
Review Article
Austin J Obstet Gynecol. 2021; 8(1): 1163.
Role of Pelvic Exenteration in the Treatment of Persistent or Recurrent Gynecological Cancers
Matteo Maruccio1*, Alessia Aloisi1, Carlo Personeni1, Michela Palumbo1, Betella Ilaria1, Achilarre Maria Teresa1, Giovanni Aletti1, Vanna Zanagnolo1, Nicoletta Colombo1, Fabio Landoni2 and Angelo Maggioni1
¹Department of Gynecologic Oncology, European Institute of Oncology IRCCS, Italy
²Department of Obstetrics and Gynaecology, University of Milan Bicocca, Italy
*Corresponding author: Matteo Maruccio, Department of Gynecologic Oncology, European Institute of Oncology, IRCCS, Italy
Received: January 15, 2021; Accepted: February 03, 2021; Published: February 10, 2021
Abstract
Objective: To assess the oncological outcomes of Persistent/Recurrent Gynaecological Cancers who underwent Pelvic Exenteration (PE) in terms of DFS and OS in a 23 years-single center experience. Secondary outcome was to identify factors associated with recurrence.
Methods: From June 1996 to March 2019, data of all patients who underwent PE were retrospectively collected. The Kaplan-Meier method was used to estimate DFS and OS. Univariable and multivariable logistic regression analysis was performed to identify potential independently associated predictors of recurrence.
Results: 192 patients were considered for final analysis. After surgery 77 women (40.1%) received a post-operative oncologic treatment. Overall 106 patients (55.2%) experienced a relapse with a median follow-up of 58 months (range, 2 to 236 months).
Presence of LVI (adjusted HR 2.2, 95% CI 1-4.9, P=0.05) was the only factor that retained an independent association with relapse at multivariable analysis. Positive lymph nodes were associated with death at univariable analysis (HR 3.9, 95% CI 1.7-9.4, P=0.002).
When stratifying patients by cervical cancer, among 115 women, 67 (58.3%) relapsed. Presence of LVI (HR 2.7, 95% CI 1.1-6.6, P=0.02) and patients with pathologic risk factors such as tumor size, positive lymph nodes and LVI (HR 3.1, 95% CI 1.4-6.8, P=0.005) were associated with recurrence both at univariable and multivariable analysis.
Conclusion: Pelvic exenteration may have a therapeutic role in cervical and endometrial tumors that recur at least 6 months after primary treatment. Patients affected by vulvar cancer or either with tumor size >5 cm, positive lymph nodes, LVI have worse oncologic outcomes.
Keywords: Gynecological cancer; Oncologic outcomes; Cervical cancer; Pelvic exenteration
Background
Pelvic exenteration is an extremely aggressive and complex surgical procedure, which consists in the complete excision of the pelvic viscera, first described by Brunschwig in 1948 [1].
In the recent years, due to the improvement of surgical techniques, devices and perioperative management, indications to perform pelvic exenteration have expanded from the classic indication of centrally persistent or recurrent cervical cancer to locally advanced primary cancers or recurrent cancers of the endometrium, vulva, vagina and ovary in selected cases [2].
Considering that up to 30% to 45% of cervical cancer recurrences are central-pelvic in a previous irradiated field [3,4], it is easily understandable that an increasing number of patients will eventually need this type of surgery.
Moreover survival rates after pelvic exenteration have been reported as 32% to 47% highlighting the importance of this surgical procedure as potentially curative [5-12].
However, it is important to underline that pelvic exenteration is an extremely aggressive surgery that leads to major physical changes that may significantly impact on patient self-image with potential physical, sexual and psychological issues [13]. For this reason a careful selection of patients is mandatory. Unfortunately there is a lack of data as regarding oncologic outcomes and especially which are the criteria (margins status, lymph node status, and tumor size, histology) to identify patients who are more likely to benefit from this surgery.
The aim of our retrospective analysis was to evaluate the oncologic outcomes in patients affected by persistent or recurrent gynecological malignancies who were submitted to exenterative surgery (anterior, posterior or total exenteration). Secondary outcome was to identify factors associated with recurrence in order to guide us in a better selection of patients suitable for surgery.
Methods
This study was approved by the Institutional Review Board at European Institute of Oncology. We identified all patients with a diagnosis of persistent or recurrent gynecologic malignancy who underwent planned pelvic exenteration at the Gynecologic Oncology Service of European Institute of Oncology from June 1996 to March 2019.
Patients’ characteristics (age, Body mass index or BMI, oncologic history, diagnosis, indication for surgery, type of procedure) were retrospectively identified from a review of medical records. Persistent disease was defined as presence of disease within 6 months from primary treatment.
Neoadjuvant treatments (defined as any treatment that was delivered within 4 weeks before surgery) such as chemotherapy, radiotherapy or chemoradiation were registered.
We included all identified patients regardless of type of pelvic exenteration (anterior, posterior or total exenteration) as originally defined by Alexander Brunschwig [1]. Removal of pelvic lymph nodes may also be part of the surgical procedure. All surgeries were performed by dedicated gynecologic oncologists.
Use of Intraoperative Radiation Therapy (IORT), which was introduced in 2001, was also recorded. IORT was used in case of positive lymph nodes or when minimal or microscopic disease persisted on the margins to the pelvic side-wall at frozen sections, at discretion of the radiotherapist based on the site and the technical possibility of dose delivery.
All histological characteristics including FIGO stage, tumor type, size, grade, lymph nodes and margins status were retrospectively identified through a review of patients’ medical records. Tumor grading was not assigned in the case of serous, melanoma, clear cell, carcinosarcoma or mixed histotypes, since, by default; these are classified as poorly differentiated and are no longer graded by pathologists [14]. All pathologic evaluation was performed by dedicated gynecologic pathologists.
All patients’ medical records were reviewed until the last recorded follow-up at our institution. We determined whether patients received any adjuvant treatment after surgery, including chemotherapy, radiotherapy, hormones, or a combination of those.
Adjuvant treatment was mainly indicated for medically fit patients with pathologic risk factors (positive or close resection margins, tumor diameter >5 cm, positive lymph-nodes or lymphatic spaces invasion, all variously associated) on the surgical specimen.
Pattern of first recurrence was recorded. Recurrences were classified as local (confined to the pelvis), distant or multisite. Disease- Free Survival (DFS) was calculated from the date of surgery to the first documented recurrence, or death from disease. Overall Survival (OS) was calculated from the date of surgery to date of death, or last followup. The Kaplan-Meier method was used to estimate DFS and OS, and estimates were compared with the Log-rank test.
Associations were analyzed using the Chi square test for categorical variables and the Mann-Whitney U Test for continuous variables. Univariable and multivariable logistic regression analyses were performed to identify factors associated with recurrence. Statistical significance was set at P>0.05. Statistical analysis was done using SPSS software.
Results
We retrospectively identified 208 women that were scheduled to undergo a planned pelvic exenteration for a persistent or recurrent gynecologic malignancy. Eight women were lost to follow-up, 5 were submitted to palliative pelvic exenteration and 3 underwent PE as primary treatment (1 melanoma, 1 sarcoma and 1 malignant amartoma), so 192 patients were considered for the final analysis. Patients’ characteristics are depicted in Table 1. The overall median age was 56 years (range 23 to 81 years) and median Body Mass Index (BMI) was 24 kg/m² (range, 13 kg/m² to 64 kg/m²). An ECOG performance score of 0 or 1 was documented in all patients. The most common oncologic diagnosis was cervical cancer (n=115, 59.9%), the most frequent histology was squamous (n=130, 67.7%). Mean tumor size was 38.9 ± 23.8 mm and Linphovascular Involvement (LVI) was found in 50 cases (26%).
Variable
median
range
Age (years)
56
23-81
Body mass index (kg/m2)
24
13-64
N
%
Type of tumor
cervical
115
59.9
vulvar
21
10.9
vaginal
29
15.1
endometrial
18
9.4
other
9
4.7
Histotypes
squamous
130
67.7
adenocarcinoma
40
20.8
adenosquamous
7
3.6
endometrioid
4
2.1
serous
2
1
clear cell
3
1.6
sarcoma
3
1.6
melanoma
3
1.6
GRADE
1
25
13.1
2
54
28.1
3
58
30.2
Not graded
55
28.6
Lymphovascular space invasion (LVI)
50
26
Previous oncologic treatment
183
95.3
chemotherapy
36
radiotherapy
50
chemoradiation
59
surgery
38
Reason for surgery
persistent disease
82
42.7
recurrent disease
110
57.3
Neoadjuvant treatment (within 4 weeks before surgery)
50
26
chemotherapy
39
-
radiotherapy
9
-
chemoradiation
2
-
Table 1: Patients’ Characteristics (N=192).
One hundred and eighty-three patients (95.3%) had previously received an oncologic treatment (chemotherapy, surgery, radiation or a combination of those) as depicted in Table 1.
Overall 50 patients (26%) received a neoadjuvant treatment (within 4 weeks before surgery): thirty-nine patients underwent neoadjuvant chemotherapy, 11 had received preoperative radiotherapy, 2 of those with concomitant chemotherapy. All the remaining patients did not receive any neoadjuvant treatment within 4 weeks before surgery.
One hundred and ten (57.3%) patients underwent surgery for recurrent disease and 82 (42.7%) for persistent disease.
Perioperative characteristics are reported in Table 2. Intraoperative Radiation Therapy (IORT) was delivered in 55 (28.6%) women. Use of IORT was introduced in 2001.
Variable
N
%
Type of exenteration
total
102
53.1
anterior
77
40.1
posterior
13
6.8
Stoma
105
91.3
temporary
27¥
permanent
78
Urinary diversion
186
91.6
Continent (Indiana pouch)
75
41.9
Incontinent
104
58.1
Bricker
22
Wallace I
4
Wallace II
72
Ureterocutaneostomy
2
Colon conduit
4
Intraoperative Radiotherapy (IORT)
55
28.6
Tumor size (mean ± DS)
38.9 ± 23.8
Margins
positive
60
31.2
negative
132
68.7
Pelvic lymph nodes (surgically assessed)
142
73.9
positive
44
31
negative
98
69
Adjuvant treatments
77
40.1
chemotherapy
61
radiotherapy
13
chemoradiation
3
Relapse
106
55.2
local
42
39.7
distant
33
31.1
multisite
31
29.2
¥ In only 16 patients the stoma was actually closed.
Table 2: Perioperative characteristics (N=192).
Intraoperative characteristics, including type of surgical procedures, are depicted in Table 2.
One hundred and thirty-two patients (68.8%) had negative margins, 60 (31.2%) had a positive margin. Lymph nodes status was surgically assessed in 142 women and 44 of those had positive pelvic lymph nodes. Twelve patients who had positive lymph nodes underwent IORT.
After surgery 77 women (40.1%) received a post-operative oncologic treatment (chemotherapy, hormones, radiation or a combination of those) as showed in Table 2.
The most common adjuvant treatment was chemotherapy (n=61), mainly indicated for medically fit patients who had already undergone radiotherapy and with pathologic risk factors (positive resection margins, tumor diameter >5 cm, positive lymph-nodes or lymphatic spaces invasion, all variously associated) on the surgical specimen.
Overall 106 patients (55.2%) experienced a relapse. Pattern of first recurrence is showed in Table 2. Most women experienced a local relapse (n=42), 33 patients developed distant metastasis and 31 had a multisite relapse. Most of these patients (n=78, 73.6%) subsequently died of disease. Stratifying by gynecological cancer, among the 115 women affected by cervical cancer, 67 (58.3%) patients relapsed.
Table 3 summarizes the univariable and multivariable analysis of factors associated with relapse: positive margins (HR 1.9 95% CI 1-3.6, P=0.05), LVI (HR 2.8, 95% CI 1.4-5.8, P=0.004) and population with one or more pathologic risk factors such as positive resection margins, tumor diameter >5 cm, positive lymph-nodes or lymphatic spaces invasion, (HR 2.2, 95% CI 1.2-4.1, P=0.01) were associated with relapse at univariable analysis. However, presence of LVI (adjusted HR 2.2, 95% CI 1-4.9, P=0.05) was the only factor that retained an independent association also in multivariable analysis. We did not insert lymph node status in multivariable analysis because lymphadenectomy was performed only in 142 patients, therefore, had all the not surgically assessed lymph nodes cases been excluded from our multivariable analysis, it would have constituted a critical bias. Positive lymph nodes were also associated with death at univariable analysis (HR 3.9, 95% CI 1.7-9.4, P=0.002) also when patients were stratified by cervical cancer only (HR 5.4, 95% CI 1.7-17.8, P=0.005). No other factors were associated with risk of death at univariable analysis.
Variable
Univariable
Multivariable
HR (95% CI)
P value
Adjusted HR (95% CI)
P value
Diagnosis
cervical
Reference
vulvar
1.01 (0.4-2.7)
0.92
vaginal
1.3 (0.6-2.9)
0.53
endometrium
1.4 (0.5-3.8)
0.51
other
0.81 (0.3-4.4)
0.87
Histology
squamous
Reference
adenocarcinoma
1.2 (0.3-4.7)
0.8
adenosquamous
0.5 (0.1-2.3)
0.4
serous
NA
0.5
endometrioid
0.5 (0.1-4.1)
0.9
other
1.2 (0.1-13.2)
0.8
Grade
1.03 (0.8-1.4)
0.82
Tumor size
= 5 cm
Reference
Reference
> 5 cm
1.4 (0.7-2.8)
0.3
1.4 (0.7-2.9)
0.4
Neoadjuvant chemotherapy
no
Reference
yes
1.1 (0.6-2.4)
0.7
Indications for surgery
persistent
Reference
relapse
0.8 (0.4-1.4)
0.4
LVI
no
Reference
Reference
yes
2.8 (1.4-5.8)
0.004
2.2 (1-4.9)
0.05
Type of pelvic exenteration
total
Reference
anterior
1.1 (0.6-1.9)
0.8
posterior
1.6 (0.5-5.1)
0.4
Lymph nodes
negative
Reference
positive
1.9 (0.9-4)
0.09
Margins
Negative
Reference
Reference
positive
1.9 (1-3.6)
0.05
1.5 (0.7-3)
0.2
IORT
Reference
Reference
1.5 (0.8-2.8)
0.2
1.4 (0.7-2.8)
0.4
One or more risk factors
no
Reference
Reference
yes
2.2 (1.2-4.1)
0.01
1.8 (0.9-3.5)
0.07
All variables were tested for multicollinearity. Clinically significant variables and variables with p <0.3 on univariable analysis were included in the multivariable analysis.
Table 3: Univariable and multivariable analysis of factors related to relapse.
In cervical cancer, squamous histology was related to a decreased risk of recurrence (HR 0.1, 95% CI 0.01-0.7, P=0.02) both at univariable analysis and multivariable analysis (adjusted HR 0.06, 95% CI 0.01-0.5, P=0.01).
When stratifying patients by cervical cancer (Table 4) also presence of LVI (HR 2.7, 95% CI 1.1-6.6, P=0.02) and patients with pathologic risk factors who underwent adjuvant treatments (HR 3.1, 95% CI 1.4-6.8, P=0.005) were associated with recurrence at univariable analysis. They both retained statistical significance on multivariable analysis and persistence of disease almost reached statistical significance as showed in Table 4.
Variable
Univariable
Multivariable
HR (95% CI)
P value
Adjusted HR (95% CI)
P value
Histology
squamous
0.1 (0.01-0.7)
0.02
0.06 (0.01-0.5)
0.01
adenocarcinoma
0.4 (0.1-2.4)
0.3
0.7 (0.1-4.3)
0.7
adenosquamous
Reference
--
Grade
Reference
--
0.9 (0.7-1.4)
0.9
Tumor size
= 5 cm
Reference
> 5 cm
1.3 (0.5-3.4)
0.6
Neaoadjuvant chemotherapy
Reference
--
1.4 (0.5-3.4)
0.5
Indications for surgery
persistent
Reference
Reference
relapse
0.6 (0.3-1.2)
0.1
0.4 (0.2-1)
0.052
L VI
no
Reference
Reference
yes
2.7 (1.1-6.6)
0.02
2.9 (1.1-7.9)
0.04
Type of pelvic exenteration
total
Reference
anterior
1.3 (0.6-2.9)
0.4
posterior
0.8 (0.1-9.2)
0.8
Margins
Negative
Reference
Reference
positive
1.6 (0.7-3.6)
0.2
0.9 (0.3-2.2)
0.8
Lymph nodes
negative
Reference
positive
1.7 (0.6-4.5)
0.3
IORT
Reference
Reference
1.8 (0.8-4.1)
0.1
2.2 (0.9-5.9)
0.1
One or more risk factors
no
Reference
Reference
yes
3.1 (1.4-6.8)
0.005
3.3 (1.3-8.1)
0.01
All variables were tested for multicollinearity. Clinically significant variables and variables with p <0.3 on univariable analysis were included in the multivariable analysis.
Table 4: Univariable and multivariable analysis of factors related to relapse in patients affected by cervical cancer (n=115).
The median follow-up was 58 months (range, 2 to 236 months). At last follow-up, 25 (13%) patients were alive with recurrent disease, 53 (27.6%) were disease-free (some of these had a recurrence but treated with complete response), and 114 (59.4%) had died.
Five year Disease Free Survival (DFS) and 5-year Overall Survival (OS), stratified by type of tumor is showed in Figure 1. Vulvar and vaginal cancer are characterized by the shortest DFS (24.6% SE+/- 13.3% and 37.7% SE+/-10.4% respectively) and OS (25.9% SE+/-12% and 40.9% SE+/-9.9% respectively).
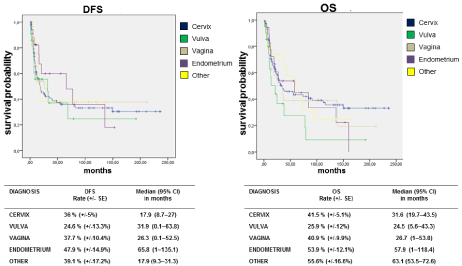
Figure 1: Five year Disease Free Survival (DFS) and 5-year Overall Survival (OS) stratified by tumor type.
As showed in Figure 2, Patients with tumor size >5 cm, presence of LVI and positive lymph nodes presented a significant worse 5 year DFS and 5 year OS.
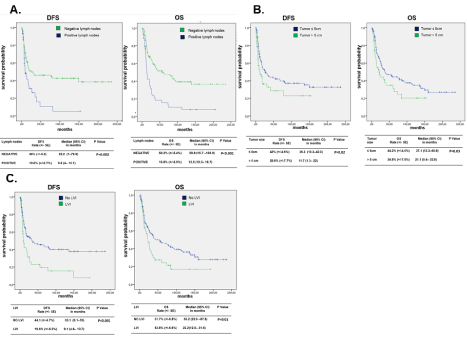
Figure 2: Five year Disease Free Survival (DFS) and 5-year Overall Survival (OS) stratified by: A) lymph nodes status B) tumor size C) LVI.
In cervical cancer population (Figure 3), patients with LVI had a significant worse 5-year DFS, and women with LVI, positive lymph nodes and tumor size >5 cm had a significant worse 5-year OS.
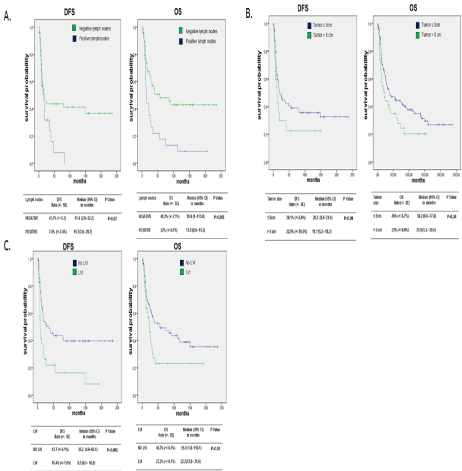
Figure 3: Five year Disease Free Survival (DFS) and 5-year Overall Survival (OS) in cervical cancer population stratified by: A) lymph nodes status B) tumor size
C) LVI.
Discussion
In the current era of minimally invasive surgery, where less is more, pelvic exenteration, an extremely extensive surgery, remains the only potentially curative treatment for selected patients with advanced or persistent/recurrent gynecologic malignancies.
Survival rates after pelvic exenteration have been reported as 32% to 47% for overall survival and 40% to 52% for recurrence-free survival in gynecologic cancers [5-12], which are in line with our cumulative data. More in particular cervical and endometrial cancers were the malignancies with the longest OS (41.5% for cervical cancer and 53.9% for endometrial cancer) confirming that these patients are appropriate candidates for pelvic exenteration. Vaginal and vulvar tumors registered shorter OS (25.9% for vulvar cancer and 40.9% for vaginal cancer) suggesting that it is uncertain whether these patients are good candidates for such an aggressive procedure.
A couple of literature reviews tried to identify prognostic factors in patients affected by gynecological malignancies who have undergone pelvic exenteration however, not all reports consistently reported all of these factors [15-17].
Tumor size of lesions >5 cm diameter have been shown to have almost no chance of cure despite complete removal of the tumor [15,17]. In our analysis, tumor size was not statistically related to recurrence, even when we stratified our cohort by cervical cancer only, probably due to the small number of cases with tumor size >5cm. However it resulted in a significant worse 5year-DFS and 5year DFS.
According to some authors, time interval between the initial treatment and recurrence (less than 2 years, between 2 and 5 years and more than 5 years) is associated with a different 5-year OS of 16.8%, 28.0% and 83.2% respectively [18]. More in particular, a relapse occurring >2 years after initial treatment is associated with a better OS in patients affected by cervical cancer [19]. We did not observe a statistically significant difference between recurrent (after six months by the end of primary treatment) versus persistent (within six months by the end of primary treatment) tumors. However when we stratified by cervical cancer, we observed a trend that almost reached statistical significance in terms of association with relapse when PE was performed within 6 months after primary treatment.
Squamous cell carcinomas have been reported as associated with a significantly worse prognosis than adenocarcinomas [15,20], even if in these studies adenocarcinomas represented only a small portion of the population and lymphovascular space invasion was more frequently observed in the squamous carcinomas. In our cohort we observed a decreased association with recurrence for squamous carcinomas on our cumulative univariable analysis in cervical cancer population, most likely because these cases were compared to very unfavorable histotypes such as undifferentiated or adenosquamous carcinomas. Moreover, these findings are consistent with those reported in the Literature for primary cervical cancer, according to which adenosquamous histology is significantly associated with poorer DFS [21,22].
The presence of lymph node metastasis is still controversial in the Literature [15], however a poorer prognosis associated with lymph node metastasis has been reported by several authors [23-26]. In the present series lymph node status appeared an important prognostic factor, associated with both risk of death and relapse. Unfortunately in our series pelvic lymphadenectomy was not systematically performed in all patients and for this reason we had to exclude lymph node status from our multivariable analysis in order to avoid selection bias.
Surgical resection margins status is reported as a major significant and independent prognostic factor associated with decreased survival [23,24,27]. Postoperative survival at two years drops from 55.2% with uninvolved margins to 10.2% with positive margins [18]. Some authors have found that the survival rate in patients with positive margins falls to 0% after three years [15]. We similarly observed a statistically significant association to relapse in patients with positive margins compared to negative margins at our univariable analysis; however we did not find any difference when we stratified our cohort for cervical cancer only.
Lymphovascular Space Invasion (LVSI) is considered in the scientific community as an independent prognostic factor which negatively impacted overall survival [6,15]. Our findings confirmed that presence of LVI is significantly associated with risk of recurrence, worse DFS and OS both in general and specifically in cervical cancer population.
The use of Intraoperative Radiation Therapy (IORT) combined with radical surgical resection in patients affected by recurrent gynecologic malignancies seems to provide some local control, especially in patients with positive or close margins [28-30]. According to our analysis IORT was not associated with reduced risk of recurrence, both on univariable and multivariable analysis. However we did not investigate, in case of a recurrence occurring after IORT, whether the recurrence was in the same site of the irradiation or elsewhere. Moreover in comparing patients who underwent IORT versus those who did not we may have occurred in several selection biases also related to the timing the procedure was implemented. For this reason, we feel that we were unable to assess the effective role of IORT, which will be further investigated in a future study.
Finally we observed that the presence of one or more pathologic risk factors (positive resection margins, tumor diameter >5 cm, positive lymph-nodes or lymphatic spaces invasion, all variously associated) may represent a selected high-risk population and it is associated with recurrence.
Points of weakness of the present study are represented by its retrospective nature and by the long time of observation (from 1996 to 2019), during which surgical performance (new devices), quality of perioperative care, treatments protocols and general life expectancy have improved over time. This long duration of time may indeed have reduced the quality of the analysis based on these points, as the population is not very homogenous.
One point of strength is represented by the number of cases. In fact, to our knowledge, this is one of the largest single centre studies of patients affected by recurrent/persistent gynecological malignancies who were submitted to pelvic exenteration. Another point of strength is our long follow up that reaches up to 236 months (median 58 months).
According to our analysis pelvic exenteration appears to have a therapeutic role in very well selected patients affected by persistent/ recurrent gynecological cancers, especially in case of cervical and endometrial tumors that recur at least 6 months after primary treatment, as it leads to an acceptable survival rate that justify such an aggressive surgical procedure in a setting where there are no other therapeutic options.
Patients who present with vulvar cancer, persistence of disease (within 6 months after primary treatment) or pathologic risk factors such as tumor size >5 cm, positive lymph nodes, presence of LVI all variously associated, seem to have worse oncologic outcomes and surgery in this particular population, even though not always identified preoperatively, should be carefully discussed case by case. In such cases neoadjuvant chemotherapy should be considered even if we did not observe a statistical significance due to extreme heterogeneity of our cohort. In particular all the conditions that could potentially lead to an intraoperative risk factor such as positive margins (like in case of large tumor size, nearly lateral disease or suspicious lymph nodes) should be evaluated preoperatively in order to consider use of intraoperative treatments such as IORT.
Conducting a randomized controlled trial in this setting is extremely unlikely, as multiple factors need to be taken into consideration. Most of these factors, such as tumor spread, histology, previous oncologic treatments and patient morbidities may exclude one treatment from the other. Therefore, we believe there is a need for prospective non-randomized comparative trials, possibly multicentre, that compare exenterative surgery versus other treatment modalities in women with recurrent gynaecological malignancies with pathologic risk factors.
Synopsis
Pelvic exenteration may have a therapeutic role in cervical and endometrial tumors that recur at least 6 months after primary treatment. Patients affected by vulvar cancer or either with tumor size >5 cm, positive lymph nodes, LVI have worse oncologic outcomes.
Acknowledgement
This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds.
References
- Brunschwig A. The surgical treatment of cancer of the cervix uteri; a radical operation for cancer of the cervix. Bull N Y Acad Med. 1948; 24: 672-683.
- Diver EJ, Rauh-Hain JA, Del Carmen MG. Total pelvic exenteration for gynecologic malignancies. Int J Surg Oncol. 2012; 2012: 693535.
- Marchiolé P, Buénerd A, Benchaib M, Nezhat K, Dargent D, Mathevet P. Clinical significance of lympho vascular space involvement and lymph node micrometastases in early-stage cervical cancer: a retrospective case-control surgico-pathological study. Gynecol Oncol. 2005; 97: 727-732.
- Grisaru DA, Covens A, Franssen E, Chapman W, Shaw P, Colgan T, et al. Histopathologic score predicts recurrence free survival after radical surgery in patients with stage IA2-IB1-2 cervical carcinoma. Cancer. 2003; 97: 1904- 1908.
- Matsuo K, Mandelbaum RS, Adams CL, Roman LD, Wright JD. Performance and outcome of pelvic exenteration for gynecologic malignancies: A population-based study. Gynecol Oncol. 2019; 153: 368-375.
- Westin SN, Rallapalli V, Fellman B, Urbauer DL, Pal N, Frumovitz MM. Overall survival after pelvic exenteration for gynecologic malignancy. Gynecol Oncol. 2014; 134: 546-551.
- Goldberg GL, Sukumvanich P, Einstein MH, Smith HO, Anderson PS, Fields AL. Total pelvic exenteration: the Albert Einstein College of Medicine/ Montefiore Medical Center Experience (1987 to 2003). Gynecol Oncol. 2006; 101: 261-268.
- Maggioni A, Roviglione G, Landoni F, Zanagnolo V, Peiretti M, Colombo N, et al. Pelvic exenteration: ten-year experience at the European Institute of Oncology in Milan. Gynecol Oncol. 2009; 114: 64-68.
- Schmidt AM, Imesch P, Fink D, Egger H. Indications and long-term clinical outcomes in 282 patients with pelvic exenteration for advanced or recurrent cervical cancer. Gynecol Oncol. 2012; 125: 604-609.
- Benn T, Brooks RA, Zhang Q, Powell MA, Thaker PH, Mutch DG, et al. Pelvic exenteration in gynecologic oncology: a single institution study over 20 years. Gynecol Oncol. 2011; 122: 14-18.
- Sharma S, Odunsi K, Driscoll D, Lele S. Pelvic exenterations for gynecological malignancies: twenty-year experience at Roswell Park Cancer Institute. Int J Gynecol Cancer. 2005; 15: 475-482.
- McLean KA, Zhang W, Dunsmoor-Su RF, Shah CA, Gray HJ, Swensen RE, et al. Pelvic exenteration in the age of modern chemoradiation. Gynecol Oncol. 2011; 121: 131-134.
- Dessole M, Petrillo M, Lucidi A, Naldini A, Rossi M, De Iaco P, et al. Quality of life in women after pelvic exenteration for gynecological malignancies: a multicentric study. Int J Gynecol Cancer. 2018; 28: 267-273.
- Denschlag D, Ulrich U, Emons G. The Diagnosis and Treatment of Endometrial Cancer. Progress and Controversies. Dtsch Arztebl Int. 2010; 108: 571-577.
- Sardain H, Lavoue V, Redpath M, Bertheuil N, Foucher F, Levêque J. Curative pelvic exenteration for recurrent cervical carcinoma in the era of concurrent chemotherapy and radiation therapy. A systematic review. Eur J Surg Oncol. 2015; 41: 975-985.
- Ang C, Bryant A, Barton DP, Pomel C, Naik R. Exenterative surgery for recurrent gynaecological malignancies. Cochrane Database Syst Rev. 2014; 2014: CD010449.
- Hockel M. Ultra-radical compartmentalized surgery in gynaecological oncology. Eur J Surg Oncol. 2006; 32: 859-865.
- Marnitz S, Kohler C, Muller M, Behrens K, Hasenbein K, Schneider A. Indications for primary and secondary exenterations in patients with cervical cancer. Gynecol Oncol. 2006; 103: 1023-1030.
- Chiantera V, Rossi M, De Iaco P, Koehler C, Marnitz S, Ferrandina G, et al. Survival after curative pelvic exenteration for primary or recurrent cervical Cancer: a retrospective multicentric study of 167 patients. Int J Gynecol Cancer. 2014; 24: 916-922.
- Baiocchi G, Guimaraes GC, Faloppa CC, Kumagai LY, Oliveira RAR, Begnami MD, et al. Does histologic type correlate to outcome after pelvic exenteration for cervical and vaginal cancer? Ann Surg Oncol. 2013; 20: 1694-700.
- Tseng JH, Aloisi A, Sonoda Y, Gardner GJ, Zivanovic O, Abu-Rustum NR, et al. Less versus more radical surgery in stage IB1 cervical cancer: A population-based study of long-term survival. Gynecol Oncol. 2018; 150: 44-49.
- Lee JY, Lee C, Hahn S, Kim MA, Kim HS, Chung HH, et al. Prognosis of adenosquamous carcinoma compared with adenocarcinoma in uterine cervical cancer: a systematic review and meta-analysis of observational studies. Int J Gynecol Cancer. 2014; 24: 289-294.
- Fleisch MC, Pantke P, Beckmann MW, Schnuerch HG, Ackermann R, Grimm MO, et al. Predictors for long-term survival after interdisciplinary salvage surgery for advanced or recurrent gynecologic cancers. J Surg Oncol. 2007; 95: 476-484.
- Park JY, Choi HJ, Jeong SY, Chung J, Park JK, Park SY. The role of pelvic exenteration and reconstruction for treatment of advanced or recurrent gynecologic malignancies: analysis of risk factors predicting recurrence and survival. J Surg Oncol. 2007; 96: 560-568.
- Barakat RR, Goldman NA, Patel DA, Venkatraman ES, Curtin JP. Pelvic exenteration for recurrent endometrial cancer. Gynecol Oncol. 1999; 75: 99- 102.
- Seagle BL, Dayno M, Strohl AE, Graves S, Nieves-Neira W, Shahabi S. Survival after pelvic exenteration for uterine malignancy: A National Cancer Data Base study. Gynecol Oncol. 2016; 143: 472-478.
- Shingleton HM, Soong SJ, Gelder MS, Hatch KD, Baker VV, Austin Jr JM. Clinical and histopathologic factors predicting recurrence and survival after pelvic exenteration for cancer of the cervix. Obstet Gynecol. 1989; 73: 1027- 1034.
- Backes FJ, Martin DD. Intraoperative radiation therapy (IORT) for gynecologic malignancies. Gynecol Oncol. 2015; 138: 449-456.
- Gemignani ML, Alektiar KM, Leitao M, Mychalczak B, Chi D, Venkatraman E, et al. Radical surgical resection and high-dose intraoperative radiation therapy (HDR-IORT) in patients with recurrent gynecologic cancers. Int J Radiat Oncol Biol Phys. 2001; 50: 687-694.
- Tran PT, Su Z, Hara W, Husain A, Teng N, Kapp DS. Long-term survivors using intraoperative radiotherapy for recurrent gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2007; 69: 504-511.