
Mini Review
Austin J Obstet Gynecol. 2019; 6(2): 1137.
Endometriosis: Role of Aromatase and Recent Advances in the Disease Treatment
Kusum1, Rai S2 and Chaube R1*
¹Department of Zoology, Institute of Science, India
²Department of Obstetrics & Gynecology, IMS, India
*Corresponding author: Radha Chaube, Department of Zoology, Assistant Professor, Institute of Science, Banaras Hindu University (BHU), Varanasi, India
Received: March 18, 2019;Accepted: April 17, 2019; Published: April 24, 2019
Abstract
Endometriosis is a disease condition where endometrial glands and tissues are present outside its uterine location mainly in pelvic peritoneum and on fallopian tubes and ovaries. It affects about 5-10% of women of reproductive phase and 20-50% of women who are suffering already with infertility. The aim of this study was the identification of causative factors, regulatory pathways, pathway related genes, factors leading to the cause or pathogenesis of endometriosis and recent advances in the field of its remedial measures. Overall updated literature survey showed that with the medicine, yoga, life style changes and ayurveda has more pronouncing effect on its treatment strategies.
Keywords: Endometriosis; Reproductive women; Remedies; Ayurvedic medicines; Yoga
Introduction
Endometriosis is presence of endometrial glands and stromal tissues outside the uterine endometrial region mostly in pelvic peritoneal region, on fallopian tubes and ovaries. It is an estrogen dependent gynecological disorder occurring in 5-10% of reproductive age women and 20-50% of women with infertility [1-4]. There are various factors which have been suggested to play an important role in the establishment and development of endometriosis. It includes sociodemographic characteristics, reproductive factors, environmental factors and personal habits [5]. All these factors have cumulative effect and help in development and progression of endometriosis.
Estrogen is the main hormone, which plays an important role in the pathogenesis of endometriosis and aromatase is the ratelimiting enzyme in its biosynthesis. For endometriosis there are many causative factors which are directly or indirectly involved in estrogen biosynthesis by affecting aromatase expression that regulates estrogen production in ovary, extra-ovarian sites and endometriotic implants. Most of the causative factors for endometriosis are related to steroidogenesis pathway, cAMP pathway or Hypothalamus- Pitutary-Gonadal Axis (HPG). These factors mainly affect genes of the pathways and thus there occurs excessive estrogen formation leading to growth and progression of endometriotic implants. However there are a number of medical therapies for endometriosis and all of these are mainly based on reduction or suppression of estrogen formation in endometrotic implants by regulating the aromatase expression (Figure 1).
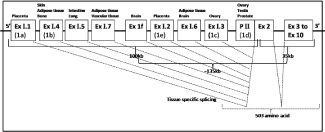
Figure 1: Gene structure of human aromatase with exon, intron and promoter II regions.
Estrogen and Aromatase
Estrogen biosynthesis occurs in a number of tissues that include ovarian granulose cells, corpus luteum of the ovary [6,7], testicular leydig cell [8], placental syncytiotrophoblast, adipose tissue [9], brain hypothalamus, amygdale and hippocampus regions [10,11].
Aromatase, the key enzyme for estrogen biosynthesis converts androstenedione into biologically active estrogen. Expression level of aromatase gene is highest in the ovarian granulosa cells in premenopausal women but later after menopause adipose tissue becomes the major aromatase gene expressing site [9]. It is immuno localized exclusively in the cytoplasm of glandular cells but immunoreactivity was not detected in the endometrial stroma [12]. It is present in the endoplasmic reticulum of cells and is composed of a specific form of cytochrome P450, called as aromatase cytochrome P450. Aromatase gene has marked homology to those of other members, notably a putative membrane-spanning region, I helix, ozoles and heme binding regions [13]. This enzyme utilizes molecular oxygen and reducing equivalents provided by a ubiquitous NADPHcytochrome P450 reductase to catalyze a series of three sequential hydroxylations that results in loss of the angular methyl group at carbon 19 and phenolization of the A ring of the steroid.
Aromatase (CYP19A1) spans about 135kb human genome with 503 amino acids sequence and is located at 15q 21.1 region of chromosome 15. This gene is very unique having 11 exons with 9 exons being translated, interrupted by 10 introns (about 80kb, exon 2a to exon 2) and consists of approximately 130kb pairs [5]. The unique sequences are present in the 5’-ends of the transcripts scattered over 100kb upstream of exon 2, whereas the translated coding sequences are present between exon 2-10 that lies about 35kb of the 3’-end of the gene [33,43]. Human aromatase gene includes multiple promoters and the aromatase transcript is tissue-specifically spliced from the multiple alternative exons available for exon 1. Presently nine unique alternative versions of exon 1 have been isolated. Each exon 1 is used in a tissue-specific manner; I.1 (1a) and I.2 (1e) in the placenta, I.3 (1c) and PII (1d) in the ovary and testis, I.4 (1b) in adipose tissue, I.5 in the fetal lung and intestine, I.6 in adipose and bone tissues, I.7 in adipose and vascular endothelial tissues, and 1f in the brain [26,44].
Aromatase Regulation in Endometriosis
In reproductive age women, Follicle Stimulating Hormone (FSH) binds to FSH receptor which is a G-protein-coupled receptor present on ovarian granulosa cell membrane that raises the intracellular cAMP levels and that further enhances the binding of two critical transcription factors. There are two most important transcription factors Steroidogenic Factor-1 (SF-1) and cAMP Response Element Binding Protein (CREB), both of these are located in proximal promoter II region of the aromatase gene (CYP19gene) [14]. These transcription factors in turn activate aromatase gene expression and consequently estrogen synthesis from the pre-ovulatory follicles [14,15]. In post menopausal women, expression of aromatase gene is regulated by the use of alternative promoter I.4 in adipose tissues and skin fibroblast by the similar regulatory cAMP pathway, which is activated by cytokines (IL-6, IL-11, TNFa) and glucocorticoides. It is proposed that aberrant aromatase expression is the only important molecular mechanism in the development and growth of pelvic endometriosis. In endometriotic implants aromatase activity was significantly stimulated by a cAMP analog, PGE2 or a combination of a glucocorticoid and a cytokines via cAMP pathway. PGE2 effect is mediated via the cAMP-inducing EP2 (receptor subtype). Estrogen increases PGE2 formation by stimulating Cyclo-Oxygenase type-2 (COX-2) enzyme in endometrotic implants. Thus a positive feedback loop for continuous local production of estrogen and prostaglandins, favouring the proliferation and inflammation in endometrotic implants is established. There may be other affecting molecular mechanisms that favour the development of endometriosis which includes abnormal expression of proteinase type enzymes that remodel tissues or their inhibitors [MMPs (Matrix Metalloproteinases), TIMPs (Tissue Inhibitor of Metalloproteinase-1)], certain cytokines (IL-6, RANTES) and Endometrial Growth Factors (EGF).
Genetical Studies Including Hormone Receptors, Steroids and Inflammatory Pathway Genes
Endometriosis is a heritable common gynaecological condition influenced by multiple genetic and environmental factors [16]. In view of this, genetic variants of genes that involves Estrogen Receptor Genes (ESRs), Progesterone Receptor genes (PRs), Steroidogenic Acute Regulatory gene (StAR), gonadotropin genes, steroid biosynthesis pathway genes mainly aromatase were extensively studied in recent years and a number of causative factors are yet to know.
It was found that both estrogen and progesterone receptors were present in endometriotic lesions. Immunocytochemical techniques demonstrated cyclic changes of Estradiol Receptors (ER) and Progesterone Receptors (PR) in human uterine tissues in several studies [17]. A differential regulation of the these receptors expression in the different uterine layers, such as the glandular epithelium and the stroma of the endometrium respectively reflects the differential functions with differential requirements of hormone effects of these tissues during different phases of the cycle [18-21]. It has been demonstrated that in normal endometrial tissue and endometriotic cysts estrogen molecule binds preferentially to ERa than to ERβ receptors [22]. Estrogen modulating enzymes in endometriotic lesions and cell lines has shown that, in contrast to normal endometrium of women without endometriosis, expression of cytochrome P450 or aromatase gene (CYP19) and 17β-Hydroxysteroid Dehydrogenase type 2 (17-HSD2) genes are present in the endometriotic lesions and uterine endometrium from patient [23,24]. But, in contrast to it, expression of 17β-Hydroxysteroid Dehydrogenase type 1 (17- HSD1) is deficient in these tissues which catalyzes the conversion of E1 (Estrone) to E2 (Estradiol) [25]. Estrogen produced by aromatase activity in the cytoplasm of leiomyoma smooth muscle cells or endometriotic stromal cells exert its effects by readily binding to its nuclear receptor within the same cell [23,26,27]. P450 aromatase is localized in the cytoplasm of glandular cells of adenomyotic tissues and eutopic endometrial obtained from patients with endometriosis [12] on the other hand disease-free endometrium and myometrium, lack its expression [27,28]. E2 and cytokines (IL-lβ, TNFa), which are increased in endometriosis induces Cyclo-Oxygenase-2 (COX- 2) gene giving rise to elevated concentrations of Prostaglandin E2 (PGE2) in these tissues. PGE2 is a most potent known stimulator of aromatase gene in endometriotic stromal cells [27] and a positive feedback loop get established in endometriosis patients causing a continuous estrogen formation leading to diseased condition (Figure 2).
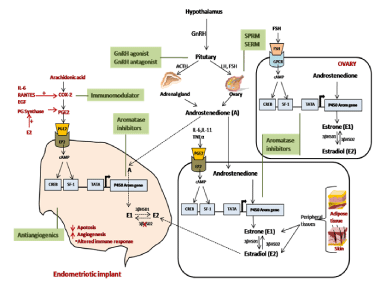
Figure 2: Pathway of aromatase (CYP19) gene action for conversion of androstenedione into estrogen in ovary, extra-ovarian tissues and in endometriotic
implants. Different treatment measures for endometriosis act at molecular level by endocrine regulatory pathways.
Remedial Measures
All medical treatments for endometriosis are designed to decrease estrogen secretion by the ovaries, extra-ovarian tissues and in endometriotic implants (Table 1). Presently endometriosis is successfully suppressed by estrogen deprivation with antiandrogenic hormone treatments with danazol, Levonorgestrel-releasing intrauterine device (LNG-IUD), Gonadotropin Releasing Hormone (GnRH) analogues, Selective Estrogen Receptor Modulators (SERM), Selective Progesterone Receptor Modulators (SPRM), aromatase inhibitors, or induction of surgical menopause [29-32]. Current evidences suggest that about 50% of endometriosis patients with chronic pelvic pain are resistant to current available treatments that causes hypoestrogenic state [33-35]. Patient’s undergone surgery often recurs with pain remaining to repeated surgical attempts and immediate response to chronic pain in 50% cases of surgical menopause [36].
Hormonal
GnRH agonist
Goserelin, Leuprolide, Nafarelin, Buserelin, Triptorelin
Garner et al., 1994, Kitawaki et al., 2008
GnRH antagonist
Cetrorelix, Abarelix, Elagolix, Danazol
Kupker et al., 2002, Altintas et al., 2008, Taniguchi et al., 2013, Diamond et al., 2014
Levonorgestrel-releasing intrauterine device (LNG-IUD)
Fedele et al., 2001
Aromatase Inhibitors (AIs)
Anastrozole, Letrozole
Ferrero et al., 2011, Agarwal et al., 2015
Selective Estrogen Receptor Modulators (SERMs)
Raloxifene, Tamoxifen, Bezedoxifene, Chloroindazole, Oxabicycloheptene
Yao et al., 2005, Yavuz et al., 2007,Stratton et al., 2008, Atlintas et al., 2010, Kulak et al., 2011, Naqvi et al., 2014, Lyu et al., 2015, Ezzat L, 2017
Selective Progesterone Receptor Modulators (SPRMs)
Mifepristone (RU-486), Onapristone, Asoprisnil, Mifepristone, Ulipristal acetate, Tanaproget
Chawlisz et al., 2005, Zhang YX , 2016, Hunaidi et al., 2013, Bruner –Tran et al., 2006
Angiogenesis inhibitors
Letrozole, Anastrazole, TNP470 endostatin, Anginex, Rapamycin, Thalidomide, Statin, Rapamycin, Dopamine cabergoline (Cb2), Quinagolide
Verma and Konje, 2009, Bilotas et al., 2010, Ferrero et al., 2011, Almassinokiani et al., 2014, Agarwal et al., 2015
Non hormonal
Immunomodulators
Loxoribine, IFN-a 2β, Etanercept, Lipoxin, Rapamycin, Infliximab, Pentoxifylline
Badawy et al., 2001, Barrier et al., 2004, Laschke et al., 2006, Koninckx et al., 2008, Kamencic and Thiel, 2008, Vlahos et al., 2010, Xu et al., 2012, Ingelmo et al., 2013, Kumar et al., 2014, Ren et al., 2016, Ezzat L, 2017
Antiantiangiogenics
Caplostatin, Endostatin, Angiostatin, Lovastatin, Atorvastatin, Simvastatin, Lodamin, Romidepsin, Icon, Cabergoline, Bromocriptine, Quinagolide, Fenofibrate, Rosiglitazone, Ciglitazone, Bentamapimod
Dabrosin et al., 2002,Backer et al., 2006, Jiang et al., 2007, Esfandiari et al., 2007, Lebovic et al., 2007, Oktem et al., 2007, Bruner-Tran et al., 2009, Onalan et al., 2009, Novella-Maestre et al., 2009, Sharma et al., 2010, Krikun et al., 2010, Becker et al., 2011, Delgado-Rosas et al., 2011, Herington et al., 2011, Imesch et al., 2011, Zhang et al., 2012, Almassinokiani et al., 2013, Lebovic et al., 2004; 2013,Chang et al., 2013, Hamid et al., 2014, Ma and He, 2014, Ercan et al., 2015, Hussein et al., 2016, Ezzat L, 2017
Table 1: Medical treatments of endometriosis.
Cyperusrotundus, Corydalis yanhusuo, Lenonurus, Curcuma phaelculis, Salvia miltiorrhiza, Typhaangustifolia, Trogopterusxanthipes, Taraxacummongolicum, Cuscutachinensis and Sparganiumstoloniferum is commonly used as single herbs for endometriosis.
Tsai et al., 2017
Herbal combinations / formulations including Cyperusrotundus, Sparganiumstoloniferum, Corydalis yanhusuo, Typhaangustifolia, Curcuma phaelculis, Meilatoosendan with anti-inflammatory & anti-oxidative activity are used for endometriosis.
Tsai et al, 2017
A formulation of Polygonumbistorata, Acacia Arabica and Santalum album is useful in menorrhagia for having anti-haemorrhagic activity.
Jahan et al., 2016
Bamboo (B. vulgaris) leaves have wound healing and anti inflammatory potential and can be used for endometriosis.
Lodhi et al., 2016
Turmeric (Curcuma longa Linn) has been proven for its anti-inflammatory, antioxidant, anti-mutagenic, anti-diabetic, anti-bacterial and anti-cancerous pharmacological activities and can be used for endometriosis.
Krup et al., 2013
Cinnamon twig, Myrrh, Chinese angelica and Licorice root are used as alternatives for pain relief in endometriosis.
Suresh et al., 2012
A specific dose of mixture of Triphala, Kanchanarguggul & Chandraprabhavati has given a new hope for treatment of ovarian cyst.
Himanshu et al., 2011
QuyuJiedu Granules raises the ova quality by reducing TNF-alpha and IL-6 levels to improve the living micro-environment for the ova.
Lian et al., 2009
Table 2: Ayurvedic herbs, formulations/compositions for endometriosis.
Pain associated with endometriosis return back in cases may be due to presence of continuous significant amount of estradiol hormone production in adipose tissues, skin and endometriotic implants during different treatments. If blockage of aromatase activity in these extra ovarian sites will be done by an aromatase inhibitor may keep larger number of patients free from recurrence of endometriosis related pain for longer period of time. The most serious side effects of the GnRH agonist treatment for endometriosis are mainly bone loss that may be due to estrogen deficiency. But oral estrogen-progesterone preparations or bisphosphonates are usually added back to minimize bone loss in these patients [37]. New strategies are needed for women with endometriosis, to live without suffering from chronic pelvic pain of decades. Now onwards with genetic factors, menstrual, environmental and lifestyle are included as pathogenic factors for disease establishment and its development [38] and which can be as new remedial measures for endometriosis.
New Approaches for Endometriosis Treatment: Yoga, Ayurvedic Medicines & Life Style Changes
Presently the approaches for treatment of endometriosis include surgery or hormonal therapy but there is a great need of new approaches that include yoga, herbal treatment and life style changes. According to WHO yoga is classified as the state of mind and body that covers contemplative techniques which strengthen our muscles, relieve stress and helps our body to integrate with mind. The recent reports are now suggesting that yoga helps to maintain the rhythm between the body and mind thus helping in the management of pelvic pain [39], which is the most prominent symptom in women suffering with endometriosis. Medicinal plants and their constituents provide a safe, effective and affordable remedy to control the progression of different diseases. There are different formulations/compositions that can be used in treatment of endometriosis. Life style factors also play an important role and have an adverse effects on cause of endometriosis. It includes dietary habits, physical activities, smoking, alcohol and coffee intake. A new study has shown that increased consumption of anti-oxidants, omega-3fatty acids, fish oils and PUFAs has a positive effect on endometriosis while higher intake of green vegetables and fresh fruits will lower the risks of endometriosis [40]. One more study has given the same view on protective role of green vegetables and fruits with negative effects of red meat, dairy products and unsaturated fatty acids [41].
Discussion and Conclusion
Estrogen hormone plays an important role in the pathogenesis of endometriosis and aromatase is the rate-limiting enzyme in its biosynthesis. The causative factors directly or indirectly affect genes of the steroidogenesis pathway and thus there occurs excessive estrogen formation leading to growth and progression of endometriotic implants.
But there are a number of medical therapies for endometriosis and all of these are mainly based on reduction or suppression of estrogen formation in endometrotic implants by regulating the aromatase expression. These medicinal treatments are effective but in most of the cases there is recurrence of the disease or side effects. So, now there is a need of putative treatment for endometriosis without any side effects and recurrence. In view of this future researches are focusing on ayurvedic medicines which will be a positive replacement for endometriosis without any side effects by regulating mechanism of expression of aromatase gene and developing efficient aromatase modulators or inhibitors for blocking estrogen production in endometrial implants. The motive of this study was the identification of causative factors, regulatory pathways, and pathway related genes, factors leading to the cause or pathogenesis of endometriosis with its new remedial measures. With this regulatory pathway genes, environmental and life style factors are also a promising candidates for future biomarker research for endometriosis diagnosis [42-46].
References
- Vessey MP, Villard-Mackintosh L, Painter R. Epidemiology of endometriosis in women attending family planning clinics. Br Med J. 1993; 306: 182-184.
- Olive DL, Schwartz LB. Endometriosis. N Engl J Med. 1993; 328: 1759-1769.
- Kjerulff KH, Erickson BA, Langenberg PW. Chronic Gynecological Conditions Reported by US women: Findings from the National Health Information Survey, 1984 to 1992. Am J Pub Health. 1996; 86: 195-199.
- Adachi S, Tajima A, Quan J, Haino K, Yoshihara K, Masuzaki H, et al. Metaanalysis of genome-wide association scans for genetic susceptibility to endometriosis in Japanese population. Journal of Human Genetics. 2010; 55: 816-821.
- Parazzini F, Esposito G, Tozzi L, Noli S, Bianchi S. Epidemiology of endometriosis and its comorbidities. Eur J Obstet Gynecol Reprod Biol. 2017; 209: 3-7.
- Natty KP, Baird DT, Bolton A, Chambers P, Corker CS, Mclean H. Concentration of oestrogens and androgens in human ovarian venous plasma and follicular fluid throughout the menstrual cycle. J Endocri. 1976; 71: 77-85.
- Doody KJ, Lorence MC, Mason JI, Simpson ER. Expression of mRNA species encoding steroidogenic enzymes in Human follicles and Corpora Lutea throughout the menstrual cycle. J Clin Endocrinol & Metab. 1990; 70: 1041-1045.
- Tsai-Morris CH, Aquilano DR, Dufau ML. Gonadotropic regulation of aromatase activity in the adult rat testes. Endocrinology. 1985; 116: 31-37.
- Grodin JM, Siiteri PK, Macdonal PC. Source of Estrogen Production in Postmenopausal Women. J Clin Endocrinol & Metab. 1973; 36: 207-214.
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, et al. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975; 31: 295-319.
- Roselli CE, Horton LE, Resko JA. Distribution and Regulation of Aromatase Activity in the Rat Hypothalamus and Limbic System. Endocrinology. 1985; 117: 2471-2477.
- Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T. Expression of Aromatase Cytochrome p450 Protein and Messenger Ribonucleic Acid in Human Endometrium and Adenomyotic tissues but Not in Normal Endometrium. Bio of Repro. 1997; 57: 514-519.
- Corbin CJ, Lorence SG, Mcphaul M, Mason IJ, Mendelson CR, Simpson ER. Isolation of a full-length cDNA insert encoding human aromatase system cytochrome P-450 and its expression in nonsteroidogenic cells (cDNA /COS- 1 cells). Proc. Natl. Acad. Sci. USA. 1998; 85: 8948-8952.
- Michael MD, Kilgore MW, Morohashi K, Simpson ER. Ad4BP/SF-1 regulates cyclic AMP-induced transcription from the proximal promoter (PII) of the human aromatase P450 (CYP19) gene in the ovary. J Biol Chem. 1995; 270: 13561-13566.
- Simpson ER, Mahendroof MS, Means GD, Kilgore MW, Hinshelwoodm MM, Graham-Lorence S, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994; 15: 342-355.
- Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: insights from metaanalysis of eight genome-wide association and replication data sets. Hum Reprod Update. 2014; 20: 702-716.
- Noe M, Kunz G, Herbertz M, Mall G, Leyendecker G. The cyclic pattern of the immunocytochemical expression of estrogen and progesterone receptors in human myometrial and endometrial layers: characterization of the endometrial-subendomerial unit. Human Reproduction. 1999; 14: 190-197.
- Domingo GC, Moreno A, Palomino P. The effect of human pregnancy serum on synthesis and action of interleukin-1. Journal of Reproductive Immunology. 1998; 13: 17-30.
- Lassey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS. Jr. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988; 67: 334-340.
- Prentice A, Randaill BJ, Weddell A, McGill A, Henry L, Horne CHW, et al. Ovarian steroid receptor expression in endometriosis and in two parent epithelia: Endometrium and peritoneal mesothelium. Hum Reprod. 1992; 7: 1318-1325.
- Snijders MPML, De Goeij AFPM, Baerts MJCDT, Rousch MJM, Koudstaal J, Bosman FT. Immunocytochemical analysis of oestrogen receptors and progesterone receptors in the human uterus throughout the menstrual cycle and the menopause. J Reprod Fert. 1992; 94: 363-371.
- Matsuzaki S, Murakami T, Uehara S, Canis M, Sasano H, Okamura K. Expression of estrogen receptor alpha and beta in peritoneal and ovarian endometriosis. Fertil Steril. 2001; 75: 1198-1205.
- Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase Expression in Endometriosis. J of Clinical Endocrinology. 1996; 81: 174-179.
- Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Honjo H. Expression of Aromatase Cytochrome P450 in Eutopic Endometrium and its Application as a Diagnostic Test for Endometriosis. Gynecol & Obstet Invest. 1999; 48: 21-28.
- Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, Johns A, Andersson S, et al. Deficient 17b-Hydroxysteroid Dehydrogenase Type 2 Expression in Endometriosis: Failure to Metabolize 17b-Estradiol. J Clin Endocrinol & Metab. 1998; 83: 4474-4480.
- Bulun SE, Simpson ER, Word ARA. Expression of the CYP19 gene and its product Aromatase Cytochrome P450 in Human Uterine Leiomyoma Tissues and Cells in Culture. J Clin Endocrinol & Met. 1994; 78: 736-743.
- Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, et al. Prostaglandin E2 Stimulates Aromatase Expression in endometriosis- Derived Stromal Cells. J Clin Endocrinol & Metab. 1997; 82: 600-666.
- Bulun SE, Price-f TM, Aitkens J, Maenroos MS, Simpson ER. A Link between Breast Cancer and Local Estrogen Biosynthesis Suggested by Quantification of Breast Adipose Tissue Aromatase Cytochrome P450 Transcripts Using Competitive Polymerase Chain Reaction after Reverse Transcription. J Clin Endocrinol & Met. 1993; 77: 1622-1628.
- Huber A, Keck CC, Hefler LA, Schneeberger C, Huber JC, Bentz EK, et al. Ten Estrogen-related polymorphisms and Endometriosis. Obstet & Gynecol. 2005; 106: 1025-1031.
- Elnashar A. Emerging treatment of endometriosis. Middle East Fertility Society Journal. 2015; 20: 61-69.
- Bedaiwy MA, Alfaraj S, Yong P, Casper R. New developments in the medical treatments of endometriosis. Fertil Sterli. 2017; 107: 555-565.
- Ezzat L. Medical treatment of endometriosis: an update. Int J Reprod Contracept Obstet Gynecol. 2017; 6: 4187-4192.
- Vercellini P, Trespidi L, Colombo A, Vendola N, Marchini M, Crosignani PG. A gonadotropin-releasing hormone agonist versus a low-dose oral contraceptive for pelvic pain associated with endometriosis. Fertil Steril. 1993; 60: 75-79.
- Vercellini P, Trespidi L, DeGiorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril. 1996; 65: 299-304.
- Waller KG, Shaw RW. Gonadotropin-releasing hormone analogues for the treatment of endometriosis: long-term follow-up. Fertil Steril. 1993; 59: 511- 515.
- Olive DL, Pritts EA. The treatment of endometriosis: a review of the evidence. Ann NY Acad Sci. 2002; 955: 360-372.
- Surrey ES, Voigt B, Fournet N, Judd HL. Prolonged gonadotropin-releasing hormone agonist treatment of symptomatic endometriosis: The role of cyclic sodium etidronate and low-dose norethindrone ‘add-back’ therapy. Fertil Steril. 1995; 63: 747-755.
- Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynecol. 2004; 18: 177-200.
- Goncalves AV, Makuch MY, Setubal MS, Barros NF, Bahamondes, L. A Qualitative Study on the Practice of Yoga for Women with Pain-Associated Endometriosis. The journal of alternative and complementary medicine. 2016; 22: 1-6.
- Thomas DS, Natarajan JS. Diet-A New Approach To Treating Endometriosis- What Is The Evidence? Journal of Nursing and Health Science (IOSR-JNHS). 2013; 1: 1959-2320.
- Parazzini F, Vigano P, Candiani M, Fedele L. Diet and endometriosis risk: A literature review. Reprod Biomed Online. 2013; 26: 323-336.
- Chen S, Besman MJ, Sparkes RS, Zollman S, Klisak I, Mohandas T, et al. Human aromatase: cDNA cloning, southern blot analysis and assignment of the gene to chromosome 15. DNA. 1988; 7: 27-38.
- Harada N, Utsumi T, Takagi Y. Tissue-specific expression of the human aromatase cytochrome P450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc Natl Acad Sci USA. 1993; 90: 11312-11316.
- Shozu M, Zhao Y, Bulun SE, Simpson ER. Multiple splicing events involved in regulation of human aromatase expression by a novel promoter, I.6. Endocrinology. 1998; 139: 1610-1617.
- Vinatier D, Orazi G, Cosson M, Dufour P. Theories of endometriosis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2001; 96: 21-34.
- Young SL. Oestrogen and progesterone action on endometrium: a translational approach to understanding endometrial receptivity. Reprod Biomed Online. 2013; 27: 497-505.