
Research Article
Austin J Obstet Gynecol. 2021; 8(5): 1183.
A Cohort Study of the Efficacy of the Dienogest and the Gonadotropin-Releasing Hormone Agonist in Women with Adenomyosis
Ji MM, Yuan M, Jiao X, Li QJ, Huang YF, Li J and Wang GY*
Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Jinan, China
*Corresponding author: Guoyun Wang, Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Jinan, 250012, China
Received: May 26, 2021; Accepted: June 08, 2021; Published: June 15, 2021
Abstract
Purpose: To study the efficacy and safety of the dienogest and the Gonadotropin-Releasing Hormone agonist (GnRH-a) in symptomatic females with uterine adenomyosis.
Methods: A total of 127 patients with adenomyosis with a chief complaint of dysmenorrhea were recruited. The first group received 2mg of Dienogest (DNG) daily, whereas the second group received Goserelin Acetate (GS) (3.6mg/4 weeks) for 12 weeks. Outpatient follow-up was undertaken after 12 weeks.
Results: Among 127 women, 56/63 (87.5%) patients completed the treatment in the DNG group, whereas 62/64 (96.9%) patients completed the treatment in the GS group. A significant decrease in dysmenorrhea symptoms as measured by the Visual Analog Scale (VAS) and Carcinoma Antigen125 (CA125) after 12 weeks of treatment was observed in both groups (P<0.001). The hemoglobin of anemic patients did not significantly improve after 12 weeks of treatment (P=0.21) and the uterine volume slightly increased without statistical significance (P=0.10) in the DNG group. Simultaneously, the hemoglobin of anemic patients significantly improved (P<0.001) and the uterine volume significantly decreased (P<0.001) in the GS group.
Conclusions: Dienogest effectively alleviates the symptoms of dysmenorrhea in patients with adenomyosis, but it cannot improve the anemia or reduce the size of the uterus. GnRH-a is more effective in improving anemia and reducing the uterine volume in patients with adenomyosis.
Keywords: Adenomyosis; Dienogest; GnRH-a
Abbreviations
GnRH-a: Gonadotropin-Releasing Hormone agonist; DNG: Dienogest; GS: Goserelin Acetate; VAS: Visual Analog Scale; CA125: Carcinoma Antigen125; ChiCTR: Chinese Clinical Trial Registration; OCs: Oral Contraceptive Pills; LNG-IUD: Levonorgestrel-Releasing Intrauterine Device; Hgb: Hemoglobin; SD: Standard Deviation; IQR: Interquartile Range; NGF: Nerve Growth Factor; IL-8: Interleukin-8
Introduction
Adenomyosis, which is a common benign gynecological disease, is distinguished by the presence of heterotopic endometrial glands and stroma with invasion of the myometrium and diffuse growth or localized hyperplasia in the myometrium. The main manifestations of adenomyosis are dysmenorrhea, menorrhagia, and infertility, which seriously affect the physical and mental health of females. The management of adenomyosis remains controversial in which the current treatment mainly includes drugs, surgery, or a combination of the two. The exhaustive treatment for adenomyosis is hysterectomy [1], but this is not feasible for patients who have fertility requirements and prefer to preserve their uterus. Several hormonal treatments, i.e., Oral Contraceptive Pills (OCs), progestin, Gonadotropin-Releasing Hormone agonist (GnRH-a), Levonorgestrel-Releasing Intrauterine Device (LNG-IUD), and nonhormonal drugs, e.g., nonsteroidal antiinflammatory drugs, are currently used to control abnormal uterine bleeding and pain in adenomyosis [2]. GnRH-a has previously been demonstrated to produce a constant hypoestrogenic state in patients with adenomyosis [3] resulting in perimenopausal symptoms, such as hot flashes, osteoporosis, and vaginal dryness, causing difficulties with prolonged use. Hence, a well-tolerated and effective drug for longterm treatment of adenomyosis and its symptoms is highly needed.
A new progestin named Dienogest (DNG), a 19-nortestosterone derivative, has strong progestational effects and increased bioavailability due to its high selectivity to the progesterone receptor [4]. Its administration results in mild hypoestrogenic effects as well as antiovulatory and antiproliferative activities on endometrial cells [5]. Daily intake of 2mg of dienogest has been reported as a treatment for symptomatic adenomyosis in Japan. DNG has long been prescribed to reduce adenomyosis-related dysmenorrhea. DNG was marketed in China in 2018; however, experience involving the treatment of adenomyosis with DNG remains limited. To study the effectiveness and safety of dienogest and GnRH-a for symptomatic adenomyosis, we conducted this prospective cohort research in premenopausal patients.
Materials and Methods
This prospective cohort study recruited premenopausal females who presented to the outpatient department of Qilu Hospital of Shandong University from September 2019 to August 2020 with a chief complaint of dysmenorrhea. The study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University, number 2019 (066). The patients that were eligible for the study were female patients aged 18 years or older, complaining of dysmenorrhea with ultrasonographic evidence of adenomyosis. The ultrasound criteria used for the diagnosis of adenomyosis are globular uterine enlargement, asymmetrical myometrial thickening, myometrial cyst, heterogenous area within the myometrium, hyperechoic regions surrounded by hypoechoic areas and unaltered uterine contour [6,7]. Simultaneously, ultrasonography was performed to compare the uterine volume, which was assessed using the formula for an ovoid object (length × width × depth × 0.52) [8]. Exclusion criteria included current pregnancy and lactation; received hormone therapy within 3 months prior to the initiation of treatment; with uterine fibroids ≥2 cm in diameter; with cervical stenosis, uterine anomalies; and with abnormal blood coagulation, ovarian tumors, and other endocrine diseases. Written informed consent was obtained from all patients. The participants were allocated by alternation to one of two treatment groups. The first group (n=63) received oral dienogest (Visanne, Bayer Pharma AG, Germany) at a dose of 2mg once daily without a break for 12 weeks. The second group (n=64) received subcutaneous Goserelin Acetate (GS) injections (Zoladex, 3.6mg/4 weeks, AstraZeneca UK Limited, England) for 12 weeks. Women were advised to use barrier methods during the study period if they required reliable contraception. At the study’s entry visit, general characteristics and clinical information were collected. Menorrhagia was assessed by Hemoglobin (Hgb) levels. Women were also requested to assess adenomyosis-related dysmenorrhea using a 100mm Visual Analog Scale (VAS) in which 0 indicated no pain and 100 indicated intolerable pain. Hematological analysis, including Carcinoma Antigen 125 (CA125) and Hgb levels was determined in each patient shortly before treatment. The patients were followed up after 12 weeks of treatment. The follow-up included VAS for dysmenorrhea, blood data, and ultrasonography. Spontaneously reported adverse events were recorded at the 12th week follow-up.
Statistical analysis
IBM® (IBM, New York, United States) SPSS® Statistics version 25 was used for the statistical analysis. Analysis was done on the recruited females who continued the study. Descriptive statisticsmean (standard deviation, SD) and median (interquartile range, IQR) were used to describe the study population. Frequency (%) was used to describe the incidence of adverse reactions. The Kruskal-Wallis Test and Student’s t-test were used for evaluation of the efficacy rate in the DNG and GS groups. P <0.05 was considered to be a statistically significant difference.
Results
In the DNG group, two patients was lost to follow-up, and two patients discontinued treatment for heavy, prolonged, and irregular genital bleeding, one for worsened dysmenorrhea, one for weight gain, and one for dizziness. In total, the number of patients analyzed was 56. In the GS group, one patient preferred surgery due to heavy, prolonged bleeding, whereas one discontinued treatment after the development of hot flushes and a sleep disorder. Thus, the number of patients analyzed was 62. Patient characteristics and dysmenorrhea are presented in Table 1. Both groups of patients had severe dysmenorrhea before treatment. Table 2 shows the results of dysmenorrhea, Hgb levels, CA 125 levels, and uterine volume before and after the treatment. VAS scores and CA 125 levels were observed to have significantly decreased after treatment in both groups (Figure 1 and 2). The hemoglobin levels of the two groups were also observed to have improved (Figure 3). Furthermore, we analyzed the hemoglobin level of patients with anemia before treatment. This study showed that the hemoglobin level of anemia patients did not improve significantly after taking dienogest (91.6 ± 8.6 vs. 95.8 ± 15.6, p=0.21). GnRH-a was found to significantly improve the anemia of patients with adenomyosis 84.0 ± 12.7 vs. 101.7 ± 14.4, p<0.001). The uterine volume before treatment in the DNG group was 144.2 (114.8–291.3) cm³, which slightly increased to 170.3 (117.1–261.5) cm³ after 12 weeks of treatment (P=0.10) (Figure 4). The uterine volume before treatment in the GS group was 222.0 (141.5–294.5) cm³, which significantly decreased to 125.3 (91.2–154.6) cm3 after 12 weeks of treatment (P<0.001) (Figure 4). Adverse events in patients during the treatment are displayed in Table 3. Hot flushes were the most common adverse event during the treatment in the GS group (40.3%), whereas it was breast discomfort in the DNG group (19.6%). Patients treated with dienogest less frequently experienced events representing other hypoestrogenic symptoms, e.g., decreased libido, backache, and vaginal dryness compared to those treated with GS. Patients in the GS group reported more headaches compared with the DNG group. Most adverse events were mild to moderate in both groups. In the dienogest group, seven patients suffered from depression, which was thought to have been possibly related to the drug, but did not need medical intervention. Other frequent adverse events reported in the dienogest group included weight gain and irritability (17.9%, 16.1%, respectively), which may have been related to reduced activity during the COVID-19 epidemic or the study drug. At 12 weeks, treatment for anemia was given to patients with anemia, and calcium supplementation was recommended for patients with backache. No patients received add-back therapy.
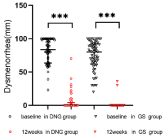
Figure 1: Dysmenorrhea in DNG and GS groups before and after 12 weeks
of treatment.
***Vs baseline; P <0.001; DNG: Dienogest; GS: Goserelin Acetate.
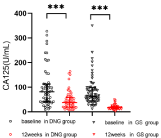
Figure 2: CA125 in DNG and GS groups before and after 12 weeks of
treatment.
***Vs baseline; P <0.001; DNG: Dienogest; GS: Goserelin Acetate; CA125:
Carcinoma Antigen 125.
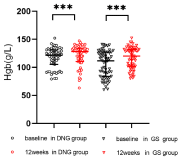
Figure 1: Hgb of in DNG and GS groups before and after 12 weeks of
treatment.
***Vs baseline; P <0.001; DNG: Dienogest; GS: Goserelin Acetate; Hgb:
Hemoglobin.
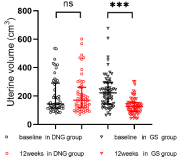
Figure 4: Uterine volume in DNG and GS groups before and after 12 weeks
of treatment.
***Vs baseline; P <0.001; ns: not significant; DNG: Dienogest; GS: Goserelin
Acetate.
DNG (56)
GS (62)
Age (years)a
39.7 ± 6.7
38.8 ± 6.8
40.5 ± 6.4
BMI (kg/m2)b
23.4 (21.8-26.2)
23.1 (21.4-25.4)
24.2 (22.0-26.5)
Parityb
1 (1-2)
1 (1-2)
1 (1-2)
82.0 (63.0-96.5)
83.5 (63.5-100.0)
80.0 (61.8-92.3)
aValues are given as mean ± SD.
bValues are given as median (1/4 quintile-3/4 quintile).
DNG: Dienogest; GS: Goserelin Acetate; BMI: Body Mass Index; SD: Standard Deviation.
Table 1: Basal characteristics in DNG and GS groups.
p-value
GS (62)
p-value
Baseline
12weeks
Baseline
12weeks
Dysmenorrheab
83.5 (63.5-100.0)
0 (0-4.0)
<0.001
80 (61.8-93.3)
0 (0-0)
<0.001
81.5 (40.5-112.8)
37.5 (19.1-59.2)
<0.001
64.2 (45.2-98.8)
17.3 (13.3-21.6)
<0.001
121.5 (105.3-130.8)
129 (110.3-134.0)
<0.001
111.5 (83.8-127.0)
120 (101.8-132.0)
<0.001
144.2 (114.8-291.3)
170.3 (117.1-261.5)
0.1
222 (141.5-294.5)
125.3 (91.2-154.6)
<0.001
Hgb (g/L) (<110g/L)a
0.21
<0.001
aValues are given as mean ± SD.
bValues are given as median (1/4 quintile-3/4 quintile).
DNG: Dienogest; GS: Goserelin Acetate; CA125: Carcinoma Antigen 125; Hgb: Hemoglobin; SD: Standard Deviation.
Table 2: Outcomes in DNG and GS groups.
GS (62)
n
%
n
%
Headache
2
3.6
12
18.8
10
17.9
6
9.7
Depression
7
12.5
3
4.8
Decreased libido
4
7.1
7
11.3
Vaginal dryness
3
5.4
6
9.7
Acne
3
5.4
0
0
Alopecia
4
7.1
7
11.3
6
10.7
25
40.3
Sick and vomit
3
5.4
4
6.5
9
16.1
14
22.6
11
19.6
3
4.8
3
5.4
9
14.5
Sleep disorder
5
8.9
6
9.7
DNG: Dienogest; GS: Goserelin Acetate.
Table 3: Numbers and proportions of women with adverse events in DNG and GS groups.
Discussion
The study confirmed DNG is an efficient method for dysmenorrhea in women with adenomyosis. VAS decreased significantly after 12 weeks of treatment with DNG, which showed a distinct effect of DNG. A previous study by Arisa Takeuchi et al. showed that dienogest could reduce nerve fiber density and Nerve Growth Factor (NGF) expression in human adenomyosis [9]. Dienogest has been reported to exhibit inhibitory effects on the secretion of cytokine interleukin-8 (IL-8) in endometriotic stromal cells [10]. Not only is this appropriate for adenomyosis, but its good therapeutic effect on pain associated with endometriosis and primary dysmenorrhea also makes dienogest a good candidate for the treatment [11,12]. CA125 is a glycoprotein antigen on the surface of body cavity epithelial cells. After entering the blood, it will cause the serum CA125 concentration to rise. The ectopic endometrium of patients with adenomyosis has a strong function of secreting CA125, and its CA125 level is 2 to 4 times that of normal. Therefore, the serum CA125 level can be measured to monitor adenomyosis. It can be seen from our research that the CA125 levels of the two groups of patients have significantly decreased.
Alternatively, our findings suggested that a risk of heavy genital bleeding during DNG treatment should be noted in patients with adenomyosis. In this study, two cases of severe genital bleeding occurred, which resulted in the discontinuation of DNG treatment? These patients had a prior history of anemia before the administration of treatment, and their Hgb was 87g/L and 97g/L, respectively. We analyzed the Hgb of 16 patients with anemia in the DNG group and 30 in the GS group at 12 weeks of treatment and found that the Hgb of anemic patients with adenomyosis increased by a nonstatistically significant amount in the DNG group. In Osuga’s study, the most common bleeding patterns reported during the period of taking dienogest were spotting and breakthrough bleeding; however, no patient ceased treatment of DNG as a consequence of the genital bleeding. Furthermore, the Hgb of the patients was at least 110g/L before the treatment [5]. Irregular bleeding has been reportedly common with administration of progestins and dienogest. This has been considered related to the breakthrough bleeding primarily derived from pseudodecidua [13]. The exact mechanisms causing severe uterine bleeding in patients with adenomyosis treated with dienogest remain unclear. Chie Nagata’s retrospective research showed that anemia, mildly suppressed or unsuppressed estradiol after treatment, and a younger age are risk factors for the cessation of treatment of DNG due to genital bleeding [14]. Sho Matsubara et al. reported that subtype I (intrinsic) patients with adenomyosis were more likely to experience serious bleeding compared to other types of adenomyosis [15,16]. Simultaneously, Kazuaki Neriishi et al. reported that subtype II adenomyosis (extrinsic) was negatively associated with severe metrorrhagia during the treatment of dienogest [17]. Consequently, DNG seems to not have any effect on the anemia of a patient with adenomyosis. Therefore, using DNG for the initial treatment of patients with adenomyosis with anemia is not recommended. Further studies on the serious bleeding factors related to dienogest are needed.
The uterus of patients with adenomyosis is usually larger than the normal uterus. Our results are consistent with previous studies that have shown that DNG has no obvious effect on the reduction of the uterus size [18,19]. In this study, the uterus enlarged in 31 of 56 patients in the DNG group and 1 of 62 patients in the GS group. For the DNG and GS groups, these therapies were ineffective for reducing uterine volume in 55.4% and 1.6% of patients, respectively. GnRH-a can reduce angiogenesis and inflammation and can induce apoptosis in adenomyosis tissues leading to its regression [20]. Unfortunately, we were unable to clarify why DNG was effective in reducing uterine swelling of some patients, but it is ineffective for others. Further studies are needed to clarify the factors predicting a patient’s response to DNG treatment.
Long-term management of adenomyosis is very important. Hysterectomy is considered as the only method that can definitively cure adenomyosis. For patients who want to preserve their uterus, conservative surgeries are available [21,22]. Adenomyosis is an estrogen-dependent disease [23], thus most of the drugs used to treat adenomyosis are hormone drugs: GnRH-a is effective in alleviating pain as well as reducing bleeding and uterine volume in adenomyosis. However, adverse effects, such as perimenopausal symptoms and bone calcium loss caused by low estrogen levels, result in limitations to its duration of use. LNG-IUS is an efficient method of relieving dysmenorrhea and menorrhagia from adenomyosis, but the ring-off and downward movement of LNG-IUS can sometimes occur, along with vaginal bleeding and pelvic and inflammatory disease. OCs can also be used to treat adenomyosis; however, the average age of adenomyosis patients is on the older side. It is important to remain cautious in administering OCs to patients over 40 years of age because it increases the risk of thrombosis. DNG alleviates pain in patients with adenomyosis, even if they are in the perimenopausal period.
However, some limitations in our study exist. The results of this study are limited to a 12-week treatment, and long-term follow-up data is still necessary. Some patients in Japan have been taking dienogest for extended periods continuously or even until menopause, which suggests that dienogest is well tolerated [17,24]. In addition, adenomyosis was diagnosed clinically and by ultrasound without histological confirmation. However, Fedele et al. showed that diagnosis by transvaginal sonography is highly precise [25]. The genital bleeding pattern was not recorded, but hemoglobin was used to assess the patient’s genital bleeding.
The treatment of adenomyosis is very personalized, according to the patient’s age, uterine size, hemoglobin level, fertility requirements and economic status to implement individualized treatment. This study has guiding significance for the individualized treatment of adenomyosis. According to our research, DNG effectively improves the symptoms of dysmenorrhea in patients with adenomyosis, although it cannot improve the anemia or reduce the size of the uterus. Therefore, dienogest is suitable for the initial treatment of patients with adenomyosis without severe anemia or a large uterus. For patients with anemia or a large uterus, the sequential use of dienogest is recommended after conservative surgery or GnRH-a treatment for 3-6 cycles.
Conclusion
Dienogest effectively alleviates the symptoms of dysmenorrhea in patients with adenomyosis but it cannot reduce the size of the uterus or improve the anemia. GnRH-a can effectively reduce the uterine volume and improve the patient’s anemia while alleviating the symptoms of dysmenorrhea in patients with adenomyosis.
Declarations
Funding: This work was supported by the National Nature Science Foundation of China (81771552) and the Technology Development Plan of Shandong Province (2019GSF108071).
Compliance with Ethical Standards Written informed consent forms were obtained from all participants and our study was approved by the Medical Ethics Committee of Qilu Hospital of Shandong University, number 2019 (066).
Consent to participate Informed consent to participate in the study was obtained from participants.
References
- Garcia L, Isaacson K. Adenomyosis: review of the literature. J Minim Invasive Gynecol. 2011; 18: 428-437.
- Vannuccini S, Luisi S, Tosti C, Sorbi F, Petraglia F. Role of medical therapy in the management of uterine adenomyosis. Fertil Steril. 2018; 109: 398-405.
- Badawy AM, Elnashar AM, Mosbah AA. Aromatase inhibitors or gonadotropinreleasing hormone agonists for the management of uterine adenomyosis: a randomized controlled trial. Acta Obstet Gynecol Scand. 2012; 91: 489-495.
- Ruan X, Seeger H, Mueck AO. The pharmacology of dienogest. Maturitas. 2012; 71: 337-344.
- Osuga Y, Fujimoto-Okabe H, Hagino A. Evaluation of the efficacy and safety of dienogest in the treatment of painful symptoms in patients with adenomyosis: a randomized, double-blind, multicenter, placebo-controlled study. Fertil Steril. 2017; 108: 673-678.
- Rizvi G, Pandey H, Pant H, Chufal SS, Pant P. Histopathological correlation of adenomyosis and leiomyoma in hysterectomy specimens as the cause of abnormal uterine bleeding in women in different age groups in the Kumaon region: A retroprospective study. Journal of mid-life health. 2013; 4: 27-30.
- Cunningham RK, Horrow MM, Smith RJ, Springer J. Adenomyosis: A Sonographic Diagnosis. Radiographics : a review publication of the Radiological Society of North America, Inc. 2018; 38: 1576-1589.
- Dueholm M, Lundorf E, Hansen ES, Sørensen JS, Ledertoug S, Olesen F. Magnetic resonance imaging and transvaginal ultrasonography for the diagnosis of adenomyosis. Fertil Steril. 2001; 76: 588-594.
- Takeuchi A, Koga K, Miyashita M, Makabe T, Sue F, Harada M, et al. Dienogest reduces proliferation, NGF expression and nerve fiber density in human adenomyosis. Eur J Obstet Gynecol Reprod Biol. 2016; 207: 157-161.
- Horie S, Harada T, Mitsunari M, Taniguchi F, Iwabe T, Terakawa N. Progesterone and progestational compounds attenuate tumor necrosis factor alpha-induced interleukin-8 production via nuclear factor kappa B inactivation in endometriotic stromal cells. Fertil Steril. 2005; 83: 1530-1535.
- Osuga Y, Hayashi K, Kanda S. Evaluation of the efficacy, safety, and clinically recommended dose of dienogest in the treatment of primary dysmenorrhea: a randomized, double-blind, multicenter, placebo-controlled study. Fertil Steril. 2020; 113: 167-175.
- Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010; 25: 633-641.
- Irahara M, Harada T, Momoeda M, Tamaki Y. Hormonal and histological study on irregular genital bleeding in patients with endometriosis during treatment with dienogest, a novel progestational therapeutic agent. Reprod Med Biol. 2007; 6: 223-228.
- Nagata C, Yanagida S, Okamoto A, Morikawa A, Sugimoto K, Okamoto S, et al. Risk factors of treatment discontinuation due to uterine bleeding in adenomyosis patients treated with dienogest. J Obstet Gynaecol Res. 2012; 38: 639-644.
- Matsubara S, Kawaguchi R, Akinishi M, Nagayasu M, Iwai K, Niiro E, et al. Subtype I (intrinsic) adenomyosis is an independent risk factor for dienogestrelated serious unpredictable bleeding in patients with symptomatic adenomyosis. Sci Rep. 2019; 9: 17654.
- Kishi Y, Suginami H, Kuramori R, Yabuta M, Suginami R, Taniguchi F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am J Obstet Gynecol. 2012; 207: 114.e1-114.e7.
- Neriishi K, Hirata T, Fukuda S, Izumi G, Nakazawa A, Yamamoto N, et al. Long-term dienogest administration in patients with symptomatic adenomyosis. J Obstet Gynaecol Res. 2018; 44: 1439-1444.
- Matsushima T, Akira S, Fukami T, Yoneyama K, Takeshita T. Efficacy of Hormonal Therapies for Decreasing Uterine Volume in Patients with Adenomyosis. Gynecol Minim Invasive Ther. 2018; 7: 119-123.
- Fawzy M, Mesbah Y. Comparison of dienogest versus triptorelin acetate in premenopausal women with adenomyosis: a prospective clinical trial. Arch Gynecol Obstet. 2015; 292: 1267-1271.
- Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, et al. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod. 2010; 25: 642-653.
- Grimbizis GF, Mikos T, Tarlatzis B. Uterus-sparing operative treatment for adenomyosis. Fertil Steril. 2014; 101: 472-487.
- Younes G, Tulandi T. Conservative Surgery for Adenomyosis and Results: A Systematic Review. J Minim Invasive Gynecol. 2018; 25: 265-276.
- Kitawaki J. Adenomyosis: the pathophysiology of an oestrogen-dependent disease. Best Pract Res Clin Obstet Gynaecol. 2006; 20: 493-502.
- Osuga Y, Watanabe M, Hagino A. Long-term use of dienogest in the treatment of painful symptoms in adenomyosis. J Obstet Gynaecol Res. 2017; 43: 1441-1448.
- Dueholm M. Transvaginal ultrasound for diagnosis of adenomyosis: a review. Best Pract Res Clin Obstet Gynaecol. 2006; 20: 569-582.