
Research Article
Austin J Obstet Gynecol. 2019; 6(4): 1144.
Fetal Liver Length and State of Maternal Glycemic Control
Gharib WF1* and Huissen WM2
¹Lecturer of Obstetrics and Gynecology Faculty of Medicine, Suez Canal University, Egypt
²Lecturer of Radiology, Faculty of Medicine, Suez Canal University, Egypt
*Corresponding author: Waleed Fouad Gharib, Lecturer of Obstetrics and Gynecology Department of Obstetrics and Gynecology, Faculty of Medicine, Suez Canal University, Round Road, Ismailia 41111, Egypt
Received: May 16, 2019; Accepted: May 29, 2019;Published: June 05, 2019
Abstract
Objective: To assess the relationship between fetal liver length as measured by ultrasound and maternal serum glucose level.
Setting: Department of Obstetrics and Gynecology, Suez Canal University Hospitals, Ismailia, Egypt.
Patients and Methods: This prospective observational study included 60 pregnant females; the participants were randomly allocated into 2 groups. The case group consisted of 30 pregnant females with either gestational or presentational diabetes, while the control group consisted of 30 healthy pregnant females. The participants were subjected to thorough medical history taking with emphasis on a detailed obstetric history, complete physical examination, investigations (Glucose Tolerance Test (GTT), glycosylated hemoglobin (HbA1c) and trans-abdominal ultrasound in which fetal parameters in addition to Fetal Liver Length (FLL) were measured.
Main Outcome Measures: Fetal liver length as measured by ultrasound among diabetic and non-diabetic pregnant females.
Results: Fetal liver length measurement at 28 weeks among the case group (48.9±3.4 mm (40.4-55) was significantly greater than the control group (41.7±3.3 mm (34.5-49.2) (p value < 0.001). Again at 37 weeks of gestation, the fetal liver length was greater among the case group compared to control (65.6±4.8 mm (56.2-72.5) vs. 54.5±3.4 mm (40.4-56.7), respectively with significant p value < 0.001).
Conclusion: Fetal liver length measurements by ultrasound correlates well with the state of maternal glycemic control among pregnant females and can be used as easy, more accurate and reproducible marker for fetal macrosomia and maternal glycemic control.
Keywords: Fetal Liver Length; Diabetes with Pregnancy
Introduction
Diabetes is a common metabolic disorder occurring during pregnancy. Gestational Diabetes Mellitus (GDM) is the most common form of impaired glycemic control during pregnancy, representing up to 90% of all cases, with progressively rising incidence that is probably due to improved screening and diagnosis rather than actual increase in incidence [1]. Gestational Diabetes Mellitus (GDM) is the occurrence of impaired glucose metabolism for the first time during pregnancy after 24 weeks [2].
Diabetes whether presentational or gestational carries several fetal and maternal possible adverse outcomes, including pre-eclampsia, preterm birth, polyhydramnios, altered fetal growth, increasing risk of fetal demise, neonatal respiratory distress syndrome, neonatal hypocalcemia, fetal cardiac hypertrophy, neonatal hyperbilirubinemia, and increased incidence of congenital anomalies which may complicate up to 10 % of diabetic pregnancies [3].
Sonographic assessment of fetal weight is inconclusive predictor of macrosomia and adverse possible complications of diabetes, with variable sensitivity and specificity. Meta-analysis of large number of formula used for assessment of expected fetal weight showed that they were all deficient in accurate detection of macrosomia. Early identification of fetuses with the potential risk of macrosomia could potentially help in antenatal management of the perinatal complications associated with diabetes mellitus [4].
As the expected fetal weight measurement does not accurately predict macrosomia or glycemic control, several alternatives have been proposed to help in more accurately predicting glycemic control and macrosomia. The aim of these alternative markers is better glycemic control and better maternal and fetal outcomes.
Maternal hyperglycemia leads to an increase in glucose transfer from the mother to the fetus through the placenta resulting in fetal hyperglycemia and hyperinsulinemia. Since the insulin acts as a growth factor, it promotes the growth of insulin-dependent tissues and organs such as the liver [5].
Fetal Liver Length (FLL) as measured by ultrasound correlates closely to the liver mass, an increased growth of the fetal liver mass as in pregnancies complicated by diabetes is expected to increase FLL. In this context, few studies have been produced to figure out the reproducibility of such measurements in the prediction of diabetes or its complications.
In 2015, Perovic and his colleagues published a paper in which they measured FLL in pregnant females and correlated these measurements to the results of oral glucose tolerance tests done to the patients. They noticed larger FLL measurements in ladies with abnormal results of the oral glucose tolerance tests [6].
The work in this research is predominantly to confirm the presence of constant and significant relation between fetal liver length and state of maternal blood glucose level.
Participants and Methods
Participants
This is an observational prospective study which was performed at the department of Obstetrics and Gynecology, Suez Canal University hospital. This study was approved by the faculty ethical committee; and all patients gave an informed consent before inclusion in the study. Pregnant women attending the outpatient clinic as well as those admitted to the inpatient were eligible for the study. 60 pregnant females between 20 and 39 years were recruited for the study; They were allocated into two groups. The control group (30 subjects) included healthy pregnant women with singleton pregnancies, sure dates and normal venous serum glucose levels. The case group (30 subjects) included pregnant women with diabetes either pregestational or gestational diabetes as evidenced by HbA1c and venous serum glucose levels. Patients (case group) with BMI equal to or more than 30, running on long term medications that could affect glucose metabolism. Also cases with chronic illnesses other than diabetes mellitus, multiple gestation, and premature rupture of membranes, other obstetric complications or unsure dates were excluded from the study. Fetuses with congenital anomalies or growth restriction were excluded from the study.
Methods
All the participants were subjected to thorough medical history (with emphasis on obstetric history), complete physical examination (including general, abdominal and obstetric examination) as well as investigations.
Oral three hour Glucose Tolerance Test (GTT) was done to all subjects except those already diagnosed with diabetes before pregnancy. In this test, fasting venous serum glucose level was measured then the patient was given 100gm oral glucose and serum glucose level was measured at 1st, 2nd and 3rd hours. Normal levels are: Fasting: 95mg/dl, 1st hour 180mg/dl, 2nd hour 155mg/dl, 3rd hour 140mg/dl, two or more of the venous serum concentrations must be met or exceeded for a positive diagnosis [7].
Venous blood samples were withdrawn in the hospital laboratory to measure HbA1c. The previous laboratory investigations were done at 28 weeks and repeated again at 37 weeks of gestation.
During each antenatal care visit, fasting (Normal up to 95g/ dl), and two hours postprandial serum glucose levels (Normal up to 140mg/dl) were determined and recorded to assess the state of glycemic control. HbA1c was assessed twice; at 28 weeks and repeated at 37 weeks of gestation.
When delivery was indicated, termination of pregnancy was done in the Obstetrics and Gynecology department delivery and operation rooms. The timing and mode of termination were according to the obstetrical indications. Immediate APGAR scores were recorded. Neonatal weight at birth was also recorded. Any complications of pregnancy, the mode of delivery and the indication for termination were all recorded.
Ultrasound examination: Ultrasound assessment was done through the trans-abdominal route, using a curvilinear probe, fetal biometry including femur length, abdominal circumference and biparietal diameter was recorded plus amniotic fluid index as one of the indicators of diabetic control were measured. The same measures were done at 28 weeks of gestation and repeated at 37 weeks.
Fetal Liver Length (FLL) measurement: This measure was done twice throughout the study, at 28 and 37 weeks of gestation. All the ultrasound scans were performed by the same operator who was not informed about the data obtained previously from the study participants, which means that he was blinded as to the risk group.
FLL was determined in the sagittal or coronal plane. To measure the FLL, the fetal aorta was identified in the longitudinal plane; the transducer is then moved along this plane until both the right hemidiaphragm and the tip of the right lobe of the liver were visualized. Length of the right lobe of the liver was measured as the longest distance from the diaphragm at the cardiopulmonary boundary to the inferior hepatic border [8].
Several measurements were obtained until three were reproducible within a 2mm range and then the average of these numbers was calculated. On-screen calibers were used. A Philips Ultrasound device (Philips health care machine HD11XE, PW 2.5-5 MHZ) was used. A curvilinear probe was used. Measurement of FLL was initially done at 28 weeks of gestation. It was repeated again at 37 weeks of gestation.
Results
Regarding the basic characteristics, both groups were comparable with no significant difference between the two groups regarding the age, parity, residence and educational level. A higher BMI was noted in the case group compared to control (28.3±0.67 vs. 27.4±0.76, respectively with significant p value = 0.03) (Table 1).
Characteristic
Control (n=40)
case (n=40)
P value
Mean ± SD
Mean ± SD
Age (years)
28.1 ± 5.5
28.3 ± 5.8
0.32
Gravidity
2.3±0.88
2.43±1.01
0.22
Parity
1.3 ± 0.8
1.13 ± 0.94
0.21
Body mass index (Kg/m2)
27.4 ± 0.76
28.3 ± 0.67
0.03*
Educated
83.30%
76.60%
0.19
Residence
Rural: 43.3%
Rural: 36.7%
0.47
Urban: 56.7%
Urban: 63.3%
* Student-t test is statistically significant at 95% confidence level.
Table 1: Baseline characteristics among studied population in both groups.
The basic parameters (fasting blood sugar, postprandial 2h blood sugar, fundal level, HBA1C, Bi-parietal Diameter, femur length, abdominal circumference, amniotic fluid index and estimated fetal weight) among both groups were assessed at 28 weeks of gestation together with FLL, with all measures showing statistically significant difference between both groups except the femur length and the biparietal diameter. FLL measurement at 28 weeks was significantly higher in the case group compared to control (48.9±3.4 mm (40.4-55) vs 41.7±3.3 mm (34.5-49.2), respectively with significant p value ‹ 0.001) (Table 2).
Characteristic
Control (n=30)
Case (n=30)
P value
Mean ± SD
Mean ± SD
Fundal level
28.5±1.1
30.15±1.9
<0.001*
HBA 1C (%)
4.5±0.4
6.5±1.2
<0.001*
Range in %
(4 - 5.4 %)
(4.5 - 9.5 %)
Femur Length (mm)
52.9±1.2
53.2±1.6
0.48
Range in mm
(50.6 - 55 mm)
(50 - 55.2 mm)
Bi-parietal Diameter (mm)
71.0±0.9
71.3±1.0
0.13
Range in mm
(69 - 72.4 mm)
(69.2 - 73.2 mm)
Abdominal circumference (mm)
246.3±17.2
253.9±7.2
0.01*
Range in mm
(229.7 - 248.2 mm)
(243 - 270 mm)
Amniotic Fluid Index (cm)
13.4±1.3
17.6±3.2
<0.001*
Range in cm
(10 - 16 cm)
(12 - 28 cm)
Fetal Liver Length (FLL) (mm)
41.7±3.3
48.9±3.4
<0.001*
Range of FLL in mm
(34.5-49.2mm)
(40.4-55mm)
Fasting blood sugar (gm/dl)
79.9 ± 5.8
110.3 ± 10.6
< 0.001*
2hr Postprandial blood sugar (gm/dl)
119.4 ± 7.8
138.3 ± 1.9
< 0.001*
* Student-t test is statistically significant at 95% confidence level.
Table 2: Main parameters in studied patients at 28 weeks of gestation.
When the same parameters were reassessed again at 37 weeks of gestation, the FLL was again greater among the case compared to control (65.6±4.8 mm (56.2-72.5) vs. 54.5±3.4 mm (40.4-56.7), respectively with significant p value < 0.001 ) (Table 3).
Characteristic
Control (n=30)
Case (n=30)
P value
Mean ± SD
Mean ± SD
Fundal level
37.8±0.9
39.3±0.8
<0.001*
HBA1C (%)
4.6±0.5
6.8±1.1
<0.001*
Range in %
(4 - 5.4 %)
(5.2 - 9.8 %)
Femur Length (mm)
72.2±0.7
72.8±0.6
0.001*
Range in mm
(70 - 73.4 mm)
(71 - 73.9 mm)
Bi-parietal Diameter (mm)
91.2±1.3
92.2±1.5
0.008*
Range in mm
(88 - 93 mm)
(90 - 94 mm)
Abdominal circumference (mm)
335.4±8.8
355±14.4
<0.001*
Range in mm
(317 - 350 mm)
(328 - 383 mm)
Amniotic Fluid Index (cm)
12.2±2.6
17.5±3.8
<0.001*
Range in cm
(10 - 18 mm)
(12 - 23 cm)
Fetal Liver Length (mm)
54.5±3.4
65.6±4.8
<0.001*
Range of FLL in mm
(40.4-56.7 mm)
(56.2-72.5 mm)
Estimated fetal weight (gm)
3090.8 ± 140
3550.6 ± 370
<0.001*
Range in gm
(2780 - 3380 gm)
(3090 - 4390 gm)
Fasting blood sugar (mg/dl)
72.7 ± 4.4
106 ± 9.8
0.001*
2hr Postprandial blood sugar (mg/dl)
115.5 ± 9.9
139.4 ± 9.2
0.001*
*Student-t test is statistically significant at 95% confidence level.
Table 3: Main parameters in studied patients at 37 weeks of gestation.
Within the case group, at 28 weeks the mean FLL was higher among those with Pre-Gestational Diabetes (PGD) than those with Gestation Diabetes (GD) (50.55±2.35 mm vs. 46.15±2.1 mm, respectively with significant p value = 0.01). Again at 37 weeks, the mean FLL was higher among those with Pre-Gestational Diabetes (PGD) than those with Gestation Diabetes (GD) (66±2.65 mm vs. 59.69±2.7 mm, respectively with significant p value = 0.01) (Table 4).
Characteristic
GDM
Mean ± SD
(n = 16)
pre-gestational DM
Mean ± SD
(n = 14)
P value
Fetal Liver Length 28 wks (mm)
46.15 ± 2.1
50.55 ± 2.35
0.01*
Fetal Liver Length 37 wks (mm)
59.69 ± 2.7
66 ± 2.65
0.01*
* Student-t test is statistically significant at 95% confidence level.
Table 4: Comparison of fetal liver length at 28 weeks and 37 weeks among the case group.
A strong positive correlation is noted between FLL and abdominal circumference (r = 0.82), AFI (r = 0.86), HBA1C level (r = 0.83), expected fetal birth weight (r = 0.82) and neonatal birth weight (r = 0.80). A moderate negative correlation was noted between fetal liver length and gestational age at termination (r = -0.34), all the previous correlations were statistically significant (Table 5).
Parameter
r
P value
Fundal level
0.69*
<0.001
Femur Length (mm)
0.28*
0.001
Bi-parietal Diameter (mm)
0.24*
0.002
Abdominal circumference (mm)
0.82*
<0.001
Amniotic Fluid Index (cm)
0.63*
<0.001
HbA1c (%)
0.83*
<0.001
Expected Fetal Birth Weight (gm)
0.82*
<0.001
Gestational Age At Termination (weeks)
-0.34*
<0.001
Birth Weight (gm)
0.80*
<0.001
*Pearson correlation is statistically significant at 95% confidence level.
Table 5: Correlation between Fetal liver length at 37 weeks and other parameters.
The ROC curve analysis for the relation between FLL measured at 28 weeks of gestation and the incidence of diabetes during pregnancy. The chosen cut-off value for fetal liver length at 28 weeks, which represented the best compromise between sensitivity and specificity, was 53.8 mm with sensitivity of 100% and specificity of 92% in prediction of diabetes with pregnancy. (AUC = 97%), with significant p value = 0.001 (Figure 1,2).

Figure 1: Ultrasound measurement of fetal liver length.
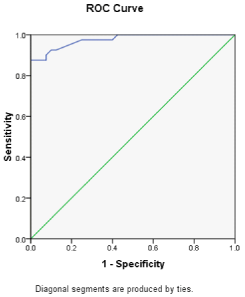
Figure 2: ROC curve analysis for the relation between fetal liver length
measured at 28 weeks of gestation and the incidence of diabetes during
pregnancy.
Discussion
Diabetes is the most common metabolic disorder that is encountered during pregnancy with gestational diabetes being the commonest form (up to 90% of cases) (9).
Diabetes with pregnancy entails an increased risk for both the mother and the fetus including major congenital malformations, increased incidence of obstetric complication, increased incidence perinatal morbidity (10).
This work was primarily carried out with the aim of determining the effect of diabetes, whether pre-gestational or gestational, on FLL measurements, with the aim of testing FLL as an accurate marker for glycemic control and macrosomia.
Pregnant women attending to the outpatient clinic as well as those admitted to the inpatient were eligible for the study. 60 pregnant females between 20 and 39 years were recruited for the study, they were allocated into two groups. The control group (30 subjects) included healthy pregnant women with normal venous serum glucose levels. The case group (30 subjects) included pregnant women with diabetes either pre-gestational or gestational diabetes as evidenced by HbA1c and GTT. Patients (case group) with BMI equal to or greater than 30, running on long term medications that could affect glucose metabolism, with chronic illnesses other than diabetes mellitus, with multiple gestation, with premature rupture of membranes or with other obstetric complications or with unsure dates were excluded from the study. Also fetuses with congenital anomalies or growth restriction were excluded from the study. Both groups were properly matched with no significant differences in mean age, gravidity or parity. Only BMI was higher among the case group compared to control.
The main outcome of this work was the greater FLL measurements among the case group compared to control. Taken at 28 weeks, it was significantly higher in the case group compared to control (48.9±3.4 mm (40.4-55) vs. 41.7±3.3 mm (34.5-49.2), respectively with significant p value < 0.001).
When the same parameters were reassessed again at 37 weeks of gestation, FLL was again greater among the case compared to control (65.6±4.8 mm (56.2-72.5) vs. 54.5±3.4 mm (40.4-56.7), respectively with significant p value ‹ 0.001).
Ultrasound FLL measurements among the case group in the present study came in agreement with those reported by Vintzileos et al. in a study done to determine a range for normal FLL in different gestational ages from 20 weeks to term [8].
The difference in FLL measurements between both groups in our work came consistent with Garabedian et al., They performed a study to detect sonographic markers that can be used to indicate fetal macrosomia. FLL and surface area were measured. In their study, the ultrasound measurements were performed four times, four weeks apart. FLL measurements were consistent with our results and were significantly higher among the case group when ultrasounds done at 30 weeks, but when repeated at 35 weeks, the difference between the two groups was less well demonstrated. This was attributed to good glycemic control as described by the authors [11].
The findings in the present study agreed the previous data described by Mirghani et al. In gestational diabetes. FLL was measured in a coronal section between weeks 21 and 24 and showed a significant difference between diabetic and control populations, with a difference of 5mm between the two groups (36mm versus 31mm, respectively; P ‹ 0.001). This increase in liver size, according to the authors, is related to hyperglycemia, which favors fat storage in the liver [12].
Anderson et al. compared two measurements of fetal liver length by the same operator (intra-operator) and by 12 operators (interoperator), and reported the reproducibility of FLL measurement in diabetic pregnancy. The intra-observer difference was 3.06mm [95% CI: 2.68-3.59] and the inter-observer difference was 2.17mm [95% CI: 0.59-4.83] [13].
Perovic et al performed a study to detect the relationship between mid-trimester ultrasound FLL and the development of diabetes mellitus detected by OGTT. Women presenting at mid trimester for ante-natal care underwent ultrasound examination in which fetal liver length was measured. OGTT was performed 1 week later. In GDM patients, there was a significant positive correlation (P ‹ 0.001) between FLL and blood glucose levels. FLL measurements in GDM patients were significantly longer than in healthy pregnant women (P ‹ 0.001) [6].
The positive correlation between FLL measurement and blood glucose level in Perovic et al. work came in accordance with our work where a strong positive and significant correlation (r = 0.83, p value ‹0.001) was found between FLL and HgA1c, both findings reflects the close relation between FLL measurements and glycemic control.
The previous data were again supported by Roberts et al. They concluded that FLL measurement could be used as a marker of fetal growth more specific to diabetes. They concluded that accelerated liver growth can be an early event but that accelerated growth can still be modified by glycemic control even in later pregnancy [14]. The increase in size was mainly because of increased cell size (increased cytoplasm), also increased hematopoiesis with raised erythropoietin levels has been reported [15]. The relative increase in FLL in that study was evident as early as the eighteenth week of gestation and became more marked with increased duration of pregnancy. This suggests that even early in pregnancy changes in FLL may reflect glycemic control [14].
This increase in fetal liver size in diabetic patients can be attributed to maternal hyperglycemia with increased glucose transfer from the diabetic mother to the fetus resulting in fetal hyperglycemia and hyperinsulinemia, promoting growth of insulin-dependent tissues and organs such as the liver through both hyperplasia and hypertrophy [7].
Hyperinsulinemia also induces the growth of hematopoietic tissue in the fetal liver which may contain more than three times as much hematopoietic tissue as the fetal liver in controls. Also prolonged hyperglycemia favors fat storage in feta liver [13].
By further analysis of FLL within the case group showed that at 28 weeks, the mean fetal liver length was higher among those with Pre- Gestational Diabetes (PGD) than those with Gestation Diabetes (GD) ( 50.55±2.35 mm vs. 46.15±2.1 mm, respectively with significant p value =0.01). Again at 37 weeks, the mean fetal liver length was higher among those with Pre-Gestational Diabetes (PGD) than those with Gestation Diabetes (GD) (66±2.65 mm vs. 59.69±2.7 mm, respectively with significant p value =0.01), the previous note indicate that PGD has more profound effect on FLL than GD.
In this work, The ROC curve analysis for the relation between FLL measured at 28 weeks of gestation and the incidence of diabetes during pregnancy concluded that, the chosen cut-off value for fetal liver length at 28 weeks, which represented the best compromise between sensitivity and specificity, was 53.8mm with sensitivity of 100% and specificity of 92% in prediction of diabetes with pregnancy. (AUC = 97%), with significant p value = 0.001.
This large AUC was noted in Perovic et al. work, where The ROC analysis established a cut-off value for FLL (measured at 24 weeks of gestation) of 39mm for the prediction GDM, which has a sensitivity of 71.76%, specificity 97.56%, positive predictive value 91.0%, and negative predictive value of 90.9%, with high area under the curve ROC (90.5%) [6]. But the difference between both works came from the different and early timing for the production of the ROC curve in Petrovic et al. work. But both works denoted the usefulness of FLL measurements in reflecting glycemic control.
Conclusion
Fetal liver length measurements by ultrasound correlates well with the state of glycemic control among pregnant females and can be used as easy, more accurate and reproducible marker for maternal glycemic control and fetal macrosomia.
References
- American Diabetes Association. “Standards of medical care in diabetes--2011”. Diabetes Care 34 Suppl 1. 2011; S11-61.
- American Diabetes Association. “Diagnosis and classification of diabetes mellitus”. Diabetes Care 33 Suppl 1. 2010; S62-69.
- Raio L, Ghezzi F, Di Naro E, Buttarelli M, Franchi M, Durig P, et al. “Perinatal outcome of fetuses with a birth weight greater than 4500 g: an analysis of 3356 cases”. Eur J Obstet Gynecol Reprod Biol. 2003; 109: 160-165.
- Combs C A, Rosenn B, Miodovnik M and Siddiqi T A. “Sonographic EFW and macrosomia: is there an optimum formula to predict diabetic fetal macrosomia?”. J Matern Fetal Med. 2000; 9: 55-61.
- de Carvalho A A, Marchiori E, Carvalho J A, Figueiredo I, Velarde L G. “Use of fetal colon thickness for auxiliary term dating of pregnancy”. Int J Gynaecol Obstet. 2011; 112: 216-219.
- Perovic M, Gojnic M, Arsic B, Pantic I, Stefanovic T, Kovacevic G, et al. “Relationship between mid-trimester ultrasound fetal liver length measurements and gestational diabetes mellitus”. J Diabetes. 2015; 7: 497- 505.
- Gojnic M, Stefanovic T, Perovic M, Arsic B, Garalejic E, Micic J, et al. “Prediction of fetal macrosomia with ultrasound parameters and maternal glycemic controls in gestational diabetes mellitus”. Clin Exp Obstet Gynecol. 2012; 39: 512-515.
- Vintzileos A M, Neckles S, Campbell W A, Andreoli J W, Jr., Kaplan B M, Nochimson D J. “Fetal liver ultrasound measurements during normal pregnancy”. Obstet Gynecol. 1985; 66: 477-480.
- Goldstein I, Lockwood C, Hobbins J C. “Ultrasound assessment of fetal intestinal development in the evaluation of gestational age”. Obstet Gynecol. 1987; 70: 682-686.
- Lipscombe L L, Gomes T, Levesque L E, Hux J E, Juurlink D N, Alter D A. “Thiazolidinediones and cardiovascular outcomes in older patients with diabetes”. JAMA. 2007; 298: 2634-2643.
- Garabedian C, Vambergue A, Salleron J and Deruelle P. “Prediction of macrosomia by serial sonographic measurements of fetal soft-tissues and the liver in women with pregestational diabetes”. Diabetes Metab. 2013; 39: 511-518.
- Mirghani H, Zayed R, Thomas L, Agarwal M. “Gestational diabetes mellitus: fetal liver length measurements between 21and 24 weeks’ gestation”. J Clin Ultrasound. 2007; 35: 34-37.
- Anderson N G, Notley E, Graham P, McEwing R. “Reproducibility of sonographic assessment of fetal liver length in diabetic pregnancies”. Ultrasound Obstet Gynecol. 2008; 31: 529-534.
- Roberts A B, Mitchell J, Murphy C, Koya H, Cundy T. “Fetal liver length in diabetic pregnancy”. Am J Obstet Gynecol. 1994; 170: 1308-1312.
- Boito S M, Struijk P C, Ursem N T, Stijnen T, Wladimiroff J W. “Assessment of fetal liver volume and umbilical venous volume flow in pregnancies complicated by insulin-dependent diabetes mellitus”. BJOG. 2003; 110: 1007-1013.