Research Article
Austin J Obstet Gynecol. 2018; 5(2): 1098.
Birth Weight/Placental Weight Ratio: Does the Association Differ Between Early- and Late-Onset Preeclampsia?
Phadungkiatwattana P* and Songphol Puttasiri
Department of Obstetrics and Gynecology, Rangsit University, Bangkok, Thailand
*Corresponding author: Podjanee Phadungkiatwattana, Department of Obstetrics and Gynecology, Rajavithi Hospital, College of Medicine, Rangsit University, Bangkok, Thailand
Received: January 16, 2018; Accepted: February 13, 2018; Published: February 27, 2018
Abstract
Purpose: Early-and late-onset preeclampsia may differ in pathophysiology, and this can be reflected in differences in birth weight/placental weight ratios. Therefore, we compared birth weight/placental weight ratios of births with earlyand those with late-onset preeclampsia.
Materials and Methods: The study included all hospital-based singleton births of 24-43 weeks’ gestation between January 2007 and December 2016. A total of 51,940 pregnant women were divided into three groups: earlyonset preeclampsia, late-onset preeclampsia, and pregnant women without preeclampsia.
Results: The mean (+SD) birth weight/placental weight (BW/Pl) ratios were significantly different in early-onset preeclampsia (PE) and late-onset PE compared with the control group(3.91+0.93 in early-onset PE, 4.85+0.91 in lateonset PE and 5.17+0.90 in the control group, p<0.001). Our study found that the factors significantly associated with BW/Pl ratios were Diabetes Mellitus (DM), gestational age, early-onset PE, late-onset PE, Small for Gestational Age (SGA) and Large for Gestational Age (LGA). After adjustment for DM, gestational age, late-onset PE, SGA and LGA, the BW/Pl ratio was still associated significantly more with early-onset PE than with late-onset PE.
Conclusion: Our study indicated that the BW/Pl ratios of preeclamptic women differed between early- and late-onset PE, and that early-onset PE may be commonly associated with placental efficiency. This suggests that preeclampsia consists of several different processes manifesting as a single disease.
Keywords: Preeclampsia; Placenta; Birth; Weight; Early Onset; Late Onset
Introduction
Birth weight/placental weight ratios (BW/Pl ratio), calculated as the grams of fetal birth weight per gram of placenta weight, reflect placental efficiency or placental function [1,2]. The ability of the placenta to maintain nutrient delivery to the fetus has an influence on fetal birth weight, and it is well established that there is a positive correlation between placental weight and birth weight [3-5]. The BW/ Pl ratio is often reduced, which may indicate a placenta that fails to adapt its nutrient transfer capacity to compensate for its small size [6].
Recent data have supported classifying Pre-eclampsia (PE) into early-onset PE, which tends to develop before 34 weeks of gestation, and late-onset preeclampsia, which develops at or after 34 weeks of gestation [7,8]. Early-and late-onset PE has been found to be associated with different pathophysiological-specific features. Early-onset PE is commonly associated with placental dysfunction, reduction in placental volume, intrauterine growth restriction and adverse maternal and neonatal outcomes [9,10]. Conversely, lateonset PE is more often associated with normal placenta, normal fetal growth and more favorable outcomes [11,12].
In this study, we hypothesized that early- and late-onset PE had different pathophysiologies. Thus, we sought to compare BW/Pl ratios of early- and late-onset pre-eclampsia in order to explore the existence of these differences.
Materials and Methods
The present study was conducted at Rajavithi Hospital, a tertiary care teaching public hospital affiliated to Rangsit University in Bangkok, Thailand, with the ethical approval of the local institutional review board. The study included all hospital-based singleton births of 24-43 weeks’ gestation between January 2007 and December 2016 (n=54,618). Deliveries after congenital anomalies (n=227), stillbirth (n=372), multiple gestations (n=994) and deliveries with missing gestational age, placental weight or birth weight (n=953) were excluded.
Descriptive analyses were performed on all study variables. Implausible values and potential errors were excluded, including birth weights above or below the mean by three Standard Deviations (SD), placental weights that were <100g or > 1000g and unknown or ambiguous genders (n= 132). The final sample was 51,940 singleton deliveries.
Preeclampsia (PE) was defined as a resting blood pressure > 140/90 mmHg and proteinuria of > 300 mg/L or a 2+ urine dipstick > 20 weeks of gestation in a previously normotensive woman [13]. Small for Gestational Age (SGA) was defined as infants with birth weight below the 10th centile for gestational age, and Large for Gestational Age (LGA) was defined as infants with birth weight above the 90th centile for gestational age.
Untrimmed placenta weight (including the membranes and umbilical cord) and birth weight of the infant were weighed in grams immediately after delivery. The Birth Weight/Placental Weight ratio (BW/Pl ratio) was then calculated.
The cases were divided into three groups: early-onset PE (preeclampsia occurring at less than 34 weeks of gestation); late-onset PE (preeclampsia occurring at 34 or more weeks of gestation); and a control group (pregnancies without preeclampsia).
The data were presented as Mean + SD (standard deviation). Student’s t-test was used to compare maternal age, nulliparous, race, infant gender, placental weight, birth weight, BW/Pl ratio in early-PE, late-PE and controls. Statistical significance was determined using multiple comparisons performed by a one-way ANOVA test among the groups. Multivariate linear regression analysis was performed to determine the significant predictive factors for BW/Pl ratio and the predictive model was developed based on a linear equation. Model fitting was carried out using a backward elimination method based on maximal likelihood estimation. Data analysis was performed using the SPSS ver.16.0 (SPSS Inc., Chicago, IL, USA). A p-value <0.05 with a 95% confidence interval was considered statistically significant.
Results
From January 2007 through December 2016, 51,940 pregnant women who had singleton hospital deliveries at 24 weeks of gestation or later and met the inclusion criteria were enrolled in the study. Those diagnosed with preeclampsia accounted for 2.7% of participants, of which 339 (0.65%) had early-onset PE, and 1111 (2.14%) had lateonset PE. The demographic data are outlined in Table 1. The mean maternal age, race, pre-gestational Diabetes Mellitus (DM) and gestational age were significantly different in early-onset PE and lateonset PE compared with the control group, while the proportion of infant gender was significantly different between early-onset PE and the control group. Gestational DM was significantly different in the late-onset PE compared to the control group.
Variables
Early onset-PE
Late onset-PE
Control
p-value
(n=339)
(n=1111)
(n=50,490)
Maternal age(yr), mean + SD
29.64+6.60a)
29.9+7.04a)
27.5+6.19
<0.001
Nulliparous (%)
197(58.1%)
592(53.3%)
26205(51.9%)
0.05
Race (%Thai)
295(87%) a)
923(83.1%) a)
38632(76.5%)
<0.001
Infant gender(%male)
150(44.2%) a)
543(48.9%)
26133(51.8%)
0.004
Pre-gestational DM (%)
10(2.9%)a)
29 (2.6%)a)
132 (3%)
<0.001
Gestational DM (%)
19(5.6%)
134(12.1%) a)
2421(4.8%)
<0.001
Gestational age (weeks), mean + SD
30.56+2.21 a)
37.15+1.88a)
37.15+1.85
<0.001
DM: Diabetes Mellitus.
a) Statistical significance (P<0.05) was tested by Student’s t-test. Early-onset PE and late-onset PE were compared with the control pregnancy group.
Table 1: Comparison of baseline characteristics data in different groups.
A comparison of mean birth weight, mean placental weight, mean BW/Pl ratio, SGA, and LGA is presented in Table 2. The mean (+SD) birth weights were 1412+479 grams in early-onset PE, 2733+612 grams in late-onset PE, and 3036+452 grams in the control group, p<0.001. Mean (+SD) placental weights were 372+127 grams in early-onset PE, 577.6+145 grams in late-onset PE, and 601+123 grams in the control group, P <0.001. Mean (+SD) BW/Pl ratios were 3.91+0.93 grams in early-onset PE, 4.85+0.91 grams in late-onset PE, and5.17+0.90 grams in the control group, p<0.001, and these values were significantly different in early-onset PE and late-onset PE compared with the control group. SGA was significantly higher in early-onset PE (41.3%) and late-onset PE(10.6%) than in the control group(1.3%),p<0.001. LGA was significantly lower in early-onset PE (9.7%) than in the control group (15.7%), p 0.009. Table 3 lists details of factors such as maternal age, race, infant gender, DM, gestational age, early-onset PE, late-onset PE, SGA and LGA that might be expected to have an influence on the BW/Pl ratio.
Variables
Early onset-PE (n=339)
Late onset-PE (n=1111)
Control (n=50,490)
p-value
Mean BW+ SD (g)
1412.4+479.0 a)
2733.0 +611.7 a)
3036.0+ 452.0
<0.001
Mean Pl+ SD (g)
372.1+127.3 a)
577.6+145.1 a)
601.2+ 122.7
<0.001
Mean BW/Pl + SD
3.9+ 0.9 a)
4.8+0.9 a)
5.2+0.9
<0.001
SGA (%)
140(41.3)a)
118(10.6)a)
662 (1.3)
<0.001
LGA (%)
33(9.7)a)
181(16.3)
7952(15.7)
0.009
BW: Birth Weight; Pl: Placenta Weight; SGA: Small for Gestational Age; LGA: Large for Gestational Age.
a) Statistical significance (P<0.05) was tested by Student’s t-test. Early-onset PE and late-onset PE were compared with the control pregnancy group.
Table 2: Comparison of BW, Pl, SGA, LGA, BW/Pl in different groups.
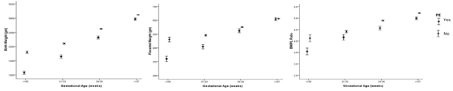
Univariate analysis indicated the factors influencing the BW/Pl ratios, and showed that race, infant gender, DM, gestational age, late onset-PE, early onset-PE, LGA, and SGA were positively associated with the BW/Pl ratios. The variables in Table 3 were then analyzed using a backward stepwise multivariate logistic regression analysis. The factors that remained significantly associated with BW/Pl ratio are shown in Table 4. To identify variables associated with BW/Pl ratio, a Cox regression analysis was performed on those variables (DM, gestational age, late onset-PE, early onset-PE, LGA, and SGA) included in the multivariate regression analysis, which were thought to be associated with the BW/Pl ratio were included in the model. Figure 1 shows that birth weight, placental weight and BW/Pl ratio were positively correlated with gestational age. Pregnancies with preeclampsia had lower birth weight, placental weight and BW/Pl ratios than pregnancies without preeclampsia.
Factors
BW/Pl ratio
Mean
SD
MD
95% CI
p-value
Maternal age (yr)
5.16
0.9
0
-0.01, 0.01
0.871
Race
Thai
5.13
0.9
Others
5.25
0.9
0.12
0.10, 0.14
<0.001
Infant gender
Male
5.2
0.9
Female
5.11
0.9
-0.8
-0.09, -0.07
< 0.001
DM
None
5.16
0.9
DM
5.04
0.9
-0.1
-0.16, -0.10
< 0.001
Gestational age (weeks)
5.16
0.9
0.1
0.10, 0.11
< 0.001
Preeclampsia
< 0.001
None
5.17
0.9
Ref
Late onset-PE
4.85
0.9
-0.3
-0.39, -0.26
< 0.001
Early onset-PE
3.91
0.9
-1.3
-1.38, -1.15
< 0.001
Fetal growth
AGA
5.17
0.9
Ref
LGA
5.13
0.9
-0.4
-0.06, -0.01
0.002
SGA
4.72
1.2
-0.5
-0.52, -0.38
<0.001
Table 3: Univariate analyses (95% Confidence Interval; CI) for BW/Pl ratio.
Factors
BW/Pl ratio
Mean
SD
MD
95% CI
p-value
Maternal age (yr)
5.16
0.9
0
-0.01, 0.01
0.871
Race
Thai
5.13
0.9
Others
5.25
0.9
0.12
0.10, 0.14
<0.001
Infant gender
Male
5.2
0.9
Female
5.11
0.9
-0.8
-0.09, -0.07
< 0.001
DM
None
5.16
0.9
DM
5.04
0.9
-0.1
-0.16, -0.10
< 0.001
Gestational age (weeks)
5.16
0.9
0.1
0.10, 0.11
< 0.001
Preeclampsia
< 0.001
None
5.17
0.9
Ref
Late onset-PE
4.85
0.9
-0.3
-0.39, -0.26
< 0.001
Early onset-PE
3.91
0.9
-1.3
-1.38, -1.15
< 0.001
Fetal growth
AGA
5.17
0.9
Ref
LGA
5.13
0.9
-0.4
-0.06, -0.01
0.002
SGA
4.72
1.2
-0.5
-0.52, -0.38
<0.001
Table 4: Independent factors associated with BW/Pl ratios after adjustment for potential confounders*.

Figure 1: Correlation between birth weight, placental weight, and BW/Pl ratio VS. Gestational age in pregnancies with and without Preeclampsia (PE).
In all pregnant women in our study, birth weight and placenta weight were correlated (r = 0.62, p<0.00).
Discussion
In this study conducted in Thailand between 2007and 2016, the mean BW/Pl ratio was significantly lower in pregnancies with earlyonset PE than in those with late-onset PE and those in the control group (3.91+ 0.93 VS. 4.85+0.91 VS. 5.17+0.90, respectively). Similarly, [14] reported that the mean BW/Pl ratio was significantly lower in the PE group than in the control group (5.1 VS. 6.0 respectively).In a previous study [15-19] variables that may affect BW/Pl ration were found to include infant gender, race, DM, gestational age, SGA, and LGA. Our study found that the factors significantly associated with BW/Pl were DM, gestational age, early-onset PE, late-onset PE, SGA and LGA. After adjustment for DM, gestational age, late-onset PE, SGA and LGA, the BW/Pl ratio was still associated with early-onset PE significantly more than with late-onset PE.
The placenta is important in providing a healthy environment for the fetus and plays a central role in the pathophysiology of preeclampsia. The placenta regulates its nutrient transfer efficiency by morphological and functional adaptations which result in optimal fetal growth [2,20,21]. In our study, mean birth weight and mean placental weight were significantly lower in the PE group, and when birth weight was divided by placental weight, it was still lower in the PE group. It has been postulated that PE is strongly associated with small placenta and that it has an influence on placental function results in a fetus that is small with respect to its genetic potential.
Preeclampsia has collectively been termed ischemic placental disease because the two types are frequently characterized by uteroplacental under perfusion, chronic hypoxia and placental ischemia, which are results of abnormal spiral artery remodeling, failed trophoblast invasion and impaired transformation of decidual spiral arteries leading to abnormal placentation and influencing placental efficiency [22-26].It has been hypothesized that placental ischemia may reduce nutrient supply so that the fetal growth may be affected. A reduction in the BW/Pl ratio may be indicative of placental dysfunction. In keeping with the results of several previous reports [26-28], the BW/Pl ratio in this report was found to be reduced in births with preeclampsia. Another important finding in the current study was that the BW/Pl ratio was still significantly lower in earlyonset PE than in late-onset PE (MD = -0.2 VS. -0.43 respectively). This finding supports the view that preeclampsia in early-PE is more commonly associated with placental dysfunction than with late-PE. The current data suggests that distinct vascular adaptation in early and late PE could reflect different pathophysiologic mechanisms [9,10,29,30].
The strength of this study was that it had adjusted data which made the outcomes more reliable, and that it was one of a large series with a big enough sample size to have the power to distinguish the outcomes. To the best of our knowledge, no previous hospital-based study on BW/Pl ratio of early- and late-onset PE has been published.
Some limitations of this study should be noted. First, there prospective nature of this study based on computer searches might be associated with some incomplete data. Secondly, the weight of placentas was considered as the sum of the weight of placenta, membrane and umbilical cord (untrimmed placenta).
In conclusion, our study indicated that the BW/Pl ratio of preeclamptic women differed in cases of early- and late-onset PE, and that early-onset PE may be commonly associated with placental efficiency. This suggests that preeclampsia is composed of several different processes manifesting as a single disease.
Compliance with Ethical Standards
Funding: The work was not supported by any fund/grant.
Disclosure statement: We declare that all authors have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Boards in March 2017. The patient’s informed consent for participation in the study was not required.
References
- Wilson ME, Ford SP. Comparative aspects of placental efficiency. Reprod Suppl. 2001; 58: 223-232.
- Fowden AL, Sferruzzi-Perri AN, Coan PM, Constancia M, Burton GJ. Placental efficiency and adaptation: endocrine regulation. J Physiol. 2009; 587: 3459-3472.
- Crawford MA, Doyle W, Meadows N. Gender differences at birth and differences in fetal growth. Hum Reprod. 1987; 2: 517-520.
- Thame M, Osmond C, Wilks R, Bennett FI, Forrester TE. Second-trimester placental volume and infant size at birth. Obstet Gynecol. 2001; 98: 279-283.
- Thame M, Osmond C, Bennett F, Wilks R, Forrester T. Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr. 2004; 58: 894-900.
- Hayward CE, Lean S, Sibley CP, Jones RL, Wareing M, Greenwood SL, et al. Placental Adaptation: What Can We Learn from Birth weight: Placental Weight Ratio? Front Physiol. 2016; 7: 28.
- Von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003; 22: 143-148.
- Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. 2011.
- Khalil A, Akolekar R, Syngelaki A, Elkhouli M, Nicolaides KH. Maternal hemodynamics at 11-13 weeks’ gestation and risk of pre-eclampsia. Ultrasound Obstet Gynecol. 2012; 40: 28-34.
- Estensen ME, Remme EW, Grindheim G, Smiseth OA, Segers P, Henriksen T, et al. Increased arterial stiffness in pre-eclamptic pregnancy at term and early and late postpartum: a combined echo cardio graphic and tonometric study. Am J Hypertens. 2013; 26: 549-556.
- Onah HE, Iloabachie GC. Conservative management of early-onset preeclampsia and feto maternal outcome in Nigerians. J Obstet Gynaecol. 2002; 22: 357-362.
- Dissanayake VH, Samarasinghe HD, Morgan L, Jayasekara RW, Seneviratne HR, Broughton Pipkin F. Morbidity and mortality associated with pre-eclampsia at two tertiary care hospitals in Sri Lanka. J Obstet Gynaecol Res. 2007; 33: 56-62.
- Anon. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000; 183: S1-S22.
- Kim HS, Cho SH, Kwon HS, Sohn IS, Hwang HS. The significance of placental ratios in pregnancies complicated by small for gestational age, preeclampsia, and gestational diabetes mellitus. Obstet Gynecol Sci. 2014; 57: 358-366.
- Lao TT, Wong WM. Placental ratio and intrauterine growth retardation. Br J Obstet Gynaecol. 1996; 103: 924-926.
- Almog B, Shehata F, Aljabri S, Levin I, Shalom-Paz E, Shrim A. Placenta weight percentile curves for singleton and twins deliveries. Placenta. 2011; 32: 58-62.
- Wallace JM, Bhattacharya S, Horgan GW. Gestational age, gender and parity specific centile charts for placental weight for singleton deliveries in Aberdeen, UK. Placenta. 2013; 34: 269-274.
- Macdonald EM, Koval JJ, Natale R, Regnault T, Campbell MK. Populationbased placental weight ratio distributions. Int J Pediatr. 2014a; 2014: 291846.
- Gloria-Bottini F, Neri A, Coppeta L, Magrini A, Bottini E. Correlation between birth weight and placental weight in healthy and diabetic puerperae. Taiwan J Obstet Gynecol. 2016; 55: 697-699.
- Coan PM, Angiolini E, Sandovici I, Burton GJ, Constancia M, Fowden AL. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J Physiol. 2008; 586: 4567-4576.
- Sandovici I, Hoelle K, Angiolini E, Constancia M. Placental adaptations to the maternal-fetal environment: implications for fetal growth and developmental programming. Reprod Biomed Online. 2012; 25: 68-89.
- Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by preeclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986; 93: 1049-1059.
- Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997; 99: 2152-2164.
- Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002; 187: 1416-1423.
- Ananth CV, Peltier MR, Chavez MR, Kirby RS, Getahun D, Vintzileos AM. Recurrence of ischemic placental disease. Obstet Gynecol. 2007; 110: 128- 133.
- Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011; 204: 193-201.
- Macdonald EM, Natale R, Regnault TR, Koval JJ, Campbell MK. Obstetric conditions and the placental weight ratio. Placenta. 2014b; 35: 582-586.
- Ganer Herman H, Miremberg H, Schreiber L, Bar J, Kovo M. The Association between Disproportionate Birth Weight to Placental Weight Ratio, Clinical Outcome, and Placental Histopathological Lesions. Fetal Diagn Ther. 2017; 41: 300-306.
- Everett TR, Mahendru AA, Mceniery CM, Wilkinson IB, Lees CC. Raised uterine artery impedance is associated with increased maternal arterial stiffness in the late second trimester. Placenta. 2012; 33: 572-577.
- Stergiotou I, Crispi F, Valenzuela-Alcaraz B, Bijnens B, Gratacos E. Patterns of maternal vascular remodeling and responsiveness in early- versus lateonset preeclampsia. Am J Obstet Gynecol. 2013; 209: 558 e1-558 e14.