
Research Article
Austin J Obstet Gynecol. 2019; 6(4): 1145.
Selenium and Sildenafil Citrate in Ovarian Torsion: An Experimental Study
Kishk EA1* and Shams TM2
¹Department of Obstetrics and Gynecology, Faculty of Medicine, Suez Canal University, Egypt
²Department of Pathology, Faculty of Medicine, Suez Canal University, Egypt
*Corresponding author: Eman A. Kishk, Department of Obstetrics and Gynecology, Faculty of Medicine, Suez Canal University, Egypt
Received: May 29, 2019; Accepted: June 24, 2019;Published: July 01, 2019
Abstract
Aim: To study the effects of co-administration of selenium and sildenafil citrate against damage induced by ovarian torsion in rats.
Materials and Methods: A total of forty- two sexually mature, virgin, female rat were divided randomly into six groups of seven each: sham group (C), ischemia group (I), ischemia reperfusion group (I/R), ischemia-reperfusion plus 0.2mg/kg selenium (I/R+Se) group, ischemia-reperfusion plus 1.4mg/kg sildenafil citrate (I/R+S) group, and ischemia-reperfusion plus combination of selenium and sildenafil citrate (I/R+S+Se). In ischemia group (I), rats exposed to ischemia for 3 hour (h). In ischemia-reperfusion group (I/R), rats exposed to ischemia for 3h followed by 6h of reperfusion. Treated groups received 0.2mg/ kg selenium or 1.4mg/kg sildenafil citrate or both 30min before reperfusion. Both ovaries were surgically removed carefully for all groups. One ovary was examined for histopathological changes and the other was subject to biochemical analysis including Malondialdehyde (MDA), Catalase (CAT) and Glutathione Peroxidase (GPx).
Results: Assessment of ovarian tissue damage using the score system showed marked vascular congestion, interstitial edema, leukocyte infiltration, hemorrhage, and follicular degeneration in ischemia and ischemia-reperfusion groups. Tissue damage score for I, IR and all treated groups were significantly higher than those of the sham group (p‹0.001), tissue damage score decreased significantly in I/R+Se and I/R+S groups compared to I/R group (p‹0.05), and notably the difference was quite significant in I/R+S+Se group (p‹0.001). There was significant increase in MDA levels and reduction in activities of CAT and GPx in I/R group compared to the sham group (P ‹ 0.05). In I/R+Se and I/R+S groups, MDA was significantly decreased compared to the I/R group (p‹0.05) and the difference was highly significant with co-administration of selenium and sildenafil (p‹0.001). CAT and GPx were higher in all treated groups compared to I/R group (p‹0.05).
Conclusion: The co-administration of selenium and sildenafil citrate is highly protective against damage induced by ovarian torsion in rats.
Keywords: Selenium; Seldenafil citrate; Ovarian torsion
Introduction
Ovarian torsion is the fifth most common gynecologic surgical emergency condition; it represents about 2.7%. Young females constitute approximately 70-75% [1]. Ovarian torsion often leads to infarction and requires urgent intervention. The ovarian recovery rate was less than 10% in adults and increase up to 27% in children [2]. Reperfusion injury is physiopathological process causes several degrees of tissue damage and occurs after restoration of circulation, so the prognosis of treatment is affected by this process [3].
Ischemia-Reperfusion (I/R) injury increases the production of free radicals as Reactive Oxygen Species (ROS) and Malondialdehyde (MDA). ROS are the main inducer of IR injury. The reduction of cellular Adenosine Triphosphate (ATP) stores is associated by conversion of ATP to hypoxanthine during ischemia. The injury is associated with increased production of ROS which results during the conversion of hypoxanthine to uric acid by Xanthine Oxidase (XO) following reoxygenation of the hypoxic tissue [4].
Selenium (Se) is an essential element and participates in maintenance of the various physiological processes. Se is incorporated into the catalytic site of antioxidant enzymes, such as glutathione peroxidase (GPx), and is involved in cell growth and development [5]. It protects cell membranes against the toxic and damaging effects caused by free radicals [6].
Phosphodiesterase type-5 (PDE5) inhibitors are vasoactive drugs that have been used in erectile dysfunction [7]. They inhibit the PDE5 enzyme resulting in increased cyclic GMP (cGMP) levels and smooth muscle relaxation [7]. These drugs are useful in the treatment of injuries in different organs, as brain [8] and heart [9]. To the best of our knowledge, the effects of co-administration of selenium and sildenafil in I/R injury on ovarian tissue has not been evaluated so our aim is to evaluate the possible effects of this novel combination against ovarian damage in rats.
Materials and Methods
Animals
This is an experimental study, where 42 sexually mature, virgin, female rats (8 weeks old) weighing 230-260 g were used throughout; they were obtained from the Laboratory Animal of Faculty of Pharmacy, Suez Canal University. All procedures were in accordance with the Guiding Principles for the Care of animals. They were housed individually for a 2-week acclimatization period prior to the experiment. Rats were fed ad libitum by standard laboratory pellet and tap water. A 12hr light: 12hr dark cycle was maintained. Room temperature was at 23 ± 2°C with a relative humidity of 45-55%. Rats were randomly divided equally into six groups.
Sham group (C): Sham operation rats were exposed to a surgical procedure as in the other groups, without any intervention to the adnexa.
Ischemia group (I): Both ovaries were torsioned 720 clockwise and fixed to the abdominal wall. After a 3h period of ischemia, ovaries were surgically removed [10].
Ischemia- reperfusion group (I/R): Ischemia for 3h followed by ovarian detorsion and reperfusion for 6h [10].
Group (I/R+S): Ischemia for 3h/reperfusion for 6h plus 1.4mg/ kg of sildenafil (El-Nile, Egypt) was injected ip, 30min before reperfusion [12].
Group (I/R_S+Se): Ischemia 3h/reperfusion 6h plus coadministration of 0.2mg/kg of selenium and 1.4mg/kg sildenafil ip 30min before reperfusion.
Selenium was obtained from JEDCO, Egypt, sodium selenite (98% powder), diluted in 0.9% NaCl (0.2mg/kg), was injected IP, 30 minutes before the reperfusion phase [11-13]. Sildenafil was obtained from Nile company, Egypt, sildenafil citrate powder was dissolved in 0.9% sodium chloride (NaCl) and was administered ip as a single dose (1.4mg/kg) 30min before reperfusion [12].
Surgical procedure
All surgical procedures were performed under sterile conditions. Rats were anesthetized with 25mg/kg, ip sodium thiopental and were placed in the supine position. A vertical midline incision (2cm) was made, and the abdomen was then closed with interrupted, size 0 vicryl sutures without any intervention to the adnexa for sham group. Torsion-detorsion model Rats underwent laparotomy procedure, both ovaries were torsioned 720 clockwise and fixed to the abdominal wall with a size 00 silk suture [14,15] and the abdomen was closed. After three hours, the suture was cut and both ovaries were detorsioned and the abdomen was closed with size 0 vicryl sutures. By the end of reperfusion period 6h, the abdomen was reopened and both ovaries were removed carefully [14,15]. The right ovaries were transferred into 10% formaldehyde solution for histopathological examination; the left ovaries were washed with 0.9% NaCl and prepared for biochemical analysis. All the surgical procedures for all rat groups were done by the gynecologist.
Histological analysis
The excised ovaries were fixed in 10% neutral buffered formalin solution and embedded in paraffin. The sections were cut at 5-μm thickness and stained with hematoxylin and eosin (H&E). Whole ovarian section was examined for each slide. The scoring system used for histopathologic evaluation of ovarian tissues was as previously described by Kara et al. [16]. Interstitial edema, congestion (vascular dilatation), hemorrhage, leukocyte infiltration and follicle degeneration were scored from 0 to 3 for each parameter. Scores represented the severity of the pathological finding; 0 represented no pathological finding, 1 represented pathological finding less than 33%, 2 for 33-66%, and finally 3 when pathological finding more than 66% of the ovarian section. The total tissue damage score was calculated by adding the score of each parameter. The sections were blindly observed under a light microscope to evaluate histopathological changes by one pathologist. All Images captured using calibrated standard digital microscope camera (Tucsen® ISH1000 digital microscope camera) using Olympus® CX21 microscope, all H&E slides captured using 100x magnification, UIS optical system (Universal Infinity System, Olympus®, Japan).
Biochemical analysis
The ovaries were washed with 0.9% NaCl, and samples were stored at -80°C until the Analysis. The frozen tissue samples were homogenized in 50mm phosphate buffer (10% W/V), pH 7.4, using Glas-Col tissue homogenizing system (Cole-Parmer, Vernon Hills, USA). Spectrophotometric measurement was performed to determine the activity of CAT, GPx and MDA [17-19]. Commercially available kits were obtained from Bio-Diagnostic, Egypt.
Statistical analysis
Data were processed using SPSS version 15 (SPSS Inc., Chicago, IL, USA). Quantitative data were expressed as mean ± SD, the experimental groups were compared using one-way Analysis of Variance (ANOVA) test and Tukey’s post hoc test. A probability value (p value) ‹0.05 was considered statistically significant.
Results
Examination of the ovarian tissue in sham operation group revealed normal cortical architecture, ovarian stroma entangling primordial follicles and normal growing mature follicles. There was no change in the appearance of interstitial cells, no infiltration or signs of hemorrhage (Figure 1A). Assessment of ovarian tissue damage using the score system of Kara et al. [16] showed marked vascular congestion, interstitial edema, hemorrhage, neutrophil infiltration and follicular degeneration in ischemia and ischemic reperfusion groups (Figure 1B, 1C).

Figure 1A: Normal ovary of a sham rat showing cellular spindle ovarian
stroma entangling few primordial follicles (black arrow) and growing maturing
follicles (green arrow) and normal blood vessels (red arrowhead).
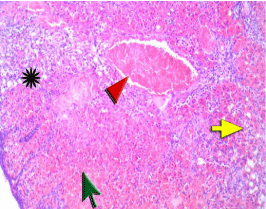
Figure 1B: The Ovary of a rat in ischemia group showed marked hemorrhage
and extravasated red cells (green arrow) within the collapsed ovarian stroma
due to ischemia resulting in marked congestion of vessels (red arrowhead)
with ischemic ovarian stroma breakdown. There are degenerated primordial
follicles and edema (asterisk) and neutrophil infiltration (yellow arrow).
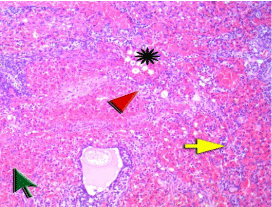
Figure 1C: Ovary of a rat in ischemia/perfusion group showed marked
hemorrhage and extravasated red cells (green arrow) within the collapsed
ovarian stroma due to post ischemia injury resulting in marked congestion of
vessels (red arrowhead) with ischemic ovarian stroma breakdown. There are
edema (asterisk) and neutrophil infiltration (yellow arrow). Next to the green
arrow there is degenerated mature follicle.
These changes were less marked in ischemic/reperfusion groups pretreated with sildenafil or selenium, the ovarian tissue showed stromal thick walled vessels showed average caliber with mild congestion and growing mature follicles with minimal residual vacuoles and degenerative changes of granulosa cells. There is residual stromal hemorrhage in a very few peripheral foci in rat treated with selenium (Figure 1D, 1E). Stromal thick walled vessels showed average caliber with slight congestion and growing maturing follicles with absence of degeneration were observed in IR+S+Se group. There is no evidence of ischemia of post ischemic reperfusion injury (Figure 1F).
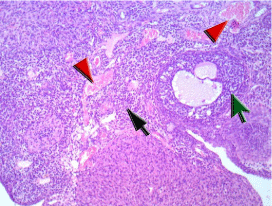
Figure 1D: The ovary of a rat in (IR+S) group showing cellular spindle
ovarian stroma entangling few primordial follicles (black arrow) and growing
maturing follicles with minimal residual vacuoles and degenerative changes
of granulosa cells (green arrow), stromal thick walled vessels showed
average caliber with slight congestion (red arrowhead). There is no evidence
of ischemia of post ischemic reperfusion injury.
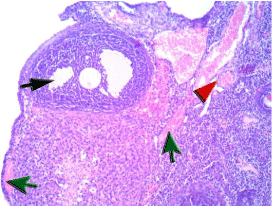
Figure 1E: The ovary of a rat in (IR +Se) group showing cellular spindle
ovarian stroma entangling few primordial follicles and growing maturing
follicles with minimal residual vacuoles and degenerative changes of
granulosa cells (black arrow), there are dilated thick walled vessels with mild
congestion (red arrowhead) and residual stromal hemorrhage in very few
peripheral foci (green arrow).
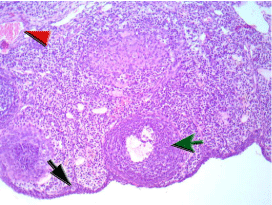
Figure 1F: The ovary of a rat in (IR+S+Se) showing cellular spindle ovarian
stroma entangling few primordial follicles (black arrow), growing maturing
follicles with foci of epithelium regeneration (green arrow) and stromal
thick walled vessels showed average caliber with slight congestion (red
arrowhead). There is no evidence of ischemia of post ischemic reperfusion
injury.
Total tissue damage score for ischemia, ischemia/reperfusion and all treated groups were significantly higher than those of the sham group (p‹0.001). Pretreatment with sildenafil, selenium or both significantly decreased the ovarian tissue damage scores in I/R+S, I/R+Se and IR+S+Se groups compared with those in I/R group, the difference was significant in I/R+S and I/R+Se groups (p‹0.05) and highly significant in I/R+S+Se group (Figure 2) (p‹0.001). The total tissue damage score was significantly lower in the I/R+S+Se group when compared to the I/R+S and I/R+Se groups (p‹0.05).
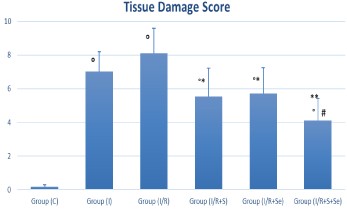
Figure 2: Total tissue damage scores of the studied groups. °p < 0.001 vs.
sham group, *p ‹ 0.05 vs. I/R group, **p ‹ 0.001 vs. I/R group and # p ‹0.05
vs. S and Se groups.
Group C=Sham group; I=Ischemia; I/R=Ischemia/reperfusion; I/
R+S=Ischemia/reperfusion + Sildenafil; I/R+Se=Ischemia/reperfusion +
Selenium; I/R+S+Se=Ischemia/reperfusion + Sildenafil + Selenium.
Biochemical studies showed significant increase in MDA levels and reduction in activities of CAT and GPx in I/R group compared to the sham group (p‹0.05). Treatment significantly lowered tissue MDA and increased CAT and GPx activities in all treated groups compared with those in IR group. In the I/R+S and I/R+Se groups, MDA was significantly decreased compared to the I/R group (p‹0.05) and the difference was highly significant with the co-administration of selenium and sildenafil (p‹0.001) (Figure 3A). Catalase and glutathione peroxidase activities were higher in all treated groups compared to the I/R group, and the difference was statistically significant (p‹0.05) (Figure 3B, 3C).
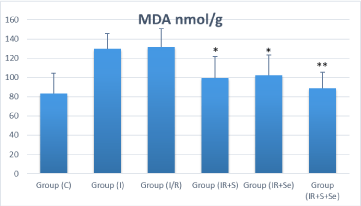
Figure 3A: Distribution of Ovarian malondialdehyde (MDA) among
the experimental groups. *p ‹ 0.05 vs. I/R group and ** p ‹ 0.001 vs. I/R
group. Group C=Sham group; I=Ischemia; I/R=Ischemia/perfusion; I/
R+S=Ischemia/perfusion+Sildenafil; I/R+Se=Ischemia/perfusion+Selenium;
IR+S+Se=Ischemia/perfusion + Sildenafil+Selenium. MDA=Malondialdehyde.
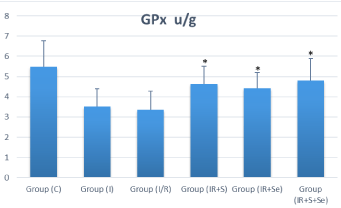
Figure 3B: Distribution of Ovarian glutathione peroxide (GPx) among
the experimental groups. *p ‹ 0.05 vs. I/R group. Group C=Sham group;
I=Ischemia; I/R=Ischemia/perfusion; I/R+S=Ischemia/perfusion + Sildenafil;
I/R+Se=Ischemia/perfusion + Selenium; I/R+S+Se=Ischemia/perfusion +
Sildenafil + Selenium. GPx=Glutathione Peroxide.
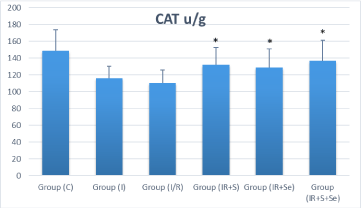
Figure 3C: Distribution of Ovarian catalase (CAT) among the experimental
groups. *p ‹ 0.05 vs. I/R group. Group C=Sham group; I=Ischemia; I/
R=Ischemia/perfusion; I/R+S=Ischemia/perfusion + Sildenafil; I/
R+Se=Ischemia/perfusion + Selenium; I/R+S+Se=Ischemia/perfusion +
Sildenafil + Selenium. CAT=Catalase.
Discussion
In the present study, the use of selenium (antioxidant), sildenafil (PDE5), and the novel combination of both protects against ovarian tissue damage induced by the ischemia-reperfusion process. The combination of both drugs proved to be more protective when compared to each alone.
Ovarian torsion is an emergency condition that usually occurs in women of reproductive age [20]. Decreased perfusion, tissue hypoxia and cell death are possible consequences of ovarian ischemia [3,21]. Early management is important to preserve the ovarian function and fertility, especially in young women [16].
In the present study, severe damage was reported in the ischemia and ischemia/reperfusion groups. Pathological
changes included marked hemorrhage, vascular congestion, edema, leukocyte infiltration and follicular degeneration. These histopathological changes decreased significantly in all treated groups and especially with the co-administration of selenium and sildenafil.
Previous studies showed that the animals exposed to ovarian torsion for 3h followed by 3h reperfusion had follicular degeneration, edema, congestion and hemorrhage [10-12]. In the current study, tissue damage score was more prominent in the I/R group compared to the I group. Our findings were in accordance with the previous study of Incebiyik et al. (2015) who demonstrated that tissue damage in I/R group was higher than I group [12].
Reperfusion injury usually ensues following ovarian detorsion. The generation of toxic oxygen free radicals with the return of blood flow following ischemia is responsible for that condition. The pathogenesis of tissue injury involves the activation of neutrophils and the release of ROS resulting in lipid peroxidation in the cell membranes which leads to the reperfusion injury [3].
In the current study, ovarian MDA levels were higher, while CAT, and GPx enzyme activities decreased significantly in I/R groups, compared to sham group. Pretreatment with sildenafil, showed reduction in MDA and increase in both CAT and GPx significantly. Similar findings were reported with that of Celik, et al. (2014), who evaluated the effects of sildenafil on ovarian rat tissue undergoing I/R injury. They found an increase in MDA and myeloperoxidase activities and a decrease in ovarian tissue damage, GPx and superoxide dismutase activities [22].
Sildenafil used in the treatment of tissue ischemia through vasodilatation and regulation of blood flow by increasing nitric oxide production [23] and decrease free oxygen radicals by inhibiting leukocyte infiltration and adhesion [24]. The protective effect of sildenafil against the ischemic/reperfusion damage results from nitric oxide production and its antioxidant properties [23-25] and its effectiveness has been shown in testicular torsion as well [26].
Our study demonstrated that selenium increased the level of CAT, and GPx, and decreased MDA which confirms the results of Bozkurt et al. [11]. An earlier study found that selenium significantly decreased MDA levels and increased the Superoxide Dismutase (SOD) activities in a rat model of testicular torsion/detorsion [13]. Selenium increased the activity and function of antioxidant enzymes, such as SOD, CAT, and GPx, so protect tissues against oxidative damage [27-30].
The novelty of the study is in the combined use of selenium and sildenafil which proved effective against ovarian tissue damage. Each act by a different mechanism; selenium through its antioxidant activity and PDE5 through vasodilation. The synergistic effect proved beneficial. In addition, sildenafil was shown to increase the serum (and brain) levels of selenium in male albino rats [31] further enhancing the synergistic effects.
Conclusion
In conclusion, co-administration of selenium and sildenafil is a novel combination which proved protective effect against damage induced by ovarian ischemia/reperfusion in rats. It might play an important role in avoiding ovariectomy and preservation of fertility in humans. However, large studies are required to elucidate the effectiveness of this new combination to protect the ovaries from the damage caused by ischemia and reperfusion injury process.
Conflict of Interest
The authors declare no conflict of interest.
References
- Asfour V, Varma R, Menon P. Clinical risk factors for ovarian torsion. J Obstet Gynaecol. 2015; 19: 1-5.
- Anders JF, Powell EC. Urgency of evaluation and outcome of acute ovarian torsion in pediatric patients. Arch Pediatr Adolesc Med. 2005; 159: 532-535.
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxiareoxygenation injury. Am J Physiol Cell Physiol. 2002; 282: C227-241.
- Abramov, A.Y., Scorziello, A., Duchen, M.R. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J. Neurosci. 2007; 27: 1129-1138.
- Hatanaka N, Nakaden H, Yamamoto Y, Matsuo S, Fujikawa T, Matsusue S. Selenium kinetics and changes in glutathione peroxidase activities in patients receiving long-therm parenteral nutrition and effects of supplementation with selenite. Nutrition. 2000; 16: 22-26.
- Clausen J, Nielsen SA, Kristensen M. Biochemical and clinical effects of an antioxidant supplementation of geriatric patients. Biological Trace Element Research. 1989; 20: 135-151.
- Shabsigh, R. Therapy of ED: PDE-5 inhibitors. Endocrine. 2004; 23: 135-141.
- Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006; 83: 1213-1219.
- Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida Ki, et al. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K (ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol. 2007; 42: 453- 458.
- Ayse N. Gungor C, Turkon H, Albayrak A, Ovali M, Islimye M, Gencer M, et al. Does Omegaven have beneficial effects on a rat model of ovarian ischemia/ reperfusion? European Journal of Obstetrics & Gynecology and Reproductive Biology. 2014; 181: 240-245.
- Bozkurt S, Arikan D, Kurutas E, Sayar H, Okumus M, Coskun A, et al. Selenium has a protective effect on ischemia/reperfusion injury in a rat ovary model: biochemical and histopathologic evaluation. J Ped. Surgery. 2012; 47: 1735-1741.
- Incebiyik A, Seker A, Camuzcuolgu H, Kocaslan S, Camuzcuoglu A, Hilali NG, et al. Does sildenafil have a beneficial effects against ovarian ischemiareperfusion injury in rats? Arch obestet Gynecol. 2015; 291: 1283-1288.
- Avlan D, Erdougan K, Cimen B, Düsmez Apa D, Cinel I, Aksöyek S, et al. The protective effect of selenium on ipsilateral and contralateral testes in testicular reperfusion injury. Pediatr Surg Int. 2005; 21: 274-278.
- Aslan MK, Boybeyi O, Senyucel MF, Ayva S, Kisa Ü, Aksoy N, et al. Protective effect of intraperitoneal ozone application in experimental ovarian ischemia/ reperfusion injury. J Pediatr Surg. 2012; 47: 1730-1734.
- Yigiter M, Halici Z, Odabasoglu F, Keles ON, Atalay F, Unal B, et al. Growth hormone reduces tissue damage in rat ovaries subjected to torsion and detorsion: biochemical and histopath-ologic evaluation. Eur J Obstet Gynecol Reprod Biol. 2011; 157: 94-100.
- Kara M, Dagloglu YK, Kuyucu Y, Tuli A, Tap O. The effect of edaravone on ischemia-reperfusion injury at ovary. Eur J Obestet Gynecol Reprod Biol. 2012; 162: 197-202.
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984; 105: 121-126.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70: 158-169.
- Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978; 86: 271-278.
- Yuk JS, Kim LY, Shin JY, Choi DY, Kim TY, Lee JH. A national populationbased study of the incidence of adnexal torsion in the Republic of Korea. Int J Gynecol Obstet. 2015; 129: 169-170.
- Halici Z, Karaca M, Keles ON, Borekci B, Odabasoglu F, Suleyman H, et al. Protective effects of amlodipine on ischemia-reperfusion injury of rat ovary: biochemical and histopathologic evaluation. Fertil Steril. 2008; 90: 2408- 2415.
- Celik M, Aksoy AN, Aksoy H, Aksoy Y, Halici Z. Sildenafil reduces ischemiareperfusion injury in rat ovary: biochemical and histopathological evaluation. Gynecol Obstet Invest. 2014; 78: 162-167.
- Inan M, Uz YH, Kizilay G, Topcu-Tarladacalisir Y, Sapmaz-Metin M, Akpolat M, et al. Protective effect of sildenafil on liver injury induced by intestinal ischemia/reperfusion. J Pediatr Surg. 2013; 48: 1707-1715.
- Yildirim A, Ersoy Y, Ercan F, Atukeren P, Gumustas K, Uslu U, et al. phosphodiesterase-5 inhibition by sildenafil citrate in a rat model of bleomycininduced lung fibrosis. Pulm Pharmacol Ther. 2010; 23: 215-221.
- Zahran MH, Hussein AM, Barakat N, Awadalla A, Khater S, Harraz A, et al. Sildenafil activates antioxidant and antiapoptotic genes and inhibits proinflammatory cytokine genes in a rat model of renal ischemia/reperfusion injury. Int Urol Nephrol. 2015; 47: 1907-1915.
- Beheshtian A, Salmasi AH, Payabvash S, Kiumehr S, Ghazinezami B, Rahimpour S, et al. Protective effects of sildenafil administration on testicular torsion/detorsion damage in rats. World J Urol. 2008; 26: 197-202.
- Ansari MA, Ahmad AS, Ahmad M, Salim S, Yousuf S, Ishrat T, et al. Selenium protects cerebral ischemia in rat brain mitochondria. Biol Trace Elem Res. 2004; 101: 73-86.
- Pucheu S, Coudray C, Tresallet N, Favier A, de Leiris J. Effect of dietary antioxidant trace element supply on cardiac tolerance to ischemia-reperfusion in the rat. J Mol Cell Cardiol. 1995; 27: 2303-2314.
- Bulger EM, Maier RV. Antioxidants in critical illness. Arch Surg. 2001; 136: 1201-1207.
- Schweizer U, Brauer AU, Kohrle J, Nitsch R, Savaskan NE. Selenium and brain function: a poorly recognized liaison. Brain Res Rev. 2004; 45: 164-178.
- Fayed AH, Gad SB. Effect of sildenafil citrate (Viagra®) on trace element concentration in serum and brain of rats. J Trace Elem Med Biol. 2011;25: 236-238.