
Case Report
Austin J Obstet Gynecol. 2018; 5(1): 1089.
Spindle Cell Sarcoma of the Uterine Corpus
Hasegawa K1,2*, Udagawa Y1,2 and Fukasawa I1
¹Department of Obstetrics and Gynecology, Dokkyo Medical University, Japan
2Department of Obstetrics and Gynecology, Fujita Health University School of Medicine, Japan
*Corresponding author: Kiyoshi Hasegawa, Department of Obstetrics and Gynecology, Dokkyo Medical University, 880 Kitakobayashi, Mibu, Japan
Received: January 05, 2018; Accepted: January 18, 2018; Published: January 25, 2018
Abstract
The different types of spindle cell sarcoma, a soft tissue neoplasm, are leiomyosarcoma, fibrosarcoma, malignant peripheral nerve sheath tumor and monophasic synovial sarcoma. Spindle cell sarcoma of the uterine corpus is extremely rare, except for leiomyosarcoma. We report herein a patient with spindle cell sarcoma of the uterine corpus that did not show any specific differentiation by morphological features or immunohistochemical staining pattern. We also conduct a review of the relevant literature.
Keywords: Spindle cell sarcoma; Uterine corpus; Fibrosarcoma; MPNST; Synovial sarcoma
Introduction
Spindle cell sarcoma is a soft tissue neoplasm. The different types are leiomyosarcoma, fibrosarcoma, Malignant Peripheral Nerve Sheath Tumor (MPNST), and monophasic synovial sarcoma [1]. Spindle cell sarcoma of the uterine corpus is extremely rare, with the exception of leiomyosarcoma. Only a few patients with fibrosarcoma, MPNST, or monophasic synovial sarcoma derived from the uterine corpus have been reported. This is a report of an extremely rare instance of undifferentiated spindle cell sarcoma of the uterine corpus. Herein, we describe the clinical, morphologic, and immunohistochemical features that typically determine the diagnosis; we also perform a review of the literature.
Case Presentation
A 44-year-old gravida 3 para 3 Japanese woman presented to Fujita Health University hospital with the complaint of continuous abdominal pain and genital bleeding. Her past history and family history were unremarkable. Pelvic examination revealed a 50 × 40 × 40 mm mass extending from the intrauterine cavity to the vaginal space through the cervical canal. The tumor had a smooth surface and appeared to be a uterine leiomyoma delivering through the cervix. Ultrasonography revealed a slender stalk of tumor located in the endometrium. The tumor was easily removed transvaginally by twisting the mass around its stalk.
The tumor was encapsulated, and the cut surface was dark red and fragile, with degeneration and necrosis apparent (Figure 1). Pathologically normal endometrial glands were present in the capsule of the tumor (Figure 2). Pathological examination demonstrated a densely trabecular arrangement of spindle cells having oval nuclei with weak nuclear pleomorphism and small, inconspicuous nucleoli (Figure 3). Hemorrhagic, degenerated, and necrotic areas were present. Mitotic figures were easily identified in the tumor cells, with a mitotic count of over 30 per 10 high-power fields (Figure 3). The tumor was composed of only non epithelial cells; apparent epithelial components were not seen.

Figure 1: Macroscopic findings. The tumor was encapsulated and cut surface
of the tumor was dark-red and fragile with degeneration and necrosis (arrow;
stalk of the tumor).

Figure 2: Pathological examination. The tumor was encapsulated and
pathologically normal endometrial glands were present in capsule (arrow;
normal endometrial glands) (a: H&E; loupe image, b: H&E original
magnification x2.5).
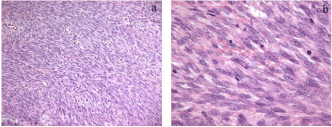
Figure 3: Pathological examination. Pathological examination of the tumor
demonstrated a densely trabecular arrangement of spindle cells having oval
nucleus with weak nuclear pleomorphism and small inconspicuous nucleoli.
Hemorrhagic, degeneration and necrotic arear were present. Mitotic figures in
tumor cells were easily identified, with a mitotic count of over 30 per 10 highpower
fields (a: H&E; original magnification x10, b: H&E x40).
Immunohistochemistry showed that the tumor cells were diffusely positive for vimentin and Cluster of Differentiation (CD99), (MIC2) (Figure 4). The tumor cells were focally and weakly positive for S-100 protein, CD56 (natural cell adhesion molecule), and nestin. They were negative for cytokeratin anion exchange protein (AE)1/ AE3, Epithelial Membrane Antigen (EMA), Smooth Muscle Actin (SMA), desmin, calponin, caldesmon, CD10, glial fibrillary acidic protein, B-cell lymphoma 2 (bcl-2), c-kit, CD34, and human melanin black 45. The labeling index of Ki-67 was 18.9%.

Figure 4: a: Immunohistochemical findings; b: The tumor cells were diffusely
positive for vimentin and CD99 (a; x10, b; x10).
From the morphological findings and immunohistochemical staining pattern, the diagnosis of this tumor was a spindle cell sarcoma, not otherwise specified. Specific differentiation was not possible because the tumor did not show any of the distinct morphological features of fibrosarcoma, MPNST, or monophasic synovial sarcoma.
For the diagnosis of monophasic synovial sarcoma, molecular analysis of SYT-SSX fusion gene transcripts was performed [2]. Therefore, we conducted SYT break-apart rearrangement Fluorescence In Situ Hybridization (FISH) using formalin-fixed, paraffin-embedded tumor tissue using a procedure described elsewhere [3]. SYT-bar FISH was performed using a LSI SYT (18q11.2) Dual Color, Break Apart Rearrangement Probe kit (Vysis, Inc., Downers Grove, IL, USA) according to the manufacturer’s instructions. A pair of split SYT signals (SYT-SSX1, SYT-SSX2, and SYT-SSX4) could not be detected in the tumor cells; therefore, this tumor could not be diagnosed as a synovial sarcoma using SYT-bar FISH.
The final diagnosis was a “general” spindle cell sarcoma. Cervical cytology was negative, and endometrial biopsy revealed a normal endometrium in the proliferative phase, without malignancy. Magnetic resonance imaging and computed tomography did not demonstrate any suspicious residual uterine tumor or metastatic lesions. Laboratory studies did not show any abnormalities and tumor makers, including a carcinoma antigen 125 level, were within normal limits.
After obtaining informed consent, we performed a hysterectomy and bilateral salpingo-oophorectomy to investigate whether any residual tumor was present. Intraoperative inspection revealed no abnormal findings in the abdominal cavity, uterus, bilateral ovaries, or fallopian tubes. The small stalk of the tumor was identified in the endometrium; however, no tumor- or vascular invasion was identified in the stalk, endometrium, myometrium, ovaries, or tubes (Figure 5). The final pathological diagnosis was a spindle cell sarcoma of International Federation of Gynecology and Obstetrics (FIGO) stage IA (pT1aNxM0). After surgery, the patient underwent close follow-up without adjuvant therapy. She is currently alive 5 years after surgery, with no evidence of recurrence.

Figure 5: Macroscopic findings. Macroscopically, uterus, bilateral ovaries and
fallopian tubes were unremarkable. The small stalk of tumor was identified in
endometrium (arrow).
Discussion
At first, this polypoid tumor appeared macroscopically to be an endometrial stromal sarcoma or carcinosarcoma, yet histopathology did not support either result. The results instead indicated a spindle cell sarcoma, an entity comprising leiomyosarcoma, fibrosarcoma, MPNST, and monophasic synovial sarcoma.
Leiomyosarcomas are generally positive for the markers of smooth muscle differentiation such as SMA, desmin, h-caldesmon, and calponin. However, our patient’s tumor was negative for all these markers, excluding leiomyosarcoma from the differential diagnosis.
A fibrosarcoma is a poorly circumscribed infiltrative spindle cell sarcoma, which shows the following histological characteristics: 1) hyperchromatic spindled cells showing no more than moderate pleomorphism; 2) a fascicular “herringbone” growth pattern and a variable degree of interstitial collagen; 3) the absence of any morphologic features of myxofibrosarcoma, low-grade fibromyxoid sarcoma, or sclerosing epithelioid fibrosarcoma; 4) absent expression of any markers other than vimentin or very minimal SMA [4]. The most common site of occurrence is in the extremities, especially in the thigh region. The tumor is characterized by local growth and has a propensity for local recurrence and hematogenous metastases, mainly to the lung or bone. Several cases of dermatofibrosarcoma protuberance of the vulva have been reported [5,6]; only a single patient with a fibrosarcoma arising from a leiomyoma nodule of the uterus has been reported [7]. Histopathologically, the tumor is composed of immature-appearing fibroblasts growing in a fascicular arrangement. In the latter report, hysterectomy was the choice of treatment; adjuvant radiotherapy was not performed because of the early stage of growth and the limited extension of the tumor [7]. Our patient’s tumor did not show a typical fascicular herringbone growth pattern and was positive for vimentin and CD99. While we could not make the definitive diagnosis of fibrosarcoma, neither could we rule it out.
An aggressive soft tissue sarcoma, MPNST invariably arises from a peripheral nerve or in the extraneural soft tissues and displays nerve-sheath differentiation. Uterine MPNST is rare; most have been reported as arising from the uterine cervix. Several patients with MPNST arising from uterine corpus have been reported [8-11]. The tumors are generally composed of compact fascicles of spindle cells arranged in a herringbone, loose fascicular, or ill-defined storiform pattern. Immunohistochemistry typically shows neuronal markers, particularly S-100 protein; however, 20% to 30% of tumors are negative for S-100 protein. Tumors are sometimes focally positive for CD57.
Synovial sarcoma is the fourth most commonly occurring sarcoma, accounting for 8% to 10% of all sarcomas. Most synovial sarcomas arise in the para-articular regions of the extremities with a predilection for the lower extremities, head, neck, and trunk [12]. However, this tumor can occur at any site, including the kidney, gastrointestinal tract, and lung. Several patients with gynecologic lesions have been reported, arising mostly in the vulva and rarely in the vagina, fallopian tube, ovary, or uterus [13-20]. Synovial sarcoma can be classified as biphasic; monophasic, fibrous type; monophasic, epithelial type; and poorly differentiated type [1]. Monophasic synovial sarcoma displays the morphologic features of spindle cell sarcoma, demonstrating fibrosarcomatous proliferation of relatively small and uniform spindle cells in sheets with collagenous stroma, with or without calcification or ossification, and a hemangiopericytic pattern [1]. One reported uterine synovial sarcoma presented as a polypoid tumor arising in the endometrium with apparent invasion into the endometrium. The tumor had a biphasic structure with a predominance of poorly differentiated, small- to medium-sized, round to oval cells arranged in diffuse sheets, with other components consisting of larger epithelioid cells with eosinophilic cytoplasm arranged in irregular nests [20]. In most cases of synovial sarcoma, the correct diagnosis can be achieved by analysis of histology and the immunohistochemical staining pattern. Synovial sarcoma mostly expresses both mesenchymal and epithelial markers, such as vimentin, transducin-like enhancer protein 1, EMA, cytokeratin, and bcl-2. It sometimes expresses S-100 protein, CD99, calponin, and, rarely, SMA [13-20]. Our patient’s tumor cells were diffusely positive for vimentin and CD99, and focally positive for S-100, but negative for epithelial markers and bcl-2. Therefore, it is difficult to say that our patient’s tumor was a synovial sarcoma by immunohistochemical analysis.
If a definitive diagnosis cannot be obtained by histological and immunohistochemical analysis, molecular analysis of specific chromosomal translocation t(X; 18) (p11; q11) or SYT-SSX fusion gene transcripts should be considered [2]. However, SYT-SSX fusion gene transcripts could not be detected using SYT-bar FISH. Of course, this technique has limitations, but it may be more reliable than reverse-transcriptase polymerase chain reaction, even using a formalin-fixed, paraffin-embedded sample.
Our case presents a few diagnostic dilemmas. First, our patient’s tumor could not be defined as a specific type of spindle cell sarcoma because it did not have any tendency toward the specific morphological manifestations of leiomyosarcoma, fibrosarcoma, MPNST, or monophasic synovial sarcoma. If pressed to make a diagnosis, it could be classified as a fibrosarcoma; however, MPNST and monophasic synovial sarcoma cannot completely be ruled out. If our patient’s tumor could be diagnosed as a monophasic synovial sarcoma, this would be the first reported case of an origin from the uterine endometrium. Our patient’s tumor has the features of an atypical spindle cell neoplasia and earns the designation of a probable low-grade sarcoma based chiefly on the nuclear pleomorphism and infiltrative borders. There seems to be a correlation between histologic features and prognosis.
In conclusion, our patient was diagnosed with a spindle cell sarcoma as a diagnosis of exclusion. We were unable to specify a histologic subtype and were forced to use the designation “not otherwise specified,” a broad diagnostic category. The rarity of spindle cell sarcoma of the uterine corpus poses a challenge in its diagnosis and treatment. Currently, case reports and case series are the most viable option for characterizing this tumor and developing optimal treatment strategies.
References
- Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. WHO Classification of Tumours of Soft Tissue and Bone. IARC press. Lyon. 2013.
- Krsková L, Sumerauer D, Stejskalová E, Kodet R. A novel variant of SYTSSX1 fusion gene in a case of spindle cell synovial sarcoma. Diagn Mol Pathol. 2007; 16: 179-183.
- Kawauchi S, Ihara K, Nishikawa K, Sugino N, Takahashi M, Sasaki K. Synovial sarcoma arising in the vulva cytogenetically confirmed by SYT break-apart rearrangement fluorescence in situ hybridization: A case report and discussion of diagnostic methods. Oncol Lett. 2012; 4: 955-959.
- Bahrami A, Folpe AL. Adult-type fibrosarcoma: A reevaluation of 163 putative cases diagnosed at a single institution over a 48-year period. Am J Surg Pathol. 2010; 34: 1504-1513.
- Soergel TM, Doering DL, O’connor D. Metastatic dermatofibrosarcoma protuberans of the vulva. Gynecol Oncol. 1998; 71: 320-234.
- Moodley M, Moodley J. Dermatofibrosarcoma protuberans of the vulva: a case report and review of the literature. Gynecol Oncol. 2000; 78: 74-75.
- Bodner-Adler B, Bodner K, Czerwenka K, Leodolter S, Mayerhofer K. Fibrosarcoma of the uterus: a case report. Anticancer Res. 2001; 21: 3651- 3652.
- de A Focchi GR, Cuatrecasas M, Prat J. Malignant peripheral nerve sheath tumor of the uterine corpus: A case report. Int J Gynecol Pathol. 2007; 26: 437-440.
- Molina CP, Putegnat BB, Logrono R. Fine needle aspiration cytology and core biopsy of malignant peripheral sheath tumor of uterus: A case report. Diagn Cytopathol. 2001; 24: 347-351.
- Gulati N, Rekhi B, Suryavanshi P, Jambhekar NA. Epithelioid malignant peripheral nerve sheath tumor of the uterine corpus. Ann Diagn Pathol. 2011; 15: 441-445.
- Sengar Hajari AR, Tilve AG, Kulkarni JN, Bharat R. Malignant peripheral nerve sheath tumor of the uterine corpus presenting as a huge abdominal neoplasm. J Cancer Res Ther. 2015; 11: 1023.
- Al-Daraji W, Lasota J, Foss R, Miettinen M. Synovial sarcoma involving the head: analysis of 36 cases with predilection to the parotid and temporal regions. Am J Surg Pathol. 2009; 33: 1494-1503.
- White BE, Kaplan A, Lopez-Terrada DH, Ro JY, Benjamin RS, Ayala AG. Monophasic synovial sarcoma arising in the vulva: a case report and review of the literature. Arch Pathol Lab Med. 2008; 132: 698-702.
- Ambani DS, White B, Kaplan AL, Alberto A. A case of monophasic synovial sarcoma presenting as a vulvar mass. Gynecol Oncol. 2006; 100: 433-436.
- Holloway CL, Russell AH, Muto M, Albert M, Viswanathan AN. Synovial cell sarcoma of the vulva: multimodality treatment incorporating preoperative external-beam radiation, hemivulvectomy, flap reconstruction, interstitial brachytherapy, and chemotherapy. Gynecol Oncol. 2007; 104: 253-256.
- Mitsuhashi A, Nagai Y, Suzuka K, Yamazawa K, Nojima T, Nikaido T, et al. Primary synovial sarcoma in fallopian tube: case report and literature review. Int J Gynecol Pathol. 2007; 26: 34-37.
- Nielsen GP, Shaw PA, Rosenberg AE, Dickersin GR, Young RH, Scully RE. Synovial sarcoma of the vulva: a report of two cases. Mod Pathol. 1996; 9: 970-974.
- Pelosi G, Luzzatto F, Landoni F, Staffa N, Maggioni A, Braidotti P, et al. Poorly differentiated synovial sarcoma of the vagina: first reported case with immunohistochemical, molecular and ultrastructural data. Histopathology. 2007; 50: 808-810.
- Smith CJ, Ferrier AJ, Russell P, Danieletto S. Primary synovial sarcoma of the ovary: first reported case. Pathology. 2005; 37: 385-387.
- Dundr P, Fischerová D, PovÝšil C, Tvrdík D, Cibula D. Primary synovial sarcoma of the uterus. Pathol Oncol Res. 2012; 18: 529-533.