96 at which MCM7 sensitivity was 100 % and
specificity was 96%.
Discussion
Ovarian cancer is the most lethal gynecologic malignancy [18]. The origin and pathogenesis of epithelial ovarian cancer (EOC) have long been investigated but still poorly understood [19]. Studies have shown that epithelial ovarian cancer is not a single disease but is composed of a diverse group of tumors that can be classified based on distinctive morphologic and molecular genetics features [20].
The mainstays of malignant cancer treatment are surgery, radiotherapy, and chemotherapy [21]. Despite progress in cancer therapy, ovarian cancer mortality has remained virtually unchanged over the past two decades [22]. Most of the patients are diagnosed at an advanced stage [2,19], and most of them have already peritoneal spread, with a 5-year survival rate of less than 30% so ovarian cancer is accepted as a “silent killer” [23].
Search is ongoing since many years for a novel, more sensitive, and more specific tumor marker or diagnostic algorithm to serve in the classification of patients with a pelvic mass and for screening in ovarian cancer. One of the promising markers is MCM7. MCM7 is one of the highly conserved Mini-Chromosome Maintenance proteins (MCM) that are essential for the initiation of eukaryotic genome replication [24]. MCM7 plays a pivotal role in the G1/S phase transition, orchestrating the correct assembly of replication forks on chromosomal DNA and ensuring that all the genome is replicated once and not more than once at each cell cycle [25].
The hexametric protein complex formed by the MCM proteins is a key component of the pre-Replication Complex (pre-RC) and involved in the formation of replication forks and in the recruitment of other DNA replication related proteins [26-27].
MCM expression represents cell cycle entry and is used as a proliferative marker superior to commonly use other proliferative markers, such as Ki67 and proliferating cell nuclear antigens (PCNAs) [28]. MCM7 is expressed in cells more than Ki67 or PCNAs, because it is expressed in cells licensed to proliferate in addition to those that are already proliferating [11].
As chemotherapy is most effective against proliferating cells, it is conceivable that MCM7 expression may indicate responsiveness to chemotherapy that may in turn affect progression-free survival [11].
So MCM7 is recently proposed as a novel potential biomarker to detect ovarian cancer cases. MCM7 expression can be used as the potential prognostic biomarker for disease recurrence or progressionfree survival in ovarian cancer [11].
The present study extended those findings by investigating MCM7 expression in human ovarian tumor tissues by quantitative real-time RT-PCR analysis, with combination between serum CA125 and RMI with MCM7 gene expression in ovarian malignancies that may be used as diagnostic tool and therapeutic target to defeat cancer cells.
In this study, there was no significant differences were found between MCM7 quantity and any of the studied clinicopathological factors (p>0.05) indicating that this protein is a good marker as it is not affected by any of the pathological factors. There was a highly significant difference between malignant and benign groups as regards expression of MCM7 gene by q-Real Time PCR (p<0.001). Using (1.96) as a cut off value for MCM7 Gene Expression measured by real time PCR area under the curve was 0.994, MCM7 sensitivity was 100% and specificity was 96%.
This results in agreement with Ota and his colleagues investigated MCM7 expression in epithelial ovarian carcinomas and correlated its expression with pathologic factors using two different MCM7 labelling indexes produced by a pathologist observer and by the automated cellular imaging system on tumor microarrays from 342 patients [11] that found (MCM7) expression is significantly higher in high-grade serous tumors compared with serous borderline tumors they also observed significantly higher expression of MCM7 in highgrade serous carcinomas compared with endometrioid and clear-cell tumors. Kobierzycki and his co-workers also investigated expression of MCM7 in ovarian cancer and they found a positive correlation of MCM-7 with Ki-67, strongly encourage consideration of the use of these proteins as proliferation markers [29].
In the current study, the median serum levels of the CA-125 in malignant patient’s 21U/ml were higher than the levels in benign patients (0.00U/ml). CA125 values showed a highly significant difference between the two groups of the study (P<0.01). This agree with other publications have described increased levels of serum CA- 125 in patients with malignant tumors [6]. Gorp and his co-operators also observed the median serum level of the CA-125 in malignant patients 276.5U/ml was significantly higher than the levels in benign patients (12.8U/ml) [30].
Regarding CA125, in the present study a cut-off value of 6.05U/ ml which was determined by the ROC curve (area under the curve, 0.947), at which the sensitivity is 96%, a specificity is 84%. This in contrast to the result of [30] who found that the ROC–AUC for CA125 was 0.856, at which the sensitivity is 73.9%, specificity is 89% with cut off value 62.5U/ml. Such difference in our results may be due to small sample size.
Regarding RMI, in the present study a cut-off value of 9.65U/ ml which was determined by the ROC curve (area under the curve, 0.976), at which the sensitivity is 100%, a specificity is 92%.
With respect to the overall performance as evaluated by area under the ROC curve (AUC), the MCM7 gene expression had the highest performance (AUC = 0,994), RMI with (AUC = 0.976) and CA125 (AUC = 0.856). So the diagnostic power of MCM7 alone was inferior to CA125 and RMI. However, by combination of MCM7 with RMI will result in absolute sensitivity (100%). The specificity (96%) and the PPV will be 96.15% while NPV will be 100% and accuracy will be 98%.
To the best of our knowledge these were novel combinations between these different parameters we are the first to correlate between MCM7 measured by real time PCR with US score, RMI, and CA125serum levels (U/ml) in all groups of the study and found highly significant correlation (p < 0.01).
In addition, we found concordance between the results of MCM7 measured by real time PCR and RMI in all groups of the study was highly significant (p < 0.01).
Conclusion
MCM7 could be a promising marker for diagnosis ovarian cancer.
References
- Chen ZH, Yan PY, Michalopoulos G, Nelson J, Luo JH. The DNA replication licensing factor miniature chromosome maintenance 7 is essential for RNA splicing of epidermal growth factor receptor, c-Met and platelet-derived growth factor receptor. J Biol Chem. 2015; 290: 8954-8958.
- Worley AB, Joseph P, Kovalenko O, Singh S, Armstrong A, Redline R, DiFeo A. Critical role of Wnt/β-catenin signaling in driving epithelial ovarian cancer platinum resistance. Oncotarget. 2015; 6: 23720-23734.
- Chien J, Sicotte H, Fan JB, Humphray S, Cunningham J M, Kalli KR et al. TP53 mutations, tetraploidy and homologous recombination repair defects in early stage high-grade serous ovarian cancer. Nucleic acids research. 2015; 43.
- Howlader N, Noone AM ,Krapcho M ,Garshell J ,Miller D, Altekruse SF, et al. SEER cancer statistics review, 1975-2011, National Cancer Institute [Internet]. Bethesda, MD: National Cancer Institute. 2011.
- Wang L, Zhu M J, Ren AM, Wu HF, Han WM, Tan RY, et al. A ten-microRN A signature identified from a genome-wide microRNA expression profiling in human epithelial ovarian cancer. PLoS One. 2014; 9: e96472.
- Sölétormos G, Duffy MJ, Hassan SOA, Verheijen RH, Tholander B, Bast Jr RC, et al. Clinical use of cancer biomarkers in epithelial ovarian cancer: updated guidelines from the European Group on Tumor Markers. International Journal of Gynecological Cancer. 2016; 26: 43-51.
- Sasaki A, Akita K, Ito F, Mori T, Kitawaki J, Nakada H. Difference in mesothelinbinding ability of serum CA125 between patients with endometriosis and epithelial ovarian cancer. International journal of cancer. 2015; 136: 1985- 1990.
- Karimi-Zarchi M, Mojaver SP, Rouhi M, Hekmatimoghaddam SH, Moghaddam RN, Yazdian-Anari P, et al. Diagnostic Value of the Risk of Malignancy Index (RMI) for Detection of Pelvic Malignancies Compared with Pathology. Electronic Physician. 2015; 7: 1505-1510.
- Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet vynaecol. 1990; 97: 922-929.
- Drissi R, Dubois ML, Douziech M, Boisvert FM. Quantitative proteomics reveals dynamic interactions of the MCM complex in the cellular response to etoposide induced DNA damage. Molecular & Cellular Proteomics. 2015; 14: 2002-2013.
- Ota T, Clayton AC, Minot DM, Shridhar V, Hartmann LC, Gilks CB, et al. Minichromosome maintenance protein 7 as a potential prognostic factor for progression-free survival in high-grade serous carcinomas of the ovary. Mod pathol. 2008; 24: 277-287.
- Xue WC, Khoo US, Ngan HY, Chan KY, Tam IY, Chiu LG. Replicative MCM7 protein as a proliferation marker in endometrial carcinoma: a tissue microarray and clinic pathological analysis. Histopathology. 2005; 46: 307-313.
- Ren B, Yu G, Tseng GC, Cieply K, Gavel T, Nelson J, et al. MCM7 amplification and overexpression are associated with prostate cancer progression. Oncogene. 2006; 25: 10908-10918.
- Hsi BL, Hung IJ, Yang CP, Lin JN, Chen JC, Tsai SF, et al. Correlation of MYCN amplification with MCM7 protein expression in neuroblastomas: a chromogenic in situ hybridization study in paraffin sections. Hum Pathol. 2004; 35: 1397-1403.
- Nishihara K, Shomori K, Fujioka S, Tokuyasu N, Inaba A, Osaki M, et al. Minichromosome maintenance protein 7 in colorectal cancer: implication of prognostic significance. Int J Oncol. 2008; 33: 245.
- Fujioka S, Shomori K, Nishihara K, Yamaga K, Nosaka K, Araki K, et al. Expression of minichromosome maintenance 7 (MCM7) in small lung adenocarcinomas (pT1): Prognostic implication. Lung Cancer; 2009; 65: 223- 229.
- American Joint Committee on Cancer: Ovary and Primary Peritoneal Carcinoma. In: AJCC Cancer Staging Manual. 7th ed. New York: Springer. 2010: 419-428.
- Kurman RJ, Shih IM. The Origin and Pathogenesis of Epithelial Ovarian Cancer- a Proposed Unifying Theory. The American journal of surgical pathology. 2010; 34: 433-443.
- Yehia M, Mansour A, Mekawy S. Human epididymis protein 4 (HE4) mRNA as a prognostic marker in ovarian tumors in relation to RMI and CA125, International Journal of Cancer Research. 2015; 11: 175-185.
- Kim A, Ueda Y, Naka T, Enomoto T. Therapeutic strategies in epithelial ovarian cancer. Journal of experimental & clinical cancer research. 2012; 31.
- Zhu X, Lang J. The significance and therapeutic potential of PD-1 and its ligands in ovarian cancer: A systematic review. Gynecol Oncol. 2016;142: 184-189.
- Yan F, Wang X, Shao L, Ge M and Hu X. Analysis of UHRF1 expression in human ovarian cancer tissues and its regulation in cancer cell growth. Tumor Biology. 2015; 36: 8887-8893.
- Worley MJ Jr, Guseh SH, Rauh-Hain JA, Esselen KM, Muto MG, Feltmate CM, et al. What is the optimal treatment for obese patients with advanced ovarian carcinoma? Am J Obstet Gynecol. 2014; 211: 231.e1-9.
- Huang TH, Huo L, Wang YN, Xia W, Wei Y, Chang SS, et al. Epidermal growth factor receptor potentiates MCM7-mediated DNA replication through tyrosine phosphorylation of Lyn kinase in human cancers. Cancer cell. 2013; 23: 796-810.
- Petrocca F, Visone R, Onelli MR, Shah MH, Nicoloso MS, de Martino I, et al. E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest and apoptosis in gastric cancer. Cancer Cell. 2008; 13: 272-286.
- Luo JH. Oncogenic activity of MCM7 transforming cluster. World Journal of Clinical Oncology. 2011; 2: 120-124.
- Wei Q, Li J, Liu T, Tong X, Ye X. Phospho-rylation of minichromosome maintenance protein 7 (MCM7) by cyclin/cyclin-dependent kinase affects its function in cell cycle regulation. J Biol Chem. 2013; 288: 19715-19725.
- Juríková M, Danihel L, Polák š, Varga I. Ki67, PCNA and MCM proteins: Markers of proliferation in the diagnosis of breast cancer. Acta histochemical. 2016; 118: 544-552.
- Kobierzycki C, Pula B, Skiba M, Jablonska K, Latkowski F, Zabel M, et al. Comparison of Minichromosome Maintenance Proteins (MCM-3, MCM-7) and Metallothioneins (MT-I/II, MT-III) Expression in Relation to Clinicopathological Data in Ovarian Cancer. Anticancer Research. 2013; 33: 5375-5384.
- Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. British Journal of Cancer. 2011; 104: 863-870.