
Rapid Communication
Austin J Obstet Gynecol. 2019; 6(3): 1142.
Tubo Ovarian Abscess: Ultrasound Findings
El-Agwany AS*
Department of Obstetrics and Gynecology, Faculty of Medicine, Alexandria University, Egypt
*Corresponding author: Ahmed S El-Agwany, Department of Obstetrics and Gynecology, El-shatby Maternity University Hospital, Faculty of Medicine, Alexandria University, Alexandria, Egypt
Received: April 18, 2019; Accepted: May 22, 2019; Published: May 29, 2019
Abstract
Tubo Ovarian Abscesses (TOAs) are common pelvic infections requiring inpatient admission in opposite to complex. We discuss here the different ultrasound findings related to be aware of by different physicians.
Keywords: Pelvic Inflammatory Disease; Tubo ovarian Abscess
Introduction
Pelvic Inflammatory Disease (PID) is a polymicrobial ascending infection that causes inflammation of the upper genital tract, including endometritis, salpingitis, pelvic peritonitis, and occasionally leading to Tubo Ovarian Abscess (TOA) and complex formation [1]. The healthcare burden of PID is generally underestimated because of cases of undiagnosed subclinical PID [2]. The incidence of PID correlates with the incidence of sexually transmitted diseases [3]. It is unclear why some women with PID develop TOA, whereas the majority of women do not. Formation of TOA may be related to prior PID infection, delay in treatment, or virulence factors of the pathogens. Among hospitalized patients with PID approximately one third have TOA [4]. TOAs are caused by an ascending infection to the fallopian tube causing endothelial damage and edema of the infundibulum resulting in tubal blockage. The ovary may become involved presumably by invasion of organisms through the ovulation site. Eventually the separation between the ovary and fallopian tube is lost. Necrosis inside this complex mass may result in 1 or more abscess cavities and an anaerobic growth environment [4]. The adherence of adjacent pelvic structures, such as the omentum and bowel, might serve a host defense mechanism to contain the inflammatory process within the pelvis. This could be a reason that some women with TOA are not overtly sick with an elevated white cell count or fever. PID has been determined to be polymicrobial in nature [5]. Sexually transmitted infections, such as N. gonorrhoeae, Chlamydia trichomatis, and Mycoplasma genitalium, have all been identified from the cervix, endometrium, and fallopian tubes from women with acute sapingitis [6]. However, endogenous, bacterial vaginosis-associated lower genital tract organisms, such as Prevotella species, Peptostreptococci sp., Gardnerella vaginitis, Escherichia coli, Haemophilus influenza, and aerobic streptococci are found in a high percentage of PID cases [7]. The most common organisms isolated for TOAs are E. coli, Bacteriodes fragilis, Bacteriodes species, Peptostreptococcus, Peptococcus, and aerobic streptococcus [8]. Importantly, E. coli is a common isolate in women with ruptured TOAs and a frequent cause of Gramnegative sepsis. TOAs that occur in women with long-term use of an IUD are often associated with Actinomyces israelii [8]. Acute PID is difficult to diagnose because of the wide variation of signs and symptoms [9]. Empiric treatment should be initiated in women at risk for sexually transmitted diseases if they are experiencing pelvic or lower abdominal pain, if other illnesses have been ruled out and if they have cervical motion tenderness, uterine tenderness, or adnexal tenderness. In addition (1) or more of the following criteria enhances the specificity of the diagnosis: fever, abnormal cervical or vaginal mucopurulent discharge, presence of abundant white blood cells on saline microscopy, elevated erythrocyte sedimentation rate, elevated C-reactive protein, and cervical infection with N. gonorrhoeae or C. trichomatis. Transvaginal ultrasound and pelvic Computed Tomography (CT) are the most common imaging modalities used to detect TOA. Transvaginal ultrasound is considered the first-line imaging modality because it provides excellent imaging of the upper genital tract, is relatively inexpensive, and does not expose the patient to radiation. Ultrasound finding suggestive of PID includes enlarged ovarian volumes or polycystic ovaries, thickened fluid-filled ovaries with incomplete septum or the “cog wheel” sign, and complex free fluid in the cul-de-sac. With more severe or progressive PID, the anatomic distinction between the ovary and the fallopian tube can no longer be identified, forming a TOA [7]. TOAs are characterized by a complex multilocular cystic mass with thick irregular walls, partitions, and internal echoes (Figure 1-5). Pelvic CT is preferred for women where the diagnosis is uncertain and there is concern for a coexisting malignancy or gastrointestinal pathology, such as appendicitis or diverticulitis [7]. Women with mild or moderate PID achieved clinical outcomes with outpatient oral antibiotics similar to those with inpatient IV antibiotics. The decision for hospitalization should be Surgical emergencies cannot be excluded; Pregnancy; Lack of response to oral antibiotics; Inability to follow or tolerate an outpatient oral regimen; Severe illness, nausea and vomiting, or high fever; Presence of TOA. Women with TOA should have direct inpatient observation for 24 hours because of risk of abscess rupture and sepsis. In acute PID: IV cefotetan or IV cefoxitin plus oral or IV doxycycline IV clindamycin plus IV gentamicin, Alternative: ampicillin/sulbactam plus doxycycline [8]. These regimens provide broad coverage for not only N. gonorrhoeae, C. trichomatis, and M. genitalium, but also for streptococcus, Gram-negative enteric bacteria (E. coli, Klebsiella spp., and Proteus spp.), and bacteria vaginosisassociated anaerobic organisms [8]. The cephalosporin based regimen is preferred because of improved tolerability. In the case of a severe penicillin allergy, clindamycin plus gentamicin is recommended. For the treatment of TOA, when comparing the first-line parenteral antibiotic regimens, none of the regimens have been shown to be superior [9]. Amnioglycosides have reduced activity in acidic, anaerobic environments with purulent debris. For the treatment of TOA, an extended-spectrum cephalosporin for the coverage of Gram-negative organisms (rather than an aminoglycoside) combined with clindamycin or metronidazole is a good option. Guidelines for the treatment of intraabdominal infections have recommended that when resistance for a specific antibiotic exceeds >10% to 20% of all isolates, then a change in the recommended antibiotic should occur. For this reason, ampicillin-sulbactam is no longer recommended for treatment of community-acquired intra-abdominal infections because of significant increased resistance in E. coli [9]. Antibiotic therapy can be switched from parenteral to oral route of administration after 24 hours of clinical improvement, resolution of nausea and vomiting and severe pain. Patients should complete an entire 14-day course of antibiotics with oral doxycycline. When a TOA is present or when the illness was preceded by gynecologic procedure greater anaerobic coverage is required, thus we recommend the addition of clindamycin or metronidazole to doxycycline. We prefer to use metronidazole because of the increased risk of Clostridium difficile colitis with clindamycin. Intrauterine Contraceptive Device (IUCD) In Situ When PID occurs with an IUCD in place removal of the IUCD is not required [10]. When the IUCD is removed, it should not be replaced until 3 months after the PID has resolved. Surgical Management and Drainage of TOAs In general, the decision to combine antimicrobial therapy with drainage or surgical excision of the TOA depends on the status of the patient and the size of the abscess. Signs of sepsis, such as hypotension, tachycardia, and tachypnea, and an acute abdomen are indicative of rupture, and such patients should immediately proceed to the operating room for surgical exploration. TOAs usually present without evidence of rupture and in these cases the role for drainage or operative management of TOA is less clear.
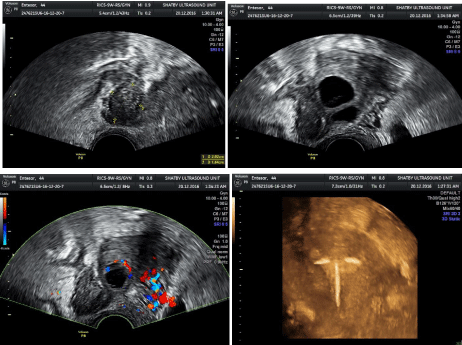
Figure 1: Tubo ovarian abcess seen as irregular complex fluid collection with ovarian cysts in center and pyosalpinx seen with turbid echogenic collection, tubo
ovarain relationship is seen with IUD intrauteirne (resolving stage, subacute).
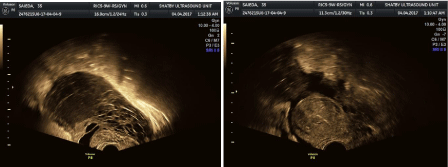
Figure 2: Honey comb apperance of liquifed ovarian and tube with multiple cysts surrounded by irregular turbd fluid collection and free fluid collection is seen
around the uterus (latr stage).
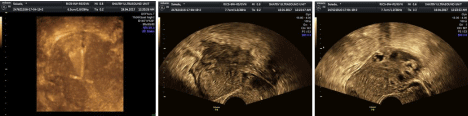
Figure 3: IUD is seen translocated on ultrasound with irregular turbid fluid collection behind the uterus and adherent large ovary with multiple echogenic cysts
(early stage, subacute).
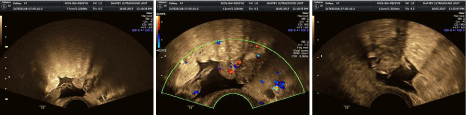
Figure 4: Acute stage with pain, where free fluid collection is seen with echogenic bowel by inflamation and irregualr tuboovarian complex.
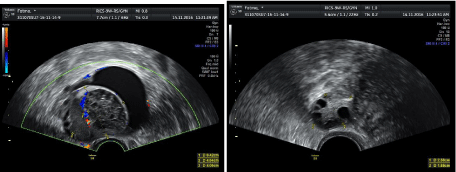
Figure 5: Unilateral multicystic ovary is seen with areas of liquifaction and surrrounded by irregular free fluid (sub acute stage, late stage).
Surgical management options for TOAs range from only drainage to unilateral salpingo-operectomy to total abdominal hysterectomy and bilateral salpingo-oopherecectomy. Historically, most women with TOA were managed aggressively with a total abdominal hysterectomy and bilateral salpingo-opherectomy. Although this approach offered high cure rates, it was at the cost of high rates of surgical complications, infertility, and hormone deficiency. With the advent of effective antimicrobial therapy, operative management has become much more conservative moving toward procedures that allow for sparing of ovarian function and if possible can even be considered in cases of rupture [10]. Options for approach can range from imaging-guided drainage to laparoscopy to laparotomy. We recommend the removal of the abscess cavity and the associated necrotic tissue and then irrigation of the peritoneum. We offer hysterectomy with bilateral salpingo-opherectomy to patients who are acutely ill and have completed child bearing. This approach may hasten recovery compared with fertility-sparing surgery. Pelvic abscess have been drained using ultrasound or CT guidance with a transabdominal, trangluteal, transrectal, or transvaginal approach. Abscesses can be drained with a catheter placement or aspiration alone with a success rate ranging between 77.8% and 100% [11]. They repeated the aspiration if abscess material was still seen on ultrasound 2 to 4 days after initial aspiration. It seems advantageous to consider transvaginal aspiration of the abscess in combination with standard antibiotics, particularly with larger abscesses, as this may increase the response rate, decrease the length of hospitalization, and improve pain control.
Conclusions
TOA contribute significantly to the number of patients with pelvic infections admitted to the hospital. These diagnoses are associated with significant long-term morbidity, including poor reproductive outcomes and chronic pain. A high level of suspicion for TOAs in women with PID is required, as many women with TOAs do not have fever or an elevated white cell count. Ultrasound Is essential diagnosis where enlarged cystic ovary with pyosalpinx, irregular turbid fluid collection are seen.
Compliance with Ethics Standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient included in the study.
Authors’ contributions
El-Agwany had done the diagnoses and surgery along with writing the article.
References
- Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010; 59: 1-110.
- Jaiyeoba O, Lazenby G, Soper DE. Recommendations and rationale for the treatment of pelvic inflammatory disease. Expert Rev Anti Infect Ther. 2011; 9: 61-70.
- Gaitan H, Angel E, Diaz R, Parada A, Sanchez L, Vargas C. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2002; 10: 171-180.
- Horrow MM. Ultrasound of pelvic inflammatory disease. Ultrasound Q. 2004; 20: 171-179.
- Gjelland K, Ekerhovd E, Granberg S. Transvaginal ultrasound-guided aspiration for treatment of tubo-ovarian abscess: a study of 302 cases. Am J Obstet Gynecol. 2005; 193: 1323-1330.
- Hiller N, Sella T, Lev-Sagi A, Fields S, Lieberman S. Computed tomographic features of tuboovarian abscess. J Reprod Med. 2005; 50: 203-208.
- Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ. Diagnosis and management of complicated intraabdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010; 50: 133-164.
- Dewitt J, Reining A, Allsworth JE, Peipert JF. Tuboovarian abscesses: is size associated with duration of hospitalization & complications? Obstet Gynecol Int. 2010; 2010: 847041.
- Granberg S, Gjelland K, Ekerhovd E. The management of pelvic abscess. Best Pract Res Clin Obstet Gynaecol. 2009; 23: 667- 678.
- Trent M, Haggerty CL, Jennings JM, Lee S, Bass DC, Ness R. Adverse adolescent reproductive health outcomes after pelvic inflammatory disease. Arch Pediatr Adolesc Med. 2011; 165: 49-54.
- Ness RB, Soper DE, Richter HE, Randall H, Peipert JF, Nelson DB, et al. Chlamydia antibodies, chlamydia heat shock protein, and adverse sequelae after pelvic inflammatory disease: the PID Evaluation and Clinical Health (PEACH) Study. Sex Transm Dis. 2008; 35: 129-135.