
Research Article
Austin J Obstet Gynecol. 2019; 6(1): 1131.
Is Long-Term Dienogest Treatment Tolerable in Korea?
Kim HG1,2, Song YJ1,2 and Na YJ1,2*
¹Department of Obstetrics and Gynecology, Pusan National University Yangsan Hospital, Yangsan, South Korea
²Research Institute for Convergence of Biomedical Science and Technology, Pusan National University, Yangsan Hospital, South Korea
*Corresponding author: Yong Jin Na, Department of Obstetrics and Gynecology, Pusan National University, Yangsan Hospital, Yangsan, South Korea
Received: November 30, 2018; Accepted: January 10, 2019; Published: January 17, 2019
Abstract
Aim: To evaluate the adverse events and tolerability of dienogest administration over an 18-month period for a prevention of recurrent symptoms and lesions after surgery for endometriosis.
Methods: Dienogest was administered to 150 patients with endometriosis for over an 18-month period after surgery. The adverse event profile and patient satisfaction regarding dienogest was determined through a questionnaire. We evaluated the adverse events of dienogest, including through laboratory tests, body weight, and bleeding patterns. Post-operation measurements of pain were applied before surgery, postoperatively, before treatment with dienogest, and after dienogest treatment. The body weight was measured during the first visit, and measurements were repeated every six months.
Results: The median duration of treatment was 24 months, with the longest follow-up duration being 60 months. Adverse events include headaches, breast discomfort, acne, constipation, hot flushes, weight gain, and mild depression. More than 70% of the patients experienced amenorrhea. However, this was not a reason for discontinuation. The vaginal bleeding pattern associated with dienogest was shown to be tolerable, and only three patients stopped taking it because of heavy menstrual bleeding resulting in anemia. Rather, the major cause of discontinuation was unwanted weight gain.
Conclusions: The data indicate that dienogest is well tolerated and has a favorable safety profile for long-term use as a prophylaxis in an effort to post-operatively obviate the recurrence of ovarian endometrioma. Our results suggest that not only atypical vaginal bleeding but also unwanted weight gain should be regarded as significant reactions, however.
Keywords: Dienogest; Endometriosis; Adverse Events; Vaginal Bleeding; Weight Gain
Introduction
Endometriosis is defined as the presence of endometrial glands and stroma outside the uterine cavity. The prevalence rate of endometriosis is approximately 5-10% of all women of reproductive age, it reduces the patient’s quality of life through the occurrence of pain, including dysmenorrhea, dyspareunia, and lower back pain [1- 3].
It is known that the primary treatment of endometriosis is surgery. The European Society of Human Reproduction and Embryology (ESHRE) guidelines state that surgical treatment is warranted for an ovarian endometrioma of larger than 3 cm, and that laparoscopic stripping of the cyst wall is considered the gold standard for treatment [4]. Because the surgical excision of lesions has shown to improve the level of pain and enhance fertility, ovarian cystectomy is preferable to oophorectomy, and most women who undergo endometriosis are of childbearing age. However, a pooled analysis of 23 studies estimated the recurrence rates as 40% to 50% 5 years after the primary surgery [5].
Medical treatment for the relief of symptoms and the prevention of recurrence after surgery include Gonadotropin-Releasing Hormone (GnRH) analogs, progestin, danazol, and estrogen/ progestin combinations. However, these treatments have adverse effects including impaired hepatic function associated with danazol, as well as a decrease in bone mineral density, which may be caused by GnRH analogs. Thus, their long-term use is limited [6,7].
Dienogest (Visanne, Bayer HealthCare, Berlin, Germany) is a selective progestin that has been approved for treating endometriosis at a low oral dose of 2 mg/day. Dienogest has many beneficial pharmacological uses, such as a potential progestogenic effect, moderate suppression of estrogen, and low concern for increased androgen and corticoid levels. Progestogenic effects lead to an effective reduction in endometrial lesions, and no significant androgenic, mineralocorticoid, or glucocorticoid activity. Dienogest has been developed to reduce side effects, such as a decreasing estradiol (E2) concentration, along with an elongation of the tolerable dose period [8-10].
Recent studies have reported that repeated surgeries for recurrent endometriomas mark a decrease in ovarian reserve and the success rate of in vitro fertilization [11,12].
Dienogest is able to prevent the recurrence of endometriosis after surgery for more than 15 months. However, most of the clinical data reflect follow-up durations of up to 6 months, and few data have been published beyond 12 months. To determine the tolerability of long-term treatment using dienogest, we evaluated patients who had undergone ovarian cystectomy for endometriosis.
Methods
Data were collected retrospectively from a chart review of 150 patients treated with 2 mg of dienogest over an 18-month period after a conservative surgery at Yangsan Hospital of Pusan National University. The subjects included 150 patients, 103 of whom had undergone laparoscopic unilateral cystectomy, 35 had undergone a laparoscopic bilateral cystectomy, and 12 had undergone a laparoscopic unilateral adnexectomy. Oral administration of dienogest was initiated on day 3 of the first menstruation following surgery. The primary modality of the diagnosis was through imaging (magnetic resonance or transvaginal ultrasonography). The Institutional Review Board of Pusan National University’s Yangsan Hospital approved this study, although written informed consent was not obtained because the present study was based only on a retrospective review of medical records. Transvaginal ultrasonography was used to measure the largest diameter of an endometriotic cyst. These measurements were repeated every 6 months after surgery. The serum cancer antigen 125 (CA125), cancer antigen 19-9 (CA19-9), and estradiol (E2) and Anti-Mullerian Hormone (AMH) concentrations were determined at baseline before treatment, and were repeatedly measured every 6 months to assist in assessing the clinical states of the patients.
The adverse event profile and patient satisfaction regarding dienogest treatment was determined through a questionnaire. Menstrual pain was determined before surgery, after surgery, before dienogest treatment, and after dienogest treatment. The severity of pain was measured on a 10-point visual analog scale (VAS; 0 mm, absence of pain; 100 mm, unbearable pain) during the outpatient visits.
Transvaginal ultrasonography was conducted on the patients every 6 months to assess the presence of endometrioma. Recurrent endometriosis was diagnosed when a round mass was identified with a thick wall, having a diameter of 2 cm or more, regular margins, and a homogenous low echogenic fluid content with scattered internal echoes. The patients were also evaluated according to the r-ASRM stage classification.
Adverse events were defined as any unfavorable or unintended signs or symptoms occurring with the use of dienogest. Serious adverse events were defined and documented according to the international standards.
Body weight was measured (at minimum) during the initial visit, and then repeatedly measured every 6 months. Weight gain was defined as an increase of over 1 kg.
The patients were instructed to record bleeding events daily on a diary card to provide information on the mean number of days, number of episodes, and duration of episodes of bleeding. Uterine bleeding was defined as bleeding for more than 10 days.
Continuous variables were analyzed using a paired t-test or Student’s t-test. A Wilcoxon signed rank test was used to compare the continuous variables if the data were not normally distributed. Categorical variables were presented as percentages and compared using a chi-square test. All statistical analyses were conducted using SPSS ver. 21.0 (IBM Co., Armonk, NY, USA), and p-values <0.05 were considered to indicate a statistical significance.
Results
Demographic characteristics of the participants who received 2 mg of dienogest are shown in Table 1. The average age of the patients was 39.4±8.1 years (range of 18-47 years in age), and the median duration of dienogest administration was 24 months (range of 18–60 months). All patients were diagnosed with endometriosis through a laparoscopy. A total of 111 patients (74%) were classified with stage III (moderate) or higher endometriosis, and 39 (26%) with stage I-II. However, the pain and staging were not correlated. The most common type of endometriosis was unilateral endometrioma (76.7%), followed by bilateral endometrioma (23.3%).
Characteristics
Patients (n = 150)
Age (yr)
39.4 ± 8.1
Body mass index (kg/m2)
19.8 ± 2.5
Previous pregnancy
68 (45)
Previous surgery for endometriosis
12 (8)
r-ASRM stage of endometriosis
Stage I-II
39 (26)
Stage III-IV
111 (74)
Type of endometriosis
Deepinfiltrating
11 (7.3)
Unilateral
115 (76.7)
Bilateral
35 (23.3)
Presence of Other diseases
Leiomyoma
56 (37.3)
Adenomyosis
28 (18.7)
Ovarian cyst
19 (12.7)
Paratubal cyst
11 (7.3)
Values are presented as mean ± standard deviation or number (%).
Table 1: Clinical characteristics.
Serum cancer antigen 125 (CA 125), cancer antigen 19-9 (CA 19- 9), and estradiol (E2) were determined at baseline prior to treatment, and were repeatedly measured every 6 months to assist in assessing the clinical state (Table 2). The mean serum cancer antigen CA 125, CA 19-9, and E2 levels before surgery were measured as 90.65±18.99 U/mL, 63.20±16.64 U/mL, and 231.82±98.13 pmol/L, respectively. During treatment of dienogest, the CA 125, CA 19-9, and E2 levels gradually decreased to 18.7±13.52 U/mL, 18.6±11.29 U/mL, and 27.56±53.29 pmol/L after 18 months.
Blood test
Pre-treatment
Post-treatment
Post-treatment
Post-treatment
P-value
6 months
12 months
18 months
CA125 (U/mL)
90.65±18.99
32.7±12.42
27.3±15.42
18.7±13.52
0.042
CA19-9 (U/mL)
63.20±16.64
28.7±17.32
21.3±9.26
18.6±11.29
0.025
E2 (pmol/L)
231.82±98.13
89.83±28.17
56.82±30.81
27.56±53.29
0.019
Value are presented as mean ± SE.
CA125, cancer antigen 125; CA19-9, cancer antigen 19-9; E2, estradiol.
Table 2: Laboratory parameters in 2 mg dienogest administered group: categorized based on treatment period.
The effects of dienogest on endometriosis-associated pain were determined by measuring pain-using VAS before surgery, after surgery, and after the dienogest treatment. The pain was significantly reduced after 18 months of medication as compared to pre-treatment (40.8±28.6 mm versus 7.3±14.6 mm, p<0.01) (Figure 1). Eleven patients showed no changes in their VAS scores after dienogest treatment during the 18-month period, whereas 139 patients showed decreased VAS scores. We observed that 92.7% of the patients demonstrated an improvement in terms of pain after surgery and long-term dienogest administration.
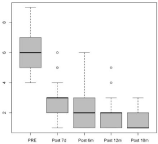
Figure 1: Changes in visual analog scale (VAS) score following dienogest
treatment for 18 months.
Because the goal of the study was to determine the effects of dienogest treatment for an 18-month period or longer, we analyzed the adverse events 18 months after dienogest administration. The most common adverse drug reaction was weight gain (24.0%), followed by headaches (6.0%), breast discomfort (5.3%), depression (2.0%), acne (1.3%), nausea (5.3%), and abdominal pain (3.3%) (Table 3). All adverse events except weight gain were generally mild to moderate, and only two women (1.3%) reported bleeding events as the primary reason for their discontinuation. However, the rate of discontinuation of treatment from weight gain was very high. The mean value of weight change during dienogest treatment was 2.8 kg, but 36 patients showed weight gain of over 4 kg during the 18-month period. Among the 36 patients, 12 women (8%) discontinued dienogest treatment on their own despite its effectiveness and an adequate explanation. A body weight analysis includes patients who provided both the baseline and highest end of the treatment data (Figure 2).
Adverse drug reactions
No (%) patients (n = 150)
Headache
9 (6.0%)
Breast discomfort
8 (5.3%)
Depressed mood
3 (2.0%)
Acne
2 (1.3%)
Nausea
8 (5.3%)
Weight gain
36 (24.0%)
Abdominal pain
5 (3.3%)
Note: Mean treatment duration: 21 months. Values are presented as number (%) of patients.
Figure 1: Changes in visual analog scale (VAS) score following dienogest treatment for 18 months.
Table 3: Adverse drug reactions for patients who received 2 mg of dienogest for more than an 18-month period.
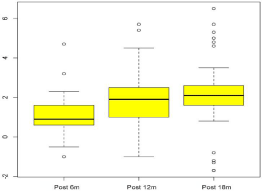
Figure 2: Change in body weight for patients who received 2 mg of dienogest
for more than an 18-month period.
Discussion
Because of an increased post-operative recurrence rate of endometrium and its related complications, secondary prevention is necessary to enhance the quality of life and fertility of those suffering from chronic endometriosis [13-16]. In the current retrospective analysis, we analyzed the efficacy and safety of long-term oral dienogest therapy for 150 patients under treatment in a single institution. In addition, we found the following effects of dienogest after 18 months or more of use.
Our study showed that, in patients who had taken dienogest treatment for at least 18 months after laparoscopic endometriosis surgery, the pain was decreased compared to the preoperative status, and the pain was relieved gradually with the use of dienogest. Our data indicate that dienogest was helpful in relieving endometriosisassociated pain based on a VAS. Laboratory findings also suggested noticeable therapeutic effects. CA125 and CA19-9 were slightly decreased after surgery, and gradually decreased with dienogest treatment during 6 months of interval follow-ups. Elevated serum CA 125 and CA 19-9 concentrations were significantly associated with endometriosis, and CA 19-9 is increased further in the more advanced stages of disease. The decrease in CA125 and CA19-9 suggest that dienogest treatment is effective in preventing the recurrence of endometriosis. Though the mechanism is not evident, dienogest treatment is suggested to inhibit extracellular signal-regulated kinase pathways, suppress the mammalian target of rapamycin, induce autophagy, and accelerate the apoptosis of endometriotic cells [17]. It is also possible that dienogest creates a high progesterone receptor ratio, and therefore enhances the responsiveness to progestin treatment in endometriotic tissue [18].
The adverse effects were mild to moderate in intensity, and were associated with low discontinuation rates. The most common adverse effects were atypical genital bleeding. It is known that the cause of atypical genital bleeding was a breakthrough of pseudodecidualized endometrium [19]. The initial increase in bleeding with dienogest treatment was followed by a progressive reduction during the continued treatment, accompanied by an increase in the amenorrhea rate. These observations suggest that the amount of atypical genital bleeding may depend on the space of the endometrium or the volume of the pseudodecidua. Thus, we can conclude that the patients in the dienogest treatment group showed irregular bleeding, but they all experienced only mild symptoms and none developed anemia. Furthermore, atypical genital bleeding rarely resulted in a discontinuation of treatment. In our cases, only two patients stopped treatment. In addition, headaches (6.0%), breast discomfort (8.7%), depression (2.0%), acne (4.7%), nausea (5.3%), weight gain (12.0%), and abdominal pain (3.3%) were also noted, but the symptoms were negligible. Weight gain turned out to be a common adverse effect of progestins [20-23]. The mean value of the weight change during dienogest treatment was 2.8 kg, but 36 of the patients showed a weight gain of over 4 kg during the 18-month period. Among the 36 patients, 12 women discontinued Progestin treatment despite the advantages of the treatment being mentioned again. Concern regarding weight gain in Korean women seems particularly high.
In other studies, the recurrence rate of ovarian endometriotic cysts was reported to be 25% for patients who underwent an operation within a 24-month period. The rate increases to 40–50% after 36 months post-operation [24]. Patients who received GnRHa as a post-operative treatment showed a 5.3% recurrence within 24 months [25]. In contrast, others have reported that GnRHa has no effect on the recurrence rate [26,27]. In contrast, a previous study with a smaller pool reported the effectiveness of administering 2 mg of dienogest for a 13-month period after an operation, suggesting its potential effectiveness as a treatment for endometriosis [28,29]. In our study, the recurrence rate after dienogest therapy was merely 4.5%. Among the group studied, 89.7% of the patients who were given dienogest continuously for 18.0±7.1 months described no complaints of any intolerable side effects, whereas 10.3% patients stopped at 2.4±1.0 months. The cause of discontinuation was weight gain for all cases, and other adverse effects were moderate.
The serum E2 levels decreased steadily until reaching lower than 60 pg/ml at 18 months. However, the E2 range was relatively broad, and reached approximately 280 pg/ml in certain cases. These elevated E2 levels were regarded as a result of follicle growth without ovulation under dienogest treatment. In this regard, dienogest appears to be a good candidate for the long-term treatment of endometriosis because it does not reduce the serum estradiol to the postmenopausal level, as does GnRH agonist. Long-term use of dienogest could be beneficial for patients who want to preserve their fertility. Dienogest prohibits an exacerbation of endometriosis, and may preserve the ovarian function. Therefore, dienogest can be considered effective for not only short-term but also long-term treatment in patients suffering from infertility. In accordance with our findings, the guidelines from the World Endometriosis Society recommends dienogest as an empirical treatment option for patients without a laparoscopic diagnosis, and as a suitable post-endometriosis surgery treatment [4].
Owing to its retrospective nature, the present study has several limitations. A large amount of data on certain important clinical outcomes in a few of the patients was missing. Because the data were solely based on a review of medical records written by clinicians, it was possible that the adverse events, such as uterine bleeding, could have been underestimated. In spite of these limitations, the present study shows that dienogest administration over an 18-month period is highly effective in preventing the recurrence of endometriosis after surgery, weakening endometriosis-associated pain, and reducing the size of recurrent endometriomas. Further prospective studies will be needed to evaluate the effects of long-term dienogest medication on the bone quality, the future risk of osteoporosis, and the ultimate reproductive outcomes in women with endometriosis.
Acknowledgements
This work was supported by a 2-year research grant from Pusan National University.
References
- D’Hooghe TM, Bambra CS, Raeymaekers BM, Koninckx PR. Increased prevalence and recurrence of retrograde menstruation in baboons with spontaneous endometriosis. Hum Reprod. 1996; 11: 2022-2025.
- Propst AM, Laufer MR. Endometriosis in adolescents. Incidence, diagnosis and treatment. J Reprod Med. 1999; 44: 751-758.
- Garry R, Diagnosis of endometriosis and pelvic pain. Fertil Steril. 2006; 86: 1307-1309.
- Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014; 29: 400-412.
- Van den Broeck U, Meuleman C, Tomassetti C, D’Hoore A, Wolthuis A, Van Cleynenbreugel B, et al. Effect of laparoscopic surgery for moderate and severe endometriosis on depression, relationship satisfaction and sexual functioning: comparison of patients with and without bowel resection. Hum Reprod. 2013; 28: 2389-2397.
- Brown J and C, Farquhar. Endometriosis: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2014.
- Laschke MW, Menger MD. Anti-angiogenic treatment strategies for the therapy of endometriosis. Hum Reprod Update. 2012. 18: 682-702.
- Koga K, Takamura M, Fujii T, Osuga Y. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil Steril. 2015. 104: 793-801.
- Oh ST. The Comparison Between 2mg Dienogest and High-Dose Medroxyprogesterone Acetate on Oral Treatment of Endometriosis. J Minim Invasive Gynecol. 2015. 22: S170.
- Park SY, Kim SH, Chae HD, Kim CH, Kang BM. Efficacy and safety of dienogest in patients with endometriosis: A single-center observational study over 12 months. Clin Exp Reprod Med. 2016; 43: 215-220.
- Roman, H. Endometriosis surgery and preservation of fertility, what surgeons should know. J Visc Surg. 2018; 155: S31-S36.
- Tinkanen H, Kujansuu E. In vitro fertilization in patients with ovarian endometriomas. Acta Obstet Gynecol Scand. 2000; 79: 119-122.
- Razzi, S, Fava A, Sartini A, Simone SD. Treatment of severe recurrent endometriosis with an aromatase inhibitor in a young ovariectomised woman. BJOG. 2004; 111: 182-184.
- Vercellini P, Bracco B, Mosconi P, Roberto A, Alberico D, Dhouha D, et al. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: a before and after study. Fertil Steril. 2016; 105: 734-743.
- Caruso S, Iraci M, Cianci S, Fava V, Casella E, Cianci A, et al, Comparative, open-label prospective study on the quality of life and sexual function of women affected by endometriosis-associated pelvic pain on 2 mg dienogest/30 microg ethinyl estradiol continuous or 21/7 regimen oral contraceptive. J Endocrinol Invest. 2016; 39: 923-931.
- Sugimoto K, Nagata C, Hayashi H, Yanagida S, Okamoto A. Use of dienogest over 53 weeks for the treatment of endometriosis. J Obstet Gynaecol Res. 2015; 41: 1921-1926.
- Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. 2009; 150: 3128-3137.
- Patel BG Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017; 96: 623-632.
- Tamura R. Tsuneki I, Yanase T. Effectiveness of the cyclic administration of dienogest in a case of pathological disappearance of intestinal endometriosis. Int J Womens Health. 2013; 5: 421-424.
- Chandra A, Rho AM, Jeong K, Yu T, Jeon JH, Park SY, et al. Clinical experience of long-term use of dienogest after surgery for ovarian endometrioma. Obstet Gynecol Sci. 2018; 61: 111-117.
- Jeng CJ, Chuang L, Shen J. A comparison of progestogens or oral contraceptives and gonadotropin-releasing hormone agonists for the treatment of endometriosis: a systematic review. Expert Opin Pharmacother. 2014; 15: 767-773.
- Jeong SH, Lee D, Kim SK, Jee BC. Symptom-alleviating effect and adverse effect of dienogest in Korean women with endometriosis. Gynecol Endocrinol. 2018; 7: 1-5.
- Kim SA, Um MJ, Kim HK, Kim SJ, Moon SJ, Jung H, et al. Study of dienogest for dysmenorrhea and pelvic pain associated with endometriosis. Obstet Gynecol Sci. 2016; 59: 506-511.
- Tanprasertkul C, Patumanond J, Manusook S, Suwannarurk K, Somprasit C, Sreshthaputra O, et al. Recurrence of Endometrioma Following Conservative Ovarian Endometrioma Cystectomy: Laparoscopy versus Laparotomy. J Med Assoc Thai. 2015; 98: S96-100.
- Dimitrijevic D, Vasiljevic M, Anicic R, Brankovic S, Ristic A, Devic A, et al. Recurrence rate of ovarian endometriosis in patients treated with laparoscopic surgery and postoperative suppressive therapy. Clin Exp Obstet Gynecol. 2015; 42: 339-343.
- Fedele L, Bianchi S, Zanconato G, Tozzi L, Raffaelli R. Gonadotropinreleasing hormone agonist treatment for endometriosis of the rectovaginal septum. Am J Obstet Gynecol. 2000; 183: 1462-1467.
- Zhong YJ, Zhang W, Zhang WT, Cheng J, Lu QY, Zeng KK, et al. [Efficacy and safety of GnRH-a combine with laparoscope conservative surgery in the treatment of the moderate or severe endometriosis]. Zhonghua Fu Chan Ke Za Zhi. 2013; 48: 180-182.
- Lee SR, Yi KW, Song JY, Seo SK, Lee DY, Cho S, et al. Efficacy and Safety of Long-Term Use of Dienogest in Women With Ovarian Endometrioma. Reprod Sci. 2018; 25: 341-346.
- Yamanaka A, Hada T, Matsumoto T, Kanno K, Shirane A, Yanai S, et al. Effect of dienogest on pain and ovarian endometrioma occurrence after laparoscopic resection of uterosacral ligaments with deep infiltrating endometriosis. Eur J Obstet Gynecol Reprod Biol. 2017; 216: 51-55.