
Research Article
Austin J Obstet Gynecol. 2020; 7(2): 1159.
The Effect of Vitamin D on Dysmenorrheic Vitamin D Deficient Females (Single Centre Experiment)
Dawod LH*, Mohmmed SJ and Seddiq WT
Department of Gynecology and Obstetrics, Al-Khanzaa Teaching Hospital, Iraq
*Corresponding author: Lubna Hazim Dawod, Department of Gynecology and Obstetrics, Al-Khanzaa Teaching Hospital, Hay Nirkal, Mosul, Iraq
Received: November 15, 2020; Accepted: December 21, 2020; Published: December 28, 2020
Abstract
Aim: The aim of this study was evaluating the effect of vitamin D in the treatment of dysmenorrhea in females who have vitamin D deficiency.
Material and Methods: A total of 40 patients between 19 and 37 years of age who were diagnosed with dysmenorrhea were included in the study in a randomized controlled manner. Cases were randomized into two groups of 5000 IU vitamin D once a day and a group did not receive vitamin D starting at the end of their menstrual cycle and continuing throughout two months study. Severity of menstrual pain was measured with Visual Analogue Scale (VAS), as the primary outcome. Need for using Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) during the study period was evaluated as the secondary outcome.
Results: There were no significant difference in age, body mass index and baseline VAS scores between groups. Pain severity of vitamin D group after treatment was found significantly lower than the group did not receive vitamin D. Median VAS scores of vitamin D group and the group did not receive vitamin D were 2 (1-4) and 7 (6-9), respectively after treatment. Requirement of NSAIDs was significantly less in vitamin D group than the group did not receive vitamin D.
Conclusion: Vitamin D has a clear effect in reducing the pain of dysmenorrhea in women with vitamin D deficiency.
Keywords: Dysmenorrhea; Vitamin D; Vitamin D deficiency
Introduction
Dysmenorrhea is pelvic or lower abdominal cyclic or recurrent pain, associated with menstruation [1]. Systemic symptoms such as nausea, vomiting, diarrhea, fatigue and insomnia frequently accompany the pain [2-6]. Dysmenorrhea has a high prevalence ranging from 45 to 93% of women of reproductive age [7]. Dysmenorrhea is classified into primary dysmenorrhea, defined as cramps originating from the uterus during menstruation without any underlying pelvic pathology, and secondary dysmenorrhea which is menstrual pain resulting from underlying pelvic pathologies [4,7]. Dysmenorrhea, when it is severe, can be associated with restriction of activity and absence from school or work [5]. Even with analgesics, many women get unsatisfactory relief of pain and uptake self-care strategies [9,10]. Pathogenesis of primary dysmenorrhea results from the increased synthesis of prostaglandins PGs which play a significant role in the development of uterine ischemia and hypoxia, resulting in dysrhythmic uterine contractions and decreased blood flow [11-14]. Both pharmacological and non-pharmacological methods have been used to alleviate pain [9]. Pharmacological management involves the use of analgesics (paracetamol & non-steroidal anti-inflammatory drugs NSAIDs) [5]. NSAIDs are superior to paracetamol in pain relief [3], they act through inhibition of prostaglandin synthesis [15] but are associated with undesirable side effects that sometimes limit their use (e.g. gastro-intestinal discomfort and even bleeding) [16]. Selective COX-2 inhibitors are sometimes used as alternatives, still they increase the risk of cardiovascular events with long term use [17].
The term “vitamin D” refers to both ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). Vitamin D3 is formed in the skin upon exposure to sunlight [18-20]. Vitamin D is enzymatically activated. First, it is hydroxylated in liver to 25-hydroxy-cholicalciferol (25-(OH)D) (calcidiol), the major circulating form of vitamin D. Then it is converted in kidneys through 1a-hydroxylation to its most active form, 1, 25-dihydroxycholecalciferol (1, 25-(OH)2D) (calcitriol) [21,22]. In tissues, 1, 25-(OH)2D binds to intracellular Vitamin D Receptors (VDR) [19]. The presence of the Vitamin D Receptor (VDR) and the expression of the 1a- hydroxylation enzyme in many cells and the large number of genes under the control of 1,25-(OH)2D suggest a broader role of the vitamin D beyond bone and calcium homeostasis [23]. It was evidenced that 1,25-(OH)2D modulates cellular growth and differentiation. It also enhances the immune system [19] and regulates the expression of several key genes involved in the PG pathway causing decreased biological activity of PGs [24]. The National Academy of Medicine considers a serum 25-hydroxyvitamin D (25-(OH)D) level of 12 to 20 ng per mL (30 to 50 nmol per L) as the normal range. Individuals with levels less than 12 ng per mL (30 nmol per L) will usually be deficient [25]. Vitamin D deficiency has a high prevalence, about 50% in both northern and southern latitudes [26]. The main reason is inadequate cutaneous vitamin D synthesis (due to inadequate sun exposure) [21,26]. Our study is focusing on the effect of vitamin D deficiency on dysmenorrhea symptoms, which have been the subject of number of studies in the last few years.
Objective and Aim
The aim of this study was evaluating the effectiveness of two months treatment with 5000 IU of vitamin D in reducing symptoms of dysmenorrhea in women with vitamin D deficiency and dysmenorrhea.
Materials and Methods
This is a randomized single blind study. The participants were unaware of the study group. The study was conducted after approval of the ethics committee at Al-Khanzaa teaching hospital. A written informed consent was obtained from 50 women aged between 19 and 37 years complaining from dysmenorrhea. The study lasted for two months; started during March 2020 and ended in May 2020.
Inclusion criteria
Eligible participants met the following inclusion criteria:
1) Women had normal menstrual periods lasting 21 to 35 days, with menstruation lasting 3 to 7 days.
2) Women had to be healthy and taking no medications including vitamins, magnesium, calcium and oral contraceptives.
3) Women had no history of gynecological disease.
4) Current and previous use of intrauterine devices for contraception within 6 months were not allowed.
Study design
A total of 50 women were identified. Participants were randomly assigned to the treatment groups. Excluded members were eight women who were unwilling to continue and two women who became pregnant. Finally, the analysis was conducted with 40 women; 20 in vitamin D group and 20 in the group did not receive vitamin D (Figure 1).
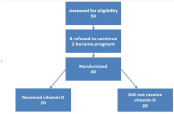
Figure 1: Study design.
List of results after randomization and throughout the study period. Severity of dysmenorrheic pain was determined based on each women’s self-perception of the pain (Table 1,2). Women were asked to mark on a 10 cm Visual Analogue Scale (VAS) anchored from zero “no pain at all” to 10 “the worst pain I have ever felt” to indicate the severity of dysmenorrheic pain. Use of NSAIDs was allowed and it had to be registrated. Participants were randomized into 2 groups by simple randomization using random numbers table. Twenty women were given 5000 IU of vitamin D once a day and 20 women did not receive vitamin D, beginning at the end of their menstrual cycle and continuing throughout two months study. The primary aim was measuring the severity of menstrual pain by a VAS. The secondary aim was the need to use NSAIDs during the study period. The severity of menstrual pain and serum 25-(OH)D3 levels were measured monthly; at the beginning of study (baseline), after one month and after two months of treatment. Serum 25-(OH) D3 was measured using electrochemiluminescence method. Statistical analysis was performed using Microsoft Office Excel 2010 program. P value of <0.05 was considered significant.
NO.
Name
Age
BMI
Serum 25-(OH)D3 before treatment nmol/L
VAS-1
Serum 25-(OH)D3 After 1 month treatment nmol/L
VAS-2
Serum 25-(OH)D3 after 2 months treatment nmol/L
VAS-3
1
E. A
26
24
8.1
6
24
4
40
1
2
S. H
26
23
8.1
8
25
5
43
4
3
S. S
19
20.7
8.1
7
27
4
44
3
4
R. K
29
30.2
8.1
9
23
6
45
4
5
A. A
22
24.1
8.3
10
25
6
42
2
6
M. K
21
26.5
8
7
26
4
41
1
7
E. E
37
29
8
8
28
6
48
3
8
S. R
30
24
9
8
27
7
46
3
9
O. I
27
19.6
8
6
24
4
49
1
10
N. M
36
27.3
8.2
9
22
6
42
2
11
F. J
31
22.5
8.1
8
29
5
46
3
12
A. G
28
20.7
8
7
24
5
44
2
13
A. H
35
21.4
8
9
30
4
47
1
14
S. F
33
28.4
8
6
22
4
48
1
15
A. Ab
19
20.6
8.1
10
26
6
46
3
16
Z. S
20
21.4
8.3
7
28
6
49
2
17
S. A
21
20.7
8.1
7
25
6
44
4
18
S. Ha
23
20.4
8
9
23
5
48
1
19
R. A
34
19.6
8
7
26
5
48
3
20
L. K
27
19.3
8
8
24
6
44
2
Mean Values
27.2
23.24
8.125
7.8
25.4
5.2
45.2
2.3
Table 1: Results of participants in vitamin D group.
NO.
Name
Age
BMI
Serum 25-(OH)D3 before treatment nmol/L
VAS-1
Serum 25-(OH)D3 After 1 month treatment nmol/L
VAS-2
Serum 25-(OH)D3 after 2 months treatment nmol/L
VAS-3
1
N. Z
21
19.7
8.2
9
9
7
8
7
2
K. M
32
25
8.5
6
12
6
9
7
3
A. G
32
20.7
9
10
8
8
9
7
4
Z. R
30
21.4
8
8
14
9
12
8
5
G. N
29
23.4
8
7
12
7
9
9
6
R. A
26
22.2
8.3
9
8
9
8
8
7
I. A
26
21.4
8.1
9
9
8
9
8
8
F. Si
24
23
8.2
6
8
8
8
9
9
M. M
30
25
7
7
9
7
8
7
10
H. S
21
33.5
8.3
6
8.5
7
8
6
11
A. N
34
32.8
8
8
12
6
9
7
12
S. A
34
21.8
8
8
10
8
9
7
13
J. J
20
20.7
8
8
12
8
11
8
14
M. S
18
21.4
8.1
9
8
7
8
7
15
H. B
20
23.5
8.3
7
9
7
8
7
16
A. E
37
22.6
6
8
12
6
9
7
17
R. M
31
24.9
8.3
9
8
8
9
7
18
F. S
24
20.9
8
8
14
9
12
8
19
R. K
18
19.9
8
7
12
7
9
9
20
M. H
34
23.8
8
10
8
9
8
8
Mean Values
27.05
23.38
8.025
7.95
10.125
7.55
9
7.55
Table 2: Results of participants in the group did not receive vitamin D.
Results
Values are shown in (Table 3). The median age was 27 (19-37) in vitamin D group and 27.5 (18-37) in the group did not receive vitamin D. The median Body Mass Index (BMI) was detected as 21.95 (19.3- 30.2) in vitamin D group and 22.4 (19.7-33.5) in the group did not receive vitamin D. The median baseline VAS scores were 8 (6-10) in vitamin D group and 8 (6-10) in the group did not receive vitamin D. There were no significant difference in terms of age, BMI and baseline VAS scores between the two groups.
Vitamin D
Did not receive vitamin D
n=20
n=20
Median
Mean ±SD
Median
Mean ±SD
(Min-Max)
(Min-Max)
Age
27 (19-37)
27.2±5.8
27.5 (18-37)
27.05±6.01
BMI
21.95 (19.3-30.2)
23.24±3.3
22.4(19.7-33.5)
23.38±3.7
VAS-1
8(6-10)
7.8±1.2
8 (6-10)
7.95±1.2
Min: Minimum, Max: Maximum; SD: Standard Deviation; BMI: Body Mass Index; VAS-1: Baseline Visual Analogue Scale.
Table 3: Baseline characteristics of the women with dysmenorrhea.
The treatment group showed significant difference in pain severity as measured on VAS score upon receiving vitamin D supplementation compared to the group did not receive vitamin D where pain severity was almost the same (Table 3,4). At the beginning of study baseline VAS score for participants in vitamin D group was (7.8±1.2SD), with treatment VAS scores decreased to (5.2±0.95SD) after one month treatment and then to (2.3±1.08SD) after two months treatment. As serum levels of 25-(OH)D3 increased throughout treatment (Figure 2) pain severity significantly decreased. The group of patients did not receive vitamin D retained serum 25-(OH) D3 at almost constant level and showed no difference in pain severity (Figure 2,3). Baseline VAS scores were (7.95±1.2SD) in the group did not receive vitamin D and did not change significantly after one month of treatment (7.55±0.99SD); or after two months of treatment (7.55±0.82SD). Requirement of NSAIDs was significantly lower in vitamin D group than the group did not receive vitamin D (4 participants vs 19 participants, p<0.001). We found significant decrease of pain severity in the vitamin D group. The median VAS scores after treatment were significantly lower in vitamin D group compared to the group did not receive vitamin D (p<0.001; Table 4).
Vitamin D
Did not receive vitamin D
n= 20
n= 20
Median
Mean ±SD
Median
Mean ±SD
(Min-Max)
(Min-Max)
VAS-1
8(6-10)
7.8±1.2
8 (6-10)
7.95±1.2
VAS-2
5 (4-7)
5.2±0.95
7.5(6-9)
7.55±0.99
VAS-3
2(1-4)
2.3±1.08
7(6-9)
7.55±0.82
P
<0.001
>0.05
Min: Minimum; Max: Maximum; SD: Standard Deviation; VAS-1: Baseline Visual Analogue Scale before treatment; VAS-2: Visual Analogue Scale after 1-month treatment; VAS-3: Visual Analogue Scale after 2 months treatment.
Table 4: VAS scores after drug regimen for each group.
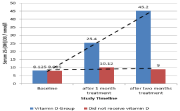
Figure 2: Average serum 25-(OH)D3 of participants in vitamin D and the
group did not receive vitamin D throughout the study.
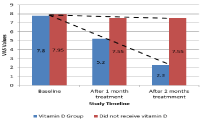
Figure 3: Average VAS scores of both groups throughout the study.
Discussion
Dysmenorrhea is one of the most common gynecological problems and is a main cause for school and work abstinence. Dysmenorrhea has been studied for long and the relationship between dysmenorrhea and vitamin D deficiency have been demonstrated in a number of studies in the few lasts years. The first study was conducted by Lasco et al., in Italy, high dose vitamin D (300,000 IU) was administered to women with primary dysmenorrhea five days before the putative date of their next cycle. In this study, significant pain reduction has been reported as serum 25-hydroxycholicalciferol levels increased after supplementation [24]. Moini et al., used the dosing schedule for treatment of vitamin D deficiency 50,000 IU weekly for 8 weeks instead of the high single dose (300,000 IU) and reported similar results. This study also showed reduction in the need to NSAIDs upon the use of vitamin D supplements [27]. Alshiagi et al., compared the effect of NSAIDs alone and the effect of combined use of vitamin D with NSAIDs for treatment of dysmenorrhea. Here, again higher degree of pain reduction have been demonstrated upon the addition of vitamin D to NSAIDs [28]. Ozel et al., compared supplementation with vitamin D, E and Ibuprofen as for their effect in the treatment of dysmenorrhea; vitamin D showed comparable efficacy to ibuprofen and superior efficacy to vitamin E supplementation [29]. Another study conducted by Karacin et al., in Turkey showed that women with low serum level of 25-hydroxy-D3 had more severe menstrual symptoms [30].
Because the vitamin D receptor is widespread and the mitochondrial cytochrome P450 enzyme 25-hydroxyvitamin D hydroxylase, is expressed in the human uterus and in immune system cells, and because vitamin D reduces the synthesis of PGs, a beneficial effect of vitamin D in the uterus pathophysiology is possible [24].
In the endometrium, the active form of vitamin D reduces the synthesis of IL-6, TNF 20 and prostaglandins by suppressing COX- 2 expression. Besides increasing prostaglandin inactivation of 15-hydroxy prostaglandin dehydrogenase, high concentrations of 1, 25(OH)-D inhibit PG21 receptor expression [31]. From all this the role of vitamin D in dysmenorrhea had become evidenced. Our study suggests the use of 5000IU of vitamin D in women with vitamin D deficiency and dysmenorrhea for two months. The results from this study were comparable to those from previous studies with women receiving vitamin D having much less menstrual pain. A daily dose of 5000IU vitamin D boosts serum levels of 25-hydroxycholecalciferol. Based on this observation more attention should be paid to the possibility of having vitamin D deficiency as the cause of dysmenorrhea. We used a different dosing schedule and obtained comparable results to those of previous work conducted about the same topic. Treatment of vitamin D deficiency markedly reduced the need for NSAIDs.
Conclusion
To our knowledge, this is the first study that used 5000IU vitamin D as treatment for dysmenorrhea. A daily dose that is lower than total doses used at different intervals in previously conducted studies. It was effective in reducing menstrual pain, reduced the need for NSAIDs. Future work should be directed towards finding if there is a difference in the degree of pain reduction upon using higher or lower doses of vitamin D and on finding what could be a better effective dosing schedule in women without vitamin D deficiency.
References
- Gerzon LR, Padilha JF, Braz MM, Gasparetto A. Physiotherapy in primary dysmenorrhea: literature review*. Rev Dor. Sao Paulo. 2014; 15:290-295.
- Stella I, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Human Reproduction Update. 2015; 21: 762-778.
- Proctor M, Farquhar C. Diagnosis and management of dysmenorrhea. BMJ. 2006; 332: 1134-1138.
- Osyande AS, Mehulic S. Diagnosis and Initial Management of Dysmenorrhea. American Family Physician. 2014; 89: 341-346.
- Burnett M, Winnipeg, Lemyre M. No. 345-Primary Dysmenorrhea Consensus Guideline. Jogc Juillet. 2017; 39: 585-595.
- Hong J, Mark J, Gita M. The Prevalence and Risk Factors of Dysmenorrhea. Epidemiol Rev. 2014; 36: 104-113.
- Bernardi M, Lazzeri L, Perelli F, Reis FM, Petraglia F. Dysmenorrhea and related disorders. F1000Research. 2017; 6: 1645.
- Sahin N, Kasap B, Kirli U, Yeniceri N, Topal Y. Assessment of anxietydepression levels and perceptions of quality of life in adolescents with dysmenorrhea. Reproductive Health. 2018 ;15.
- Armour M, Smith CA, Steel KA, Macmillan F. The effectiveness of self-care and lifestyle interventions in primary dysmenorrhea: a systematic review and meta-analysis. BMC Complementary and Alternative Medicine. 2019; 19: 22.
- Armour M, Parry K, Al-Dabbas MA, Curry C, Holmes K, MacMillan F, et al. Self-care strategies and sources of knowledge on menstruation in 12,526 young women with dysmenorrhea: A systematic review and meta-analysis. PLoS One. 2019; 14: e0220103.
- Sharghi M, Mansourkhani SM, Ashtary-Larky D, Kooti W, Niksefat M, Firoozbakht M, et al. An update and systematic review on the treatment of primary Dysmenorrhea. JBRA Assisted Reproduction. 2019; 23: 51-57.
- Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol. 2011; 31: 986-1000.
- Sugimoto Y, Inazumi T, Tsuchiya S. Roles of prostaglandin receptors in female reproduction. J Biochem. 2015; 157: 73-80.
- Dehnavi ZM, Jafarnejad F, Kamali Z. The Effect of aerobic exercise on primary dysmenorrhea: A clinical trial study. Journal of Education and Health Promotion. 2018; 7: 1-2.
- Oladosu FA, Frank F Tu, Hellman KM. NSAID resistance in dysmenorrhea: epidemiology, causes, and treatment. Am J Obstet Gynecol. 2018; 218: 390- 400.
- Sharghi M, Mansourkhani SM, Ashtary-Larky D, Kooti W, Niksefat M, Firoozbakht M, et al. An update and systematic review on the treatment of primary Dysmenorrhea. JBRA Assisted Reproduction. 2019; 23: 51-57.
- Feng X, Wang X. Comparison of the efficacy and safety of non-steroidal antiinflammatory drugs for patients with primary dysmenorrhea: A network metaanalysis. Molecular Pain. 2018; 14: 1744806918770320.
- Zhang R, Naughton DP. Vitamin D in health and disease: Current perspectives. Nutrition Journal. 2010; 9: 65.
- Pludowski P, Holick, Michael FH, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, et al. Vitamin D supplementation guidelines. Journal of Steroid Biochemistry & Molecular Biology. 2018; 175: 125-135.
- Pilz S, Zittermann A, Trummer C, Theiler-Schwetz V, Lerchbaum E, Keppel MH, et al. Vitamin D testing and treatment: a narrative review of current evidence. Endocrine Connections. 2019; 8: R27-R43.
- Chang S-W, Lee H-C. Vitamin D and health - The missing vitamin in humans. Pediatrics and Neonatology. 2019; 60: 237-244.
- Aatsinki S-M, Elkhwanky M-S, Kummu O, Karpale M, Buler M, Viitala P, et al. Fasting-Induced Transcription Factors Repress Vitamin D Bioactivation, a Mechanism for Vitamin D Deficiency in Diabetes. Diabetes. 2019; 68: 918- 931.
- Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer- Pietsch B, Bianchi ML, et al. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. European Journal of Endocrinology. 2019; 180: P23-P54.
- Lasco A, Catalano A, Benvenga S. Improvement of Primary Dysmenorrhea Caused by a Single Oral Dose of Vitamin D: Results of a Randomized, Double-blind, Placebo-Controlled Study. Arch Intern Med. 2012; 172: 366- 367.
- LeFerve ML, LeFerve NM. Vitamin D Screening and Supplementation in Community-Dwelling Adults: Common Questions and Answers. American Family Physician. 2018; 97: 254-260.
- Bashmakova NV, Lisovskaya TV, Vlasova VY. Pathogenetic role of vitamin D deficiency in the development of menstrual dysfunction in pubertal girls: a literature review. Gynecological Endocrinology. 2017; 33: 52-55.
- Moini A, Ebrahimi T, Shirzad N, Hosseini R, Radfar M, Bandarian F, et al. The effect of vitamin D on primary dysmenorrhea with vitamin D deficiency: a randomized double blind controlled clinical trial. Gynecol Endocrinol. 2016; 32: 502-504.
- Lama A, Najla A, Azzah A, Areej A, Alaa E, Alsuwaidan S. Vitamin D Supplements as Adjunctive Therapy with Analgesics for Primary Dysmenorrhea: A Randomized Clinical Trial. International Journal of Reproductive Medicine & Gynecology. 2019.
- Ozel A, Ates S, Sevket O, Demir M, Ilhan G, Davutoglu E. A Randomized Controlled Study of Vitamin D in the Treatment of Primary Dysmenorrhea. Duzce Medical Journal. 2019; 21: 33-36.
- Karacin O, Mutlu I, Kose M, Celik F, Kanat-Pektas M, Yilmazer M. Serum vitamin D concentrations in young Turkish women with primary dysmenorrhea: A randomized controlled study. Taiwanese Journal of Obstetrics & Gynecology. 2018; 57: 58-63.
- Halpern G, Schor E, Kopelman A. Nutritional aspects related to endometriosis. Rev Assoc Med Bras. 2015; 61: 519-523.