
Research Article
Austin J Obstet Gynecol. 2019; 6(2): 1136.
Effect of Topical Vaginal Antimicrobials on Recurrence Rate of Vaginitis in a Rural Community in Ismailia Governorate
Madny EH*, Ibrahim NM, Shahira A. Ahmed, Elwahed HAA and Khattab MS
¹Department of Obstetrics and Gynecology with Department of Medical Parasitology and Department of Family Medicine, Suez Canal University, Egypt
*Corresponding author: Elham Hussien Madny, Department of Obstetrics and Gynecology at Faculty of Medicine, Suez Canal University, Egypt
Received: March 18, 2019; Accepted: April 03, 2019; Published: April 10, 2019
Abstract
Background: vaginitis is common gynecological complain and recurrent vaginitis would have a great impact on women health and quality of life therefore it is immensely needed to test new regimens that could decrease recurrence rate of vaginitis in women.
Objectives: To assess the effect of using monthly prophylactic combined vaginal suppositories (metronidazole 750 mg + miconazole 200 mg) in prevention of recurrent vaginitis in rural communities.
Methods: A double blinded randomized controlled clinical trial was conducted on 200 patients with recurrent vaginitis attending EL Mahsama family practice center affiliated to Suez Canal University in Ismailia city. Patients selected randomly and allocated by using a computer program (Random Number Generator RNG) into control group including 100 patients receiving placebo and intervention group including 100 patients receiving co-formulated vaginal ovules (750 mg Metronidazole + 200 mg miconazole) each group receives their ovules 5 days monthly for 6 months.
Results: The interventional group has significantly less symptoms than control group after implementation of the intervention (p‹0.001).
Also there were29 cases of vaginitis less in interventional group per 100 persons compared with that in control groups with a number needed to treat of four persons. Thus, on average 4 patients would have to receive the monthly combined vaginal suppositories to prevent one excess event of vaginitis.
Conclusion: Co-formulated vaginal ovules (750 mg metronidazole+200 mg miconazole) 5 days/month for 6 months duration is effective in decreasing recurrence of vaginitis in patient with recurrent B.V and V.V candidacies by about 29 percent
Keywords: Infectious vaginitis; Recurrent vaginitis; Recurrent; Vulvovaginal candidiasis; Trichomoniasis
Background
Vaginitis is defined as a spectrum of conditions that cause vaginal and sometimes vulvar symptoms, such as itching, burning, irritation, odor, and vaginal discharge. Vulvovaginal complaints are one of the most common reasons for women to seek medical advice [1].
There are three common causes of infectious vaginitis bacterial vaginosis, trichomoniasis and candidiasis, of which trichomoniasis is a sexually transmitted infection [2].
The normal postmenarchal and premenopausal vaginal pH is 3.8- 4.2. At this pH, growth of pathogenic organisms usually is inhibited. Disturbance of the normal vaginal pH can alter the vaginal flora, leading to overgrowth of pathogens [3].
Recurrent infectious vaginitis is highly important and preventable health problem encountered in our daily practice. It was estimated that 75% of women will experienced at least one episode of vulvovaginal candidiasis, and 40 to 45% will have two or more [4].
Bacterial Vaginosis (BV) is the commonest cause of abnormal vaginal discharge in woman of childbearing age, but may also be encountered in menopausal women. It represents 33% of Egyptian women vaginal infections [5]. Recurrent infections even when asymptomatic, is associated with a high incidence of endometritis and pelvic inflammatory disease. Bacterial vaginosis is associated with late miscarriages, premature rupture of membranes, and preterm birth [6].
According to guidelines, metronidazole is the recommended treatment of BV, used orally twice daily for 7days and also recommended for management of tichomoniasis in the same way or as a single 2 gm. dose. Miconazole is an antifungal used topically, in a dose of 200-400 mg for treatment of (VVC) for 3 consequitive days [1,2]. Recommended maintenance regimens for recurrent vulvovaginal candidiasis after initial treatment include fluconazole 100,150 or 200mg orally once weekly for six months [1]. This systemic antifungal has many contraindications and side effects as well as of much more cost to the patient [7,8].
One Randomized Clinical Trial (RCT) on persistent bacterial vaginosis indicated that metronidazole gel 0.75% (Metrogel), used twice weekly for six months after initial treatment, effectively maintained a clinical cure for six months [9].
Another study in Kenya recommended combined metronidazole and miconazole vaginal suppository to prevent recurrent vaginitis generally in HIV negative women [10].
Materials and Methods
Abstract
We conducted a randomized clinical trial in EL-Mahsama family practice center affiliated to Suez Canal University, Ismailia, Egypt. This trial was conducted from July 2016 to May 2018, after being approved by the Medical Ethical Committee at Faculty of Medicine, Suez Canal University. In addition, an informed consent was obtained from each patient. The participants were aged between 18 and 40 years old females diagnosed with recurrent vaginitis. Recurrent vaginitis was diagnosed according to clinical and lab criteria. Box 1 demonstrates the exclusion criteria.
Study procedure
We enrolled two hundred patients in this trial. Patients were randomly allocated to two groups; the interventional or control, by simple random technique, where each group consisted of 28 patients. Data describing the socioeconomic status, education, occupation, and income were obtained from the participants. Then, all patients were subjected to full medical history taking and clinical examination. Patients in the interventional group received their treatment for the current presenting infection according to American academy of family physicians guidelines. as mentioned above in addition to maintenance regimen in the form of co-formulated vaginal ovule (miconazole 200 + meronidazole 750 mg) once daily for 5 consecutive days each month for six months.
The control group received their treatment for the current presenting infection according to American academy of family physicians guidelines. The maintenance regimen after treatment of acute infection was in the form of placebo vaginal suppository, same time as in intervention group.
Outcome measures
1-Recurrence rate of vaginal infections: it had been assessed monthly during 6 months of therapy using history and clinical criteria but lab criteria had been re-assessed only after 6 months at the end of the study.
Table 1 shows baseline characteristics of patients in both groups before intervention. In the interventional group, 44% of the females were included in (31-35) age group, while in control group, 54% of them were included in the same age group. Illiteracy was the most frequent educational level among both interventional (36%) and control (40%) groups. Most females in both groups were housewives, 60% in interventional group and 64% in control. Most females in both groups had free governmental health care source, 84% in interventional group and 77% in control. In addition, most females in both groups didn’t have habitual vaginal douching, 74% in interventional group and 71% in control. About 80% of females in the interventional group didn’t have any co-morbid diseases, while 77% of females in the control group didn’t have any co-morbid diseases as well. Family stressors were present in about 60% of females in both groups. Finally, there was no significant difference between the two groups in any of the baseline characteristics (>0.05).
Variables
Total population
Study groups
test value
p-value
n=200
Intervention group (n=100)
Control group (n=100)
n (%)
n (%)
n (%)
Age groups
25-30
25 (12.5)
16 (16)
9 (9)
0.48
0.213a
31-35
98 (49)
44 (44)
54 (54)
36-40
77 (38.5)
40 (40)
37 (37)
Occupation
House wife
124 (62)
60 (60)
64 (64)
0.88
0.331a
Unskilled manual worker
76 (38)
40 (40)
36 (36)
Educational level
Illiterate
75 (37.5)
39 (39)
36 (36)
0.41
0.551b
Read and write
53 (26.5)
25 (25)
28 (28)
Intermediate
67 (33.5)
35 (35)
32 (32)
University
5 (2.5)
1 (1)
4 (4)
The usual source of health care
Free governmental HS
161 (80.5)
84 (84)
77 (77)
1.24
0.142a
More than one source
39 (19.5)
16 (16)
23 (23)
Use of vaginal douching by the patients
Habitual use
55 (27.5)
26 (26)
29 (29)
0.47
0.376a
No use
145 (72.5)
74 (74)
71 (71)
Co-morbid diseases
No comorbid disease
156 (78)
79 (79)
77 (77)
0.34
0.432a
Diabetes
44 (22)
21 (21)
23 (23)
Smoking
Non-smoker
81 (40.5)
39 (39)
42 (42)
0.24
0.378a
Passive smoker
119 (59.5)
61 (61)
58 (58)
Family stressor
Present
120 (60)
62 (62)
58 (58)
0.68
0.333a
Absent
80 (40)
38 (38)
42 (42)
aP-values are based on Chi-square test. Statistical significance at p <0.05.
bP-values are based on Fisher exact test. Statistical significance at p <0.05.
Table 1: Baseline sociodemographic characteristics of study groups.
Table 2 shows that the most prominent presentation in the interventional and control groups were combined symptoms of discharge, itching and dysparunia (68%) and (30%), respectively. Moreover, the most widely used contraceptive method in both groups was the pills with proportion (45%) and (50%), respectively. Meanwhile, there was no significant association between study groups and presenting clinical symptoms (p=0.84) or different contraceptive methods (p=0.829).
Variables
Total population n=200
Study groups
test value
p-value
Intervention group (n=100)
Control group (n=100)
n (%)
n (%)
n (%)
Clinical symptoms
Vaginal discharge
23 (11.5)
9 (9)
23 (23)
0.24
0.842 a
Itching
26 (13)
14 (14)
26 (26)
Dyspareunia
21 (10.5)
9 (9)
21 (21)
Combined symptoms
130 (65)
68 (68)
30 (30)
Contraceptive methods
IUD
75 (28.5)
38 (38)
37 (37)
0.44
0.829 b
Pills
95 (47.5)
45 (45)
50 (50)
Implants
20 (10)
11 (11)
9 (9)
Injections
10 (5)
6 (6)
4 (4)
aP-values are based on Chi-square test. Statistical significance at p <0.05.
bP-values are based on Fisher exact test. Statistical significance at p <0.05.
Table 2: Clinical characteristics and used contraceptive methods of study groups.
Table 3 shows that the most presenting organism by wet mount test was VV Candidiasis (about 40% in each group), while most results appeared by KOH testing (whiff test) was positive in both interventional (62%) and control (61%) groups. On the other hand, there was no significant association between the study groups and both results of wet mount test (p=0.96) or whiff test results (p=0.887).
Variables
Total population
Study groups
test value
p-value
n=200
Intervention group (n=100)
Control group
(n=100)
n (%)
n (%)
n (%)
Wet mount testing
BV
59 (29.5)
29 (29)
30 (30)
0.11
0.96a
VV candidiasis
77 (38.5)
38 (38)
39 (39)
TV
12 (6)
6 (6)
6 (6)
Mixed vaginal infection
52 (26)
27 (27)
25 (25)
KOH testing (whiff test)
Positive whiff test
123 (61.5)
62 (62)
61 (61)
0.02
0.887a
Negative whiff test
77 (38.5)
38 (38)
39 (39)
BV: Bacterial Vaginosis, VV: Vulvovaginal Candidiasis., TV: Trichomonas Vaginalis
aP-values are based on Chi-square test. Statistical significance at p <0.05.
Table 3: Diagnostic testing of both interventional and control groups before intervention.
Post intervention comparison between study group
Table 4 shows that, interventional group has significantly more cured cases than in control group after implementation of the intervention. (p‹0.001).
Variables
Total population n=200
Study groups
test value
p-value
Intervention group (n=100)
Control group (n=100)
n (%)
n (%)
n (%)
Cured (negative test)
47
38
9
0.11
<0.001a
BV
47
19
28
VV candidiasis
56
20
36
TV
10
5
5
Mixed vaginal infection
40
18
22
BV: Bacterial Vaginosis; VV: Vulvovaginal Candidiasis; TV: Trichomonas Vaginalis
aP-values are based on Chi-square test. Statistical significance at p <0.05.
bP-values are based on Fisher exact test. Statistical significance at p <0.05.
Table 4: Effect of combination regimen on both interventional and control groups after intervention.
Figure 1 illustrates that recurrence rate of infection in interventional group was significantly less than in control group after intervention (p=0.001).
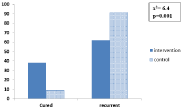
Figure 1: The effect of the intervention on the recurrence of infection of the
study groups.
Table 5 shows that there are 29 cases of vaginitis less in interventional group per 100 persons compared with that in control groups with a number needed to treat of four persons. Thus, on average 4 patients would have to receive the monthly combined vaginal suppositories to prevent one excess event of vaginitis.
Groups
No. per group
Event No.
Incidence rate
Attributable Risk
NNT
Interventional
100
62
0.62
0.29
4
Controls
100
91
0.91
Table 5: Incidence Rates, Attributable Risk (Rate Differences), and Numbers needed to treat for recurrence of vaginitis in females treated with monthly combined vaginal suppositories as a prophylaxis vs. placebo.
Discussion
The study sample included 200 patients with recurrent vaginitis cumbering the co-formulated vaginal ovules with placebo.
For BV, it represented (29-30%) in intervention and control group, respectively with no statistical significant difference between both groups. this is consistent with another study that found bacterial vaginosis is the most common cause of vaginal symptoms among women. The prevalence in the United States was estimated to be 29.2% among women ages 14-49 years [5].
In Egypt; another study [11]. Rasheed M. Salah and colleagues reported a prevalence of BV in the control women of 15.4% (59/ 382) compared to 45.5% (398/874) in infertile women.
This prevalence is consistent with another study [12] that found the overall prevalence of BV was 24.4% among symptomatic patients.
A systematic review in 2013 reported that BV prevalence varies between and within countries worldwide [13] Women from South and East Africa have higher rates of BV compared to women from West Africa. Women in Latin America and the Caribbean have lower rates of BV, except in rural and antenatal populations in Jamaica and Peru.
Regarding trichomoniasis, this study found that it represent 6% of each study groups and this is consistent with a study [14] that compare different prevalence of trichomoniasis and also mentioned the prevalence among males and females.
After the intervention this study found that there is statistical significant difference (p‹0.05) between groups regarding wet mount testing especially in B.V and V.V candidacies .Infection recurred in 62% of intervention group versus 91% in control group. This is consistent with many studies [7,15,16] that found metronidazole gel 0.75% used twice weekly for 4 months was reducing recurrence rate for BV.
One Randomized Clinical Trial (RCT) on persistent bacterial vaginosis indicated that metronidazole gel 0.75% (Metrogel), used twice weekly for six months after initial treatment, effectively maintained a clinical cure for six months [9].
Another study in Kenya [10] recommended combined metronidazole and miconazole vaginal suppository to prevent recurrent vaginitis generally and mainly BV in HIV negative women. This regimen decrease BV by about 19%. In our study the recurrence rate is markedly decrease in both BV and VV candidiasis as well after the intervention by about 29 percent. This have the same conclusion discussed earlier [10].
Limitations of the Current Study
• No staining used in diagnosis of BV we depend only on amsel criteria for diagnosis.
• No culture used for diagnosis of VV candidiasis to differentiate candida albicans from other candida species.
Conclusion
Co-formulated vaginal ovules (750 mg metronidazole+200mg miconazole) 5 days/month for 6 months duration is effective in decreasing recurrence of vaginitis in patient with recurrent B.V and V.V candidacies by about 29 percent.
References
- Hainer BL, Gibson MV, Md P. American Family Physician. Guideline on the Management of Vaginal infecions. 2011; 83: 807-815.
- Sherrard J, Donders G, White D, Jensen JS, European IUSTI. European (IUSTI/WHO) guideline on the management of vaginal discharge, 2011. Int J Std Aids. 2011; 22: 421-429.
- Vaginal infections. Medscape.
- Corsello S, Spinillo A, Osnengo G, Penna C, Guaschino S, Bettrame A, et al. An epidemiological survey of vulvovaginal candidiasis in Italy. Eur J Obstet Gynecol Reprod Biol. 2003; 110: 66-72.
- Akinbiyi AA, Watson R F-WP. Prevalence of Candida albicans and bacterial vaginosis in bacterial vaginosis, and candidiasis in women of reproductive age in rural Northeast Brazil: a population based study. Mem Inst Oswaldo Cruz. 2007; 102: 751-756.
- Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: A meta-analysis. Am J Obstet Gynecol. 2003; 189: 139-147.
- Boris J, Påhlson C, Larsson PG. Six year’s observation after successful treatment of bacterial vaginosis. Infect Dis Obstet Gynecol. 1997; 5: 297-302.
- Flagyl (metronidazole) treatment uses. Medscape.
- Sobel JD, Ferris D, Schwebke J, Nyiriesy P, Wiesenfeld HC, Peipert J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006; 194: 1283-1289.
- McClelland RS, Balkus JE, Lee J, Anzala O, Kimani J, Schet al. Randomized trial of periodic presumptive treatment with high-dose intravaginal metronidazole and miconazole to prevent vaginal infections in HIV-negative women. J Infect Dis. 2015.
- Salah RM, Allam AM, Magdy AM, Sh A. European Journal of Obstetrics & Gynecology and Reproductive Biology Bacterial vaginosis and infertility: cause or association ? Eur J Obstet Gynecol. 2013; 167: 59-63.
- Ranjit E, Raghubanshi BR, Maskey S, Parajuli P. Prevalence of bacterial vaginosis and its association with risk factors among nonpregnant women: A hospital based study. Int J Microbiol. 2018; 2018: 8349601.
- Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: A systematic review. Am J Obstet Gynecol. 2013; 209: 505-523.
- Bowden GGF. Trichomonasvaginalis epidemiology: parameterising and analyzing a model of treatment interventions. Sex Transm Infect. 2000; 76: 248-256.
- Girerd PH. Bacterial vaginosis workup. Medescape.
- Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009; 3: CD006055.