
Case Report
Austin J Obstet Gynecol. 2022; 9(2): 1202.
Multidisciplinary Management of Placenta Accreta Avoiding Maternal Mortality in a High-Risk Center- Case Report
Vallejo GM, Uriel M and Romero XC*
Department of Maternal Fetal Medicine, El Bosque University, Colombia
*Corresponding author: Romero XC, Av Department of Maternal Fetal Medicine, El Bosque University, Carrera 9 # 131a-02, Ecodiagnóstico El Bosque S. A. S, Calle 134 #7B – 83, Bogotá – Colombia, 110121000
Received: August 02, 2022; Accepted: August 26, 2022; Published: September 02, 2022
Abstract
Background: Placenta Accreta (PA) constitute a spectrum of abnormal placentation associated with a significant increase in maternal morbidity and mortality. Antenatal diagnosis of PA has transcendental importance in the orientation of the multidisciplinary management showing a substantial reduction in hemorrhagic complications.
Case Presentation: A patient of 36.4 weeks of gestation with ultrasound findings of placenta previa and a high probability of placenta increta was attended in our high-risk center. Cesarean section with hysterectomy was performed at 37 weeks. During the surgical procedure, it was found infiltration of the placenta up to the uterine serosa at the level of the uterine segment and adjacent bladder. A hysterotomy at the corpus of the uterus was performed and a healthy newborn was obtained. Ligation of the hypogastric arteries bilaterally and total abdominal hysterectomy was achieved. The patient received a transfusion of red blood cells, platelets, cryoprecipitates and fresh frozen plasma and required vasopressor support with favorable evolution and avoiding fetal and maternal complications. The review of this case highlights that the management by a multidisciplinary team reduces the complications and morbidity-mortality of patients with placenta accreta as it is currently a public health problem.
Conclusions: The maternal-perinatal outcomes not only depend on the availability of prenatal diagnosis but also on the organization of this multidisciplinary group with the application of standardized management and providing care in high-risk centers.
Keywords: Placenta accrete; Postpartum hemorrhage; Ultrasonography; Doppler; Surgical procedures
Introduction
Placenta Accreta (PA) and its variants constitute a spectrum of abnormal placentation associated with a significant increase in maternal morbidity and mortality [1-3]. The prevalence rate of this condition is between 0.01 and 0.1% according to the most recent meta-analysis and it has been increasing since the last decades, probably secondary to the increased cesarean section rate [3-5]. In Colombia, some studies have reported an incidence of approximately 1 in 539 deliveries [6]. PA is classified according to the depth of villous invasiveness in relation to the myometrium into 3 categories: PA when the villi simply adhere to the myometrium, Placenta Increta (PI) when the villi invade the myometrium, and Placenta Percreta (PP) when the villi invade the full thickness of the myometrium reaching the uterine serosa with or without invasion of adjacent organs (most commonly the urinary bladder or colon) [7-10].
The most accepted hypothesis regarding the physiopathology is a defect in the myometrium-endometrium interface, mainly due to a scar, which generates an inadequate decidualization of this area and excessive trophoblastic invasion [5,8,12]. The most common risk factor is the history of cesarean delivery with a clear association between the number of cesarean sections and the subsequent risk of PA [5,8,10]. Another important risk factor is the placenta previa [5,10,13,14].
Antenatal diagnosis of PA spectrum has transcendental importance since it allows adequate planning of the delivery in a highrisk center with access to maternal and neonatal intensive care [5,15]. Several abnormal findings have been described in the image grayscale ultrasound that are associated with PA: multiple vascular lacunae within the placenta, loss of the normal hypoechoic zone between the placenta and myometrium, decreased retro placental myometrial thickness (less than 1 mm), abnormalities of the uterine serosabladder interface, and extension of placenta into myometrium, serosa, or bladder [5,15,16]. The most recent literature reviews revealed that ultrasonography with Doppler evaluation reaches a high sensitivity and specificity of 97% for antenatal detection of PA spectrum [15,19]. Prenatal MRI has also shown to be highly accurate in diagnosing PA [21] and it might be useful for evaluating lateral extension and depth of myometrial invasion when the placenta is posterior or in obese patients, however, the cost and limited access to MRI makes it impractical as a screening tool [15,19-22].
PA is associated with multiple complications such as catastrophic obstetric hemorrhage requiring massive transfusion, admission to the ICU, need for hysterectomy, injury to adjacent organs, reinterventions, coagulopathy, respiratory complications, infection, multi-organ failure and maternal death in up to 7% of cases [5,23]. When the prenatal diagnosis is available, the management of PA and the planning of the delivery by a multidisciplinary team in a high-risk center will reduce the maternal morbidity and mortality [23,24].
It is unclear the appropriate time for delivery, therefore, the decision must be individualized considering the risks and benefits for the mother and the fetus [5]. Several management strategies for PA have been used. The conservative treatment includes all procedures in order to avoid the performance of peripartum hysterectomy [5,10,11,15,21,23,25]. It can be a reasonable alternative option to planned cesarean hysterectomy in well-selected cases for uterus preservation, [23] with the risk of developing a catastrophic hemorrhage. Four types of conservative management have been described (1) the extirpative technique with manual removal of the placenta; (2) the expectant approach or “leave the placenta in situ”; (3) one-step conservative surgery in which only the accreta area is removed; and (4) the Triple-P procedure that involves performing a suture around the accreta area after resection [23,25]. However, in most cases, non-conservative management is performed in which the uterus is removed.
The most frequent surgery is cesarean section-hysterectomy with placenta left in situ, in which hysterectomy is performed after delivery without trying to remove the placenta [26]. An alternative option is to postpone the hysterectomy (planned delayed or secondary hysterectomy) that arises as an emergent procedure and that is considered a hybrid approach in order to prevent complications that may occur with either immediate hysterectomy or prolonged placental retention. It is believed that the risk of hemorrhage at the time of hysterectomy can be reduced by allowing spontaneous regression of some of the placental bulk [11,23,25]. The optimal timing of delayed hysterectomy is unclear, therefore, there are no precise recommendations with this type of management [15,23]. Adjuvant therapies such as the use of methotrexate (MTX) that were used in the past are no longer recommended, since, the low rate of cell division in the third trimester compared to early pregnancy, does not allow a significant effect of MTX on placental resorption. Besides, MTX can cause serious maternal damage such as neutropenia or medullary aplasia, even with a single dose [15,23,25].
The review of this case highlights that the ultrasound is a broadly available with high sensitivity and specificity for diagnosis of PA and that the management by a multidisciplinary team in a high-risk center may reduce the complications and morbidity-mortality of patients with this prevalent health problem.
Case Presentation
41-year-old pregnant woman G4 P2 A1 C1V2, with a history of cesarean section and myomectomy on two occasions was admitted in high-risk center at Bogotá-Colombia, with 36.4 weeks of gestation and prenatal diagnosis of placenta previa with high probability of placenta increta from week 34according to results of Doppler ultrasonography and MRI. Ultrasonography reported anterior and posterior placenta with implantation at isthmus level, covering completely the internal OS, and with a portion above the C-section scar. In this area it was not possible to determine the hypoechoic line of separation between the placenta and the uterine wall, additionally, it was found vascular lacunae confluent and blood vessels of low resistance. At the color Doppler examination on the maternal surface of the placenta, there were no vessels protruding into the bladder (Figure 1).
The patient was evaluated by a multidisciplinary team (surgeon, urologist, a specialist in maternal-fetal medicine, gynecologic oncology and anesthesiologist), reserve of blood products and planning a cesarean section-hysterectomy was made. At 37 weeks a cystoscopy was performed with ureteral catheter placement by the urologist before the procedure (cesarean section hysterectomy). A supra and infraumbilical median incision was made and a uterus of 40 cm with multiple subserosal and intramyometrial myomas, irregular infiltration of the placenta up to the serosa at the level of the uterine segment and adjacent bladder were finding. A hysterotomy incision at corpus was performed and newborn with a weight of 2970g, 51 cm size and APGAR 8-9-10 was obtained. Cord ligation as close as possible to the placenta and hysterorrhaphy were carried out. Then, the surgeon ligated hypogastric arteries bilaterally without complications and total abdominal hysterectomy was performed. A total of 3000 cc of bleeding was quantified and during the procedure the patient received a transfusion of red blood cells, platelets, cryoprecipitates, fresh frozen plasma and vasopressor support.
At the end of the intervention, the patient was transferred to intensive care unit where she had a favorable evolution, with hospital discharging after 6 days. Macroscopic pathology reported an internal cervical OS not visualized by the interposition of vellus-like tissue and the microscopic pathology revealed a thinning cervix with extensive decidualization adjacent and some areas of chorionic villi with fibrin in the myometrium of the uterine segment which confirmed diagnosis of PA.
Discussion
In the case reported above, the patient had a prenatal diagnosis of PA at 34 weeks, which allows the planning of multidisciplinary management. The patient had the following risk factors for PA reported in the literature: the history of cesarean section, actual placenta previa, [27] two myomectomies (which alter the myometrium-endometrial interface and can modify the decidualization of this area favoring impaired placentation when pregnancy occurs) [8] and advanced maternal age [27].
Given the above-mentioned risk factors mainly placenta previa, a more detailed evaluation of the placenta was performed with the Doppler study of the insertion site as recommended in the scientific literature [15] and the MRI carried out supported the diagnostic suspicion of PA.
The comparison of the recommendations for ultrasound findings of the EW-AIP and the signs found in the patient are summarized in (Table 1), and it is possible to determine that the same criteria were used to make the suspected diagnosis. The (Figures 1, 2, 3 and 4), show examples of ultrasonographic findings suggestive of PA.
US finding ( EW-AIP)
US finding case
2D grayscale
Loss of ‘clear zone’
Abnormal placental lacunae
Bladder wall interruption
Myometrial thinning
Placental bulge
Focal exophytic mass2D grayscale
Loss of hypoechoic line of separation between the placenta and uterine wall
Confluence of vascular lacunae2D color Doppler
Uterovesical hypervascularity
Subplacental hypervascularity
Bridging vessels
Placental lacunae feeder vessels2D color Doppler
Blood vessels of low resistance
No vessels protruding into the bladder3D ultrasound ± Power Doppler
Intraplacental hypervascularity
Placental bulge
Focal exophytic mass
Uterovesical hypervascularity
Bridging vessels3D ultrasound ± power Doppler
3D ultrasound was not performed
Table 1: Unified descriptors, as suggested by the European Working Group on Abnormally Invasive Placenta (EW-AIP), for ultrasound (US) findings in PA vs findings in the patient’s ultrasound. Ultrasound Obstet Gynecol. 2016; 47: 276–278.
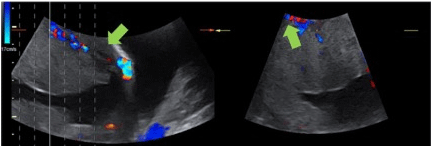
Figure 1: Ultrasound of a patient with suspected PA. The green arrow shows bridging vessels between placenta and wall bladder and yellow arrow shows
uterovesical and subplacental hypervascularity.
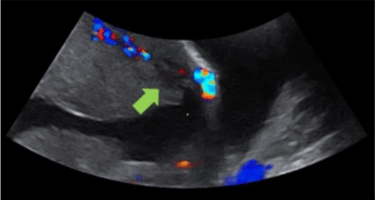
Figure 2: Ultrasound of a patient with suspected PA that shows placental lacunae.
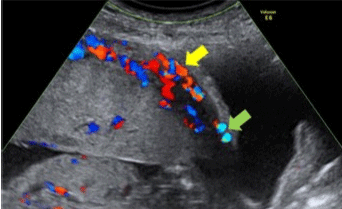
Figure 3: Ultrasound of a patient with suspected PA that shows loss of ‘clear zone’.
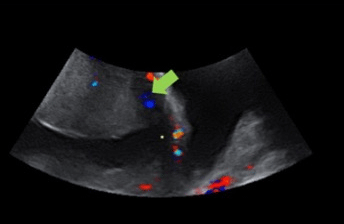
Figure 4: Ultrasound of a patient with suspected PA that shows myometrial thinning.
During the patient’s follow-up, at 36 weeks it was decided to admit her for planning the management (cesarean section and hysterectomy) by a multidisciplinary team.
In the literature, It has been described that during peripartum hysterectomy, the rate of unintentional injuries of the urinary tract is approximately 29%, with bladder involvement being more frequent than ureter involvement, therefore, a preoperative cystoscopy and the placement of ureteric stents have been recommended [15,26]. In the case of this patient, cystoscopy and placement of ureteral catheters were performed and no injuries of the urinary tract were reported.
On the multidisciplinary team, the general surgeon performed the ligation of the hypogastric arteries, with the objective of reducing the hemorrhage and the need for transfusion, however, studies in relation to this procedure in PA context have not shown benefits compared with non-prophylactic vascular intervention [26]. During the surgical procedure of this patient, the bleeding was considerable and required a massive transfusion. The patient had a successful outcome with a short ICU stay and without major complications.
Finally, as a summary, in the case of having a patient with risk factors for placenta accreta, the followed algorithm (Figure 5) is proposed for the assessment and management in order to guide prenatal.
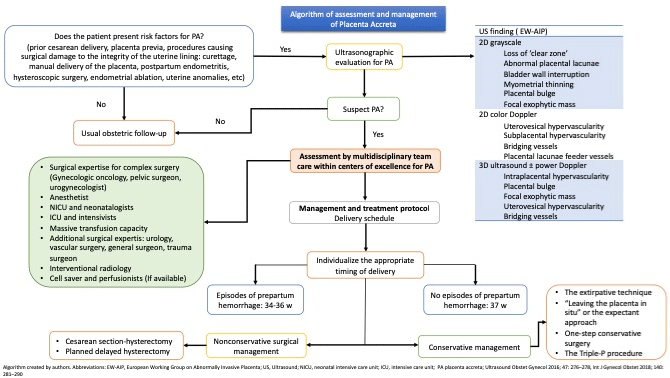
Figure 5: Algorithm of assessment and management of Placenta Accreta.
References
- Henske WC. A study of placenta accreta: Irving, Frederick C, and Hertig, Arthur T: Surg. Gynec. Obst. 64: 178, 1937. American Journal of Obstetrics & Gynecology. 1939; 38: 1088.
- Bailit JL, Grobman WA, Rice MM, Reddy UM, Wapner RJ, Varner MW, et al. Morbidly Adherent Placenta Treatments and Outcomes. Obstetrics & Gynecology. 2015; 125: 683-689.
- Jauniaux E, Bunce C, Grønbeck L, Langhoff-Roos J. Prevalence and main outcomes of placenta accreta spectrum: a systematic review and metaanalysis. American journal of obstetrics and gynecology. 2019; 221: 208-218.
- Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Incidence and Risk Factors for Placenta Accreta/Increta/Percreta in the UK: A National Case-Control Study. PLoS ONE. 2012; 7: e52893.
- Cahill AG, Beigi R, Heine RP, Silver RM, Wax JR. Placenta Accreta Spectrum. American journal of obstetrics and gynecology. 2018; 219: B2-B16.
- Davis Sjogreen A, Sánchez LM, Rubio Romero JA. Placenta Acreta in the Instituto MaternoInfantil 1994-1999. Colombian Journal of Obstetrics and Gynecology. 2002; 53: 327-334.
- Luke RK, Sharpe JW, Greene RR. Placenta accreta: the adherent or invasive placenta. American journal of obstetrics and gynecology. 1966; 95: 660-668.
- Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. American Journal of Obstetrics and Gynecology. 2018; 218: 75-87.
- Silver RM, Barbour KD. Placenta accreta spectrum: accreta, increta, and percreta. Obstetrics and gynecology clinics of North America. 2015; 42: 381- 402.
- Silver RM, Branch DW. Placenta Accreta Spectrum. New England Journal of Medicine. 2018; 378: 1529-1536.
- Sentilhes L, Goffinet F, Kayem G. Management of placenta accreta. Acta Obstetricia et Gynecologica Scandinavica. 2013; 92.
- Fox KA, Shamshirsaz AA, Carusi D, Secord AA, Lee P, Turan OM, et al. Conservative management of morbidly adherent placenta: expert review. American journal of obstetrics and gynecology. 2015; 213: 755-760.
- Bowman ZS, Eller AG, Bardsley TR, Greene T, Varner MW, Silver RM. Risk factors for placenta accreta: a large prospective cohort. American journal of perinatology. 2014; 31: 799-804.
- Chen L, Zhang H, Wang Q, Xie F, Gao S, Song Y, et al. Reproductive Outcomes in Patients With Intrauterine Adhesions Following Hysteroscopic Adhesiolysis: Experience From the Largest Women’s Hospital in China. Journal of minimally invasive gynecology. 2017; 24: 299-304.
- Jauniaux E, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, et al. Placenta Praevia and Placenta Accreta: Diagnosis and Management. BJOG: An International Journal of Obstetrics & Gynaecology. 2019; 126: e1-e48.
- D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound in Obstetrics & Gynecology. 2013; 42: 509-517.
- Collins SL, Ashcroft A, Braun T, Calda P, Langhoff-Roos J, Morel O, et al. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound in Obstetrics & Gynecology. 2016; 47: 271-275.
- Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016; 215: 712-721.
- Jauniaux E, Bhide A. Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: a systematic review and metaanalysis. American Journal of Obstetrics and Gynecology. 2017; 217: 27-36.
- Prayer D, Malinger G, Brugger PC, Cassady C, Catte LD, Keersmaecker BD, et al. ISUOG Practice Guidelines: performance of fetal magnetic resonance imaging. Ultrasound in Obstetrics & Gynecology. 2017; 49: 671-680.
- D’Antonio F, Iacovella C, Palacios-Jaraquemada J, Bruno CH, Manzoli L, Bhide A. Prenatal identification of invasive placentation using magnetic resonance imaging: systematic review and meta-analysis. Ultrasound in Obstetrics & Gynecology. 2014; 44: 8-16.
- Jauniaux E, Bhide A, Kennedy A, Woodward P, Hubinont C, Collins S. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. International Journal of Gynecology & Obstetrics. 2018; 140: 274-280.
- SENTILHES L, KAYEM G, SILVER RM. Conservative Management of Placenta Accreta Spectrum. Clinical Obstetrics and Gynecology. 2018; 61: 783-794.
- Shamshirsaz AA, Fox KA, Erfani H, Clark SL, Salmanian B, Baker BW, et al. Multidisciplinary team learning in the management of the morbidly adherent placenta: outcome improvements over time. Am J Obstet Gynecol. 2017; 216: 612.e-612.e5.
- Sentilhes L, Kayem G, Chandraharan E, Palacios-Jaraquemada J, Jauniaux E. FIGO consensus guidelines on placenta accreta spectrum disorders: Conservative management ,. International Journal of Gynecology & Obstetrics. 2018; 140: 291-298.
- Allen L, Jauniaux E, Hobson S, Papillon-Smith J, Belfort MA. FIGO consensus guidelines on placenta accreta spectrum disorders: Nonconservative surgical management ,. International Journal of Gynecology & Obstetrics. 2018; 140: 281-290.
- Eshkoli T, Weintraub AY, Sergienko R, Sheiner E. Placenta accreta: risk factors, perinatal outcomes, and consequences for subsequent births. American journal of obstetrics and gynecology. 2013; 208: 219.e1-219.e7.