
Research Article
Austin J Obstet Gynecol. 2019; 6(2): 1140.
Selenium Supplementation and Gonadotropin Releasing Hormone Agonist Alleviate the Gonadotoxicity of Cyclophosphamide in Adult Mice
Kishk EA1* and Shams TM2
¹Department of Obstetrics and Gynecology, Faculty of Medicine, Suez Canal University, Egypt
²Department of Pathology, Faculty of medicine, Suez Canal University, Egypt
*Corresponding author: Kishk EA, Department of Obstetrics and Gynecology, Faculty of Medicine, Suez Canal University, Egypt
Received: March 28, 2019; Accepted: April 27, 2019; Published: May 04, 2019
Abstract
Purpose: To assess the effect of coadministration of selenium and gonadotropin releasing hormone (GnRH) agonists against the cyclophosphamide induced gonadal toxicity in mice.
Methods: A controlled, experimental study, whereas forty- two female mice were divided randomly and equally into seven groups; control group (C), triptorelin acetate (T) group, selenium group (Se), cyclophosphamide (Cy) group, triptorelin plus cyclophosphamide treated (T+Cy) group, Selenium plus cyclophosphamide treated (Se+Cy) group and cyclophosphamide group pretreated with selenium and treptorelin (T+Se+CPA) group. Mice in group (T) were subcutaneously injected with GnRH agonist (triptorelin acetate) in a dose of 0.5 mg/kg daily for 21 days and 75mg/kg of cyclophosphamide (Cy) was injected intraperitoneally on day 14 in (Cy) group, (T+Cy) and (T+Se+Cy) group where mice were injected with 0.5mg/kg selenium once daily for three weeks intraperitoneal (IP) in (Se), (Se+Cy) and (T+Se+Cy) groups. After 21 days, all mice were sacrificed and both ovaries were removed carefully to assess ovarian follicles and ovarian tissue glutathione peroxidase (GPx) for all groups.
Results: There was a significant reduction in the number of primordial, primary, secondary, and antral follicles in the Cy group compared with the control group confirming the gonadotoxic effect of cyclophosphamide (P‹0.001). In the T+Cy groups there was significant increase in primordial, primary, secondary and antral follicles compared with the Cy group (P‹0.05), meanwhile; the improvement was only in secondary and antral follicles for Se +Cy group. The co-administration of GnRH agonist and selenium, the integrity of ovarian tissue was spared. The number of primordial and primary follicles was statistically highly significant than Cy group (p‹0.001) and significant for secondary and antral follicles (p‹0.05). Surprisingly, no significant difference was found in primordial, primary, secondary and antral follicles in comparison to control group (p>0.05).
Conclusion: Cyclophosphamide causes destruction of the ovarian follicles, pretreatment with combination of triptorelin and selenium has marked ovarian protection in mice.
Keywords: GnRH agonist; Selenium; Cyclophosphamide; Ovarian toxicity
Introduction
Fertility preservation is one of the most important issues especially with improvement in diagnosis and treatment of cancer. As the survival rates increase, the reproductive function of young women should be protected against the cytotoxic effect of chemotherapy. Fertility preservation strategies have been developed to prevent Premature Ovarian Failure (POF) following chemotherapy [1,2]. Cyclophosphamide (Cy) is an alkylating agent that are used widely in treatment of cancer, these agents are not cell-cycle specific, so cell proliferation is not essential for their cytotoxic action. Cyclophosphamide induced gonado-toxicity by DNA alkylation and subsequent disruption of normal cellular processes [3]. The effectiveness of gonadotropin-releasing hormone agonists (GnRHa) in protection against chemotherapy induced Premature Ovarian Failure (POF) is still controversial [4-7]. A recent meta-analysis study reported the advantage of GnRHa in patients with breast cancer [8]. While others found no benefits [5,9,10]. Selenium (Se); an essential trace element; It is necessary for the maintenance of various physiological processes [11]. Se is incorporated into the catalytic site of antioxidant enzymes, such as Glutathione Peroxidase (GPx), protects the cells against Reactive Oxygen Species (ROS), prevents cell damage and enhance growth and development [12]. Glutathione plays a critical part in cytoplasmic maturation of the oocyte [13]. So, we aimed to assess the effect of coadministration of Ronadotropin Releasing Hormone (GnRH) agonists and selenium against the cyclophosphamide induced gonadal toxicity in mice.
Materials and Methods
Forty-two sexually mature, virgin, female mice (8 weeks old) were used throughout the study, they were obtained from the Egyptian Organization for Biological Products and Vaccines (Vacsera, Egypt). All procedures were in accordance with the Guiding Principles for the Care of animals. They were housed individually for a 2-week acclimatization period prior to the experiment. Mice were fed ad libitum by standard laboratory pellet and tap water. A 12hr light: 12hr dark cycle was maintained. Room temperature was at 23±2oC with a relative humidity of 45-55%. Mice were randomly divided equally into seven groups:
Group (1): Control (C) group.
Group (2): triptorelin (T) group: where mice were injected with 1mg/kg triptorelin acetate (Decapeptyl®, FERRING pharmaceuticals) subcutaneously (SC) daily for 21 days [14].
Group (3): Selenium (Se) group: where mice were injected with 0.5 mg/kg selenium once daily for three weeks intraperitoneal (IP); sodium selenite, 98% powder, diluted in 0.9% NaCl (Gedco Pharmaceutical Industrial Co. Egypt) [15].
Group (4): Cyclophosphamide treated (Cy) group: where mice were injected with cyclophosphamide (Endoxan®, Baxter, Oncology, GmbH) at a dose of 75 mg/kg once IP on day 14 [16].
Group (5): triptorelin plus cyclophosphamide treated (T+Cy) group: where mice were injected with 1mg/kg triptorelin acetate (Decapeptyl®, FERRING pharmaceuticals) SC for 21 days. The animals were further injected with cyclophosphamide at a dose of 75mg/kg once intraperitoneally on day 14.
Group (6): Selenium plus cyclophosphamide treated (Se+Cy) group: where mice were injected with 0.5mg/kg selenium daily IP. The animals were further injected with cyclophosphamide at a dose of 75mg/kg once IP on day 14.
Group (7): triptorelin plus selenium plus cyclophosphamide treated (T+Se+CPA) group: where mice were injected with 1mg/kg triptorelin (Decapeptyl, Ferring Pharmaceuticals) subcutaneously plus 0.5mg/kg selenium daily IP. The animals were further injected with cyclophosphamide at a dose of 75mg/kg once IP on day 14.
On 21st day, all animals, including the age-matched controls, were sacrificed by an overdose of ether and their ovaries were removed from each mouse carefully; right ovary for histological studies and left ovary for homogenizing process.
Preparation of paraffin sections: The excised ovaries were fixed in formalin overnight and embedded in paraffin. Five -micrometer serial sections were cut with a microtome and stained with hematoxylin and eosin. The ovarian PMF number was counted in every fifth section by the same examiner to reduce the interobserver error. To calculate the total number of PMF, the numbers of PMF were then multiplied by 5. The same was done for primary, secondary and antral follicles counting.
Morphometric study
The degree of morphological involvement in ovarian toxicity was determined using light microscope. The number of primordial, primary, secondary, and antral follicles was counted, the follicles were classified into four stages, as follows: a primordial follicle contains a partial or complete layer of flattened granulosa cells encircling the oocyte, in the primary follicle, the oocyte is surrounded by a single layer of cuboidal granulosa cells, the secondary follicle contains multiple layers of cuboidal granulosa cells surrounding the oocyte with no antral space, in an early antral follicle, generally only one or two small antra are present, whereas an antral follicle contains a single large antral space adjacent to the oocyte [17].
All slides were observed by the same pathologist using light microscope (CX21, Olympus optical co. LTD, Japan) with resolution of 10MP (megapixels) (3656x2740-pixel average), captured by Tucsen ISH1000 digital microscope camera at magnification of 100x.
Tissue Collection and measurement of GPx activity. Ovaries were washed with 0.9% NaCl, Samples of ovarian tissues were homogenized in 50mM phosphate buffer (10% W/V), pH 7.4, using Glas-Col tissue homogenizing system (Cole-Parmer, Vernon Hills, USA). Supernatants obtained upon centrifugation at 10,000g for 15 minutes, stored at -80°C until measurement of the GPx activity using standard spectrophotometric assay at 340nm [18].
Statistical analysis
Data were processed using SPSS version 21 (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as means±SD and qualitative data were expressed as numbers and percentages. Oneway analysis of variance (ANOVA) and Tukey’s test was used to test significance of difference for quantitative variables. A probability value (p-value) ‹0.05 was considered statistically significant.
Results
Examination of the ovarian tissue in control group revealed normal ovary showing ovarian cortex contain functional structures of the ovarian follicles in various stages of development. The layers of the surrounding granulosa cells were integral, granulosa cells in all follicles are oval to rounded cells with small central oval to rounded nucleus with rim of cytoplasm. The medullary area showed stroma formed of cellular spindle cells and blood vessels are also evident with average caliber (Figure 1a).
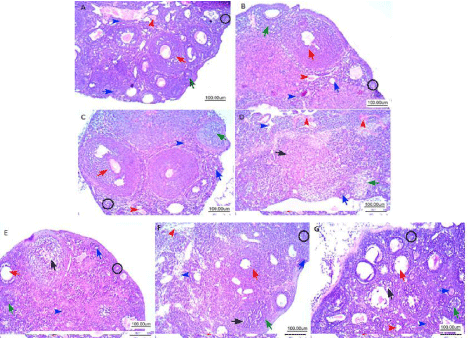
Figure 1: Representative photomicrographs of hematoxylin and eosin-stained ovarian tissue. Histological sections of control (A) showing normal ovarian cortex:
Primordial follicle (black circle) contains a partial or complete layer of flattened granulosa cells encircling the oocyte; Primary follicle, (blue arrow) the oocyte is
surrounded by a single layer of cuboidal granulosa cells; Secondary follicle (green arrow) contains multiple layers of cuboidal granulosa cells; Antral follicle (red
arrow) contains a single large antral space adjacent to the oocyte. The medullary area showed stroma formed of cellular spindle cells (blue arrowhead) and
blood vessels are evident with average caliber (red arrowhead). B & C represent T and Se groups with normal ovarian cortex and medulla, (Cy) group revealed
destruction of the ovarian structure, reduction in the number of follicles, degenerated follicles in various stages of degeneration (D). T+Cy group showing mild
preservation of primordial follicles with evidence of degeneration of granulosa cells of primary follicles (E), Se+Cy group showing minimal primordial follicles (F),
(T+Se+CY) group showed marked protective effect; Primordial, primary, secondary and antral follicle are seen with marked improvement of their number; one antral
follicle seen showed mild residual degeneration of granulosa cells, normal strom and vessels (G).
Examination of the ovarian tissue of triptorelin and selenium group revealed that the cortex was occupied by follicles in various stages of development. The layers of the surrounding granulosa cells, medulla stroma and vessels were normal (Figure 1b and 1c).
Examination of the ovarian tissue in the (Cy) group revealed destruction of the ovarian structure, reduction in the number of follicles, degenerated follicles in various stages of degeneration accompanied with collapsed follicles on the surface of the ovary and lost integrity of surrounding granulosa layer. Ovary showed marked decrease in cortex thickness indicating toxic effect on follicles: primordial follicles showed marked decrease of their number, primary follicles decreased in number, but residuals are seen, secondary follicles are markedly decreased with evident follicle showed markedly degenerated granulosa cells and antral follicles are not seen due to marked decrease in their numbers with evident atretic follicles. The medullary area showed stroma formed of collapsed degenerated spindle cells and congested blood vessels with areas of early hemorrhage (Figure 1d).
Ovarian tissue of cyclophosphamide group pretreated with treptoreline (T+CY) showed moderate protective effect; few primordial follicle are seen with moderate improvement; Primary follicle, are mildly improved with evidence of degeneration of granulosa cells; Secondary follicle are markedly improved with multiple layers of unremarkable granulosa cells; Antral follicle markedly improved contains a single large antral space adjacent to the oocyte, one antral follicle seen showed mild residual degeneration of granulosa cells. The medullary area showed stroma formed of cellular spindle cells with focal residual edema (Figure 1e).
Ovarian tissue of cyclophosphamide group pretreated with selenium (Se+CY) showed moderate focal protective effect; Primordial follicle are few with minimal improvement of their numbers; Primary follicle, are minimally improved with degenerated cells; Secondary follicle are moderately improved with increased numbers with residual degeneration of granulosa cells; Antral follicle, moderately improved contains a single large antral space adjacent to the oocyte. The medullary area showed stroma formed of cellular spindle cells showed moderate edema with dilated congested vessels (Figure 1f).
Ovarian tissue of cyclophosphamide group pretreated with triptorelin and selenium (T+Se+CY) showed marked protective effect; Primordial follicle are seen with moderate improvement of their number; Primary follicle, are markedly improved with normal numbers; Secondary follicle are markedly improved with multiple layers of unremarkable granulosa cells; Antral follicle, moderately improved contains a single large antral space adjacent to the oocyte, one antral follicle seen showed mild residual degeneration of granulosa cells. The medullary area showed stroma formed of cellular spindle cells with normal vessels (Figure 1g).
Obvious destruction of ovarian structure and significant depletion of primordial, primary, secondary and antral follicles were demonstrated in the Cy group compared with the control group, affirming the ovarian toxicity of cyclophosphamide. There was a significant reduction in the number of primordial, primary, secondary, and antral follicles in the Cy group compared with the control group (P‹0.001).
In the T+Cy groups there was significant increase in primordial, primary, secondary and antral follicles compared with the Cy group (P‹0.05) showing the effect of triptorelin before and during the course of chemotherapy on ovarian protection meanwhile; the improvement was only in secondary and antral follicles for Se+Cy group. Significant reduction was found in different follicles in T+Cy and Se+Cy groups in comparison to control group (P‹0.05).
Regarding, the co-administration of GnRH agonist and selenium, the integrity of ovarian tissue was spared. The number of primordial and primary follicles was statistically highly significant than Cy group (p‹0.001) and significant for secondary and antral follicles (p‹0.05). Surprisingly, no significant difference was found in primordial, primary, secondary and antral follicles in comparison to control group (p>0.05) showing the great effect of the new combination of triptorelin and selenium against the chemotherapy induced gonadotoxicity (Figure 2).
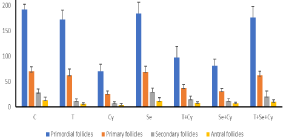
Figure 2: Effect of GnRH agonist and selenium on the number of
primordial, primary, secondary and antral follicles after administration of
Cyclophosphamide.
Each column represents the mean±SD of the experimental groups; P ‹0 .05
was considered to be statistically significant. T+Cy VS Cy group, all follicles
P< 0.05, T+Se+Cy group vs Cy group, all follicles P‹0.001, Se+Cy vs Cy
group secondary & antral follicles P‹0.05, T+Se+Cy vs control group p>0.05,
using one-way ANOVA followed by Tukey’s post-hoc test.
GPx was lower significantly in Cy group than control group (p‹0.05), meanwhile, it was increased significantly in Se+Cy and T+SE+CY groups compared to Cy group (p‹0.05). There was no significant difference between selenium pretreated groups and control group (p>0.05) (Figure 3).
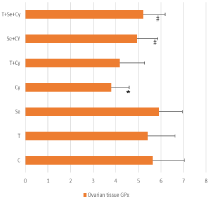
Figure 3: Distribution of Ovarian GPx among the experimental groups.
Data was expressed as mean±SD P ‹0 .05 was considered to be statistically
significant. *p‹0.05 vs control group. #p‹0.05 vs Cy group.
Discussion
In the present study, we have studied gonadotoxic effect of cyclophosphamide in mice through evaluation of the integrity of granulosa cells and assessment of different stages of ovarian follicles especially the primordial follicles which reflect the ovarian reserve and consequently the future fertility.
This study showed destructive effect of cyclophosphamide on ovarian follicles in mice which agree with previous study of Oktem and Oktay who reported atrophy with a marked loss of primordial follicles and oocyte depletion following chemotherapy [19]. This could be explained that alkylating agents’ effect on mammalian cells is through two mechanisms: cell killing and delay in cell cycle progression. Cytotoxic agents damage the pre-granulosa cells of the primordial follicle [20]. Cell staining has revealed that apoptosis is one of the pathways that causes follicle loss [21]. Blood vessels injury and focal cortical fibrosis are involved in ovarian damage and depletion of the reserve [22]. Cyclophosphamide activates upregulation of the PI3K pathway, initiating follicles recruitment and growth leads to reduction of primordial follicles [23].
Oktem and Oktay [19] showed that apoptosis affected primarily on primordial follicle rapidly post injection of cyclophosphamide (12%, 53% and 93% of follicle loss at 12, 24 and 48h after the first injection, respectively). Human oocytes were destructed (100% at 12h post injection), then 63% of granulosa cells were damaged within 12h. The fact that alkylating agents are not cell-cycle specific, this explain why resting follicles are also damaged. [20,24,25].
We studied pre-treatment with triptorelin, a GnRH agonist alone and in combination with selenium. In (T+Cy) and (T+Se+Cy) groups, the number of primordial, primary secondary and antral follicles was significantly greater than in the CPA group. Surprisingly, the integrity of ovarian tissue was spared with a novel combination of GnRH agonist and selenium.
Previous animal studies seemed to confirm our finding regarding the protective effect of GnRH analogue against the gonadoxicity of cyclophosphamide and inhibition of depletion of primordial follicles [26-28]. However, conflicting results were found, Letterie did not show any improvement in ovarian reserve, he assessed suppression of ovulation by combination of oestrogen and progesterone or leuprolide in mice injected with cyclophosphamide, both methods failed to protect the mice against gonadal toxicity [29]. Recently, the German Hodgkin Study Group prematurely stopped a prospective study on hormonal treatment with combined oral contraception or GnRH owing to a lack of effectiveness. The AMH levels after 12 months showed no benefit of GnRH or combined oral contraception [30].
The possible protective mechanisms of GnRH agonist through suppression of gonadotrophins levels in the ovary as direct effect of GnRH agonist and decreased ovarian blood flow. According to this explanation GnRH agonist preserve only the growing follicle [31]. GnRH therapy decreases ovarian blood flow, and consequently, reduces the dose of chemotherapy reaching the ovary so decrease the damage to the ovarian reserve. Pervious study showed that ovarian flow decreased by administration of GnRH agonist in rats [32]. On the other hand, three-dimensional power Doppler ultrasound of the ovarian stromal blood flow did not show significant changes post administration of GnRH agonist [33].
In the human ovary, GnRH receptors were detected only in the preovulatory follicle and corpus luteum (CL) meanwhile, in the nonprimate ovary they have been found at all stages [34]. It was shown that GnRH agonists protected the human granulosa cells from damage caused from doxorubicin [35].
GnRH agonist may exert antiapoptotic effect through upregulation of an intragonadal anti-apoptotic molecule such as Sphingosine-1-Phosphate (S1P) and protection of the undifferentiated germline stem cells [36]. An indirect influence through the transforming growth factor-b superfamily members expressed by ovarian somatic cells and oocytes. The development of primordial to primary follicle (which is gonadotrophin-independent) might be regulated through ligands, which originate from growing follicles (which are gonadotrophin-dependent). This could explain the indirect effect of GnRH agonist in recruitment of primordial follicles [37].
Despite the promising results in rats where GnRH agonists inhibited chemotherapy-induced ovarian damage, application of this option to preserve fertility in human is still limited [20,24].
The present study showed the protective effect of selenium against cyclophosphamide in agreement with the study of said et al who investigated the mechanism of potential radio protective effect of sodium selenite on radiation-induced ovarian failure in rats.
Treatment of animals with sodium selenite before exposure to irradiation protect significantly against radiation-induced oxidative stress in ovarian and uterine tissues. Selenium improved folliculogenesis, it increased granulosa cells proliferation, hormones secretions (estradiol and FSH), activation of GPx, which act as antioxidant, and inhibition of apoptosis through reduction of lipid peroxidation and oxidative stress [38].
The present study showed improvement in the antral and secondary follicles significantly in mice pretreated only with selenium, adding this element to GnRh agonist rescue the ovarian follicles, surprisingly, no significant difference was found between T+Se+Cy group and control group. Mandle Showed that sodium selenite preserves primordial follicles’ stock, preantral and antral follicles and stimulates follicular maturation in rats exposed to radiation [39]. Se improves follicular development as it increases GPx activity and reduces ROS which causes cell damage [12,40-42]. No one investigated the effect of selenium on ovarian reserve and fertility preservation in human or animal model receiving cytotoxic agents, so Se treatment alone or in combination with GnRH analogue for young women with cancer need to be further studied. To the best of our knowledge, this is the first study assess the co-administration of GnRH agonist and selenium as antioxidant in protection against gonadotoxic effect of cyclophosphamide in mice.
Conclusion
This study proved that this novel combination protects the mice ovary against the cytotoxic effect of cyclophosphamide, GnRH agonists and selenium are widely available drugs, effective, simple method and could be considered as an ideal strategy for fertilitypreservation. Further studies are needed to assess these therapies, if proven effective; they would be an ideal fertility-preserving therapy.
References
- Maltaris T, Seufert R, Fischi F, Schaffrath M, Pollow K, Koelbl H, et al. The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol. 2007; 130: 148-155.
- Marhhom E, Cohen I. Fertility preservation options for women with malignancies. Obstet. Gyneco. Surv. 2007; 62: 58-72.
- Mohammadnejad D, Tayefi-nasrabadi H, Naghibi M, Abedelahi A. Cetrorelix preserves follicular viability in Cyclophosphamid-induced ovarian toxicity. International Journal of Research in Applied and Basic Medical Sciences. 2015; 1: 56-60.
- Turner NH, Partridge A, Sanna G, Di Leo A, Biganzoli L. Utility of gonadotropinreleasing hormone agonists for fertility preservation in young breast cancer patients: The benefit remains uncertain. Ann Oncol. 2003; 24: 2224-2235.
- Oktay K, Turan V. Failure of ovarian suppression with gonadotropin-releasing hormone analogs to preserve fertility: An assessment based on the quality of evidence. JAMA Oncol. 2016; 2: 74-75.
- Del Mastro L, Lambertini M. Temporary ovarian suppression with gonadotropin-releasing hormone agonist during chemotherapy for fertility preservation: Toward the end of the debate? Oncologist. 2015; 20: 1233- 1235.
- Peccatori F, Demeestere I. GnRH analogue for chemotherapy-induced ovarian damage: Too early to say? Fertil Steril. 2009; 92: e33.
- Lambertini M, Ceppi M, Poggio F, Peccatori FA, Azim HA Jr, Ugolini D, et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: A metaanalysis of randomized studies. Ann Oncol. 2015; 26: 2408-2419.
- Elgindy E, Sibai H, Abdelghani A, Mostafa M. Protecting ovaries during chemotherapy through gonad Suppression: A systematic review and metaanalysis. Obstet Gynecol. 2015; 126: 187-195.
- Gerber B, Ortmann O. Prevention of Early Menopause Study (POEMS): Is it possible to preserve ovarian function by Gonadotropin Releasing Hormone analogs (GnRHa)? Arch Gynecol Obstet. 2014; 290: 1051-1053.
- Zhang J, Robinson D, Salmon P. A novel function for selenium in biological system: selenite as a highly effective iron carrier for Chinese hamster ovary cell growth and monoclonal antibody production. Biotechnol Bioeng. 2006; 95: 1188-1197.
- Abedelahi A, Salehnia M, Allameh AA. The effects of different concentrations of sodium selenite on the in vitro maturation of preantral follicles in serum-free and serum supplemented media. J Assist Reprod Genet. 2008; 25: 483-488.
- Chattopadhyay S, Pal Ghosh S, Ghosh D, Debnath J. Effect of dietary coadministration of sodium selenite on sodium arsenite-induced ovarian and uterine disorders in mature albino rats. Toxicol Sci. 2003; 75: 412-422.
- Li X, Kang X, Deng Q, Cai J and Wang Z. Combination of a GnRH agonist with an antagonist prevents flare-up effects and protects primordial ovarian follicles in the rat ovary from cisplatin-induced toxicity: a controlled experimental animal study. Reproductive Biology and Endocrinology. 2013; 11: 16.
- Pontual ML, Tuji FM, Barros SP, Boscolo FN, Novaes PD, de Almeida SM. Ultrastructural evaluation of the radio protective effect of sodium selenite on submandibular glands in rats. J Appl Oral Sci. 2007; 15: 162-168.
- Meirow D, Lewis H, Nugent D and Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999; 14: 1903-1911.
- Myers M, Britt KL, Wreford Ng, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2004; 127: 569-80.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70: 158-169.
- Oktem O, Oktay K. Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer. 2007; 10: 2222-2229.
- Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000; 169: 123-131.
- Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001; 7: 535-543.
- Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007; 22: 1626-1633.
- Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, et al. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013; 5: 185.
- Blumenfeld Z, Dann E, Avivi I, Epelbaum R, Rowe JM. Fertility after treatment for Hodgkin’s disease. Ann Oncol. 2002; 13: 138-147.
- Lutchman Singh K, Davies M, Chatterjee R. Fertility in female cancer survivors: pathophysiology, preservation and the role of ovarian reserve testing. Hum Reprod Update. 2005; 11: 69-89.
- Ataya K, Ramahi-Ataya A. Reproductive performance of female rats treated with cyclophosphamide and/or LHRH agonist. Reprod Toxicol. 1993; 7: 229- 235.
- Bokser L, Szende B, Schally AV. Protective effects of D-Trp6-luteinising hormone-releasing hormone microcapsules against cyclophosphamideinduced gonadotoxicity in female rats. Br J Cancer. 1990; 61: 861-865.
- Ataya K, Rao LV, Lawrence E, Kimmel R. Luteinizing hormone releasing hormone agonist inhibits cyclophosphamide-induced ovarian follicular depletion in rhesus monkeys. Biol Reprod. 1995; 52: 365-372.
- Letterie GS. Anovulation in the prevention of cytotoxic-induced follicular attrition and ovarian failure. Hum Reprod. 2004; 19: 831-837.
- Behringer K, Wildt L, Mueller H, Mattle V, Ganitis P, van den Hoonaard B, et al. No protection of the ovarian follicle pool with the use of GnRH-analogues or oral contraceptives in young women treated with escalated BEACOPP for advanced stage Hodgkin lymphoma. Final results of a phase II trial from the German Hodgkin Study Group. Ann Oncol. 2010; 21: 2052-2060.
- Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004; 10: 251-266.
- Kitajima Y, Endo T, Nagasawa K, Manase K, Honnma H, Baba T, et al. Hyper stimulation and a gonadotropin-releasing hormone agonist modulate ovarian vascular permeability by altering expression of the tight junction protein claudin-5. Endocrinology. 2006; 147: 694-699.
- Yu Ng EH, Chi Wai Chan C, Tang OS, Shu Biu Yeung W, Chung Ho P. Effect of pituitary down regulation on antral follicle count, ovarian volume and stromal blood flow measured by three-dimensional ultrasound with power Doppler prior to ovarian stimulation. Hum Reprod. 2004; 19: 2811-2815.
- Choi JH, Gilks CB, Auersperg N, Leung PC. Immunolocalization of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and type I GnRH receptor during follicular development in the human ovary. J Clin Endocrinol Metab. 2006; 91: 4562-4570.
- Imai A, Furui T. Chemotherapy-induced female infertility and protective action of gonadotropin-releasing hormone analogues. J Obstet Gynaecol. 2007; 27: 20-24.
- Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy? The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries. Oncologist. 2007; 12: 1044- 1054.
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006; 132: 191-206.
- Said R, Nada A, El-Demerdash E. Sodium Selenite Improves Folliculogenesis in Radiation-Induced Ovarian Failure: A Mechanistic Approach. PLOS. 2012; 7; 12, e50829; 1-12.
- Mandl AM. A quantitative study of the sensitivity of oocytes to irradiation. Proc R Soc Lond B Biol Sci.1959; 150: 53-71.
- Feinendegen LE. Reactive oxygen species in cell responses to toxic agents. Hum Exp Toxicol. 2002; 21: 85-90.
- Abedelahi A, Salehnia M, Allameh AA, Davoodi D. Sodium selenite improves the in vitro follicular development by reducing the reactive oxygen species level and increasing the total antioxidant capacity and glutathione peroxide activity. Hum Reprod. 2010; 25: 977-985.
- Basini G, Tamanini C. Selenium stimulates estradiol production in bovine granulosa cells: possible involvement of nitric oxide. Domest Anim Endocrinol. 2002; 18: 1-17.