
Research Article
Austin J Obstet Gynecol. 2022; 9(2): 1204.
BNT162b2 COVID-19 Vaccine has no Adverse Effect on Women’s In Vitro Fertilization Outcomes and Fertility
Safrai M1*, Rottenstreich A1, Herzberg S1, Imbar T1, Reubinoff B1,2 and Ben-Meir A1
1Department of Obstetrics and Gynecology, Hadassah-Hebrew University Medical Center and Faculty of Medicine, Israel
2The Sydney and Judy Swartz Embryonic Stem Cell Research Center of The GoldyneSavad Institution of Gene Therapy, Hadassah Hebrew University Medical Center, Israel
*Corresponding author: Myriam Safrai, Department of Obstetrics and Gynecology, Hadassah-Hebrew University Medical Center, Ein-Kerem, P.O. Box 12000, Jerusalem, Israel
Received: August 18, 2022; Accepted: September 12, 2022; Published: September 19, 2022
Abstract
Purpose: To investigate the effect of BNT162b2 COVID-19 vaccine on women’s fertility.
Methods: We prospectively collected data of women patients undergoing In Vitro Fertilization (IVF) treatment after completion of 2 doses of BNT162b2 vaccination between February and April 2021 (POST vaccine). For comparison, we reviewed records of the same patients before the vaccination (PRE vaccine) up to February 2019. Each woman served as self-control before and after vaccination. Study outcomes were compared between the PRE- and POSTvaccination groups. Clinical pregnancy values were assessed if data were available for both cycles.
Results: 47 women were eligible, with a mean interval of 362 ± 368 days between the two ovum pick-ups. The numbers of oocytes retrieved, matured oocytes, fertilization rates, and numbers and qualities of embryos at day 3 before-and-after vaccinations were similar for all parameters. The numbers and percentages of clinical pregnancies did not differ significantly between the two vaccination groups.
Conclusion: From our findings, the vaccine does not affect women’s in vitro outcomes and, therefore, fertility. This study repudiates misinformation from unreliable sources, reassuring patients to improve compliance and promote COVID-19 eradication.
Trial registration number: IRB(HMO-21-054), January 2020.
Keywords: BNT162b2; mRNA vaccine; COVID-19; Fertility; Women; SARS-CoV-2
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for causing Coronavirus Disease 19 (COVID-19), has affected over 160 million people worldwide since it was declared a pandemic in March 2020 by the World Health Organization (WHO) [1] and has caused over 3 million deaths [2] worldwide. The resulting urgent need for practical tools to combat COVID-19 has led to the accelerated development and recent approval of the BNT162b2 mRNA vaccine launched by BioNTech and Pfizer [3]. In a large trial, the two-dose regimen of BNT162b2 vaccine was assessed and found to confer a 95% protection rate against SARS-CoV-2 among individuals aged 16 and older [4,5]. Based on this information, a mass vaccination campaign using the BNT162b2 vaccine began in Israel [6], recommending vaccinate the entire population aged 16 and above [5]. The vaccine’s safety profile was previously assessed using self-reporting of local and systemic adverse events, the use of antipyretic or pain medication to treat minor side-effects of the vaccine, and unsolicited adverse severe events infrequently reported [3,7]. However, the effect of the vaccine on fertility has not been initially investigated.
Reproductive-aged women are considered a special population and often are not included in clinical. Indeed, pregnant women and women trying to conceive were excluded from the pivotal clinical trials evaluating the mRNA-based vaccines [3,8,9], resulting in many unanswered questions about the safety of the BNT162b2 vaccine on fertility. Since the vaccine’s launch, vaccination hesitancy has been a significant challenge in COVID eradication [10]. The social media panic has significantly increased the fear and hesitancy to receive the COVID-19 vaccine [11,12] in large parts of the population [10,13]. The impact of the vaccine on fertility has also been the subject of many rumors and misinformation.
The adult female population has another unique and challenging aspect to the morbidity of COVID-19: Pregnant women are at a higher risk of complications and increased risk of perinatal complications if they become infected with COVID-19 [9,14-19]. Therefore the American Society of Reproductive Medicine Task Force does not recommend withholding the vaccine from patients planning to conceive [20]. Despite recent studies showing no adverse effect of the vaccine on women’s fertility [21,22], long-term follow-ups from folliculogenesis through embryo formation and pregnancy rate are scarce. Due to the lack of information and clinical relevance, we aimed to investigate the possible impact of the BNT162b2 COVID-19 vaccine on women’s fertility.
Methods
This study was carried out in a large tertiary center. Our medical center is a university hospital with an In Vitro Fertilization (IVF) unit, which runs an average of 1000 fresh IVF cycles per year. Data were collected from all patients treated in the IVF unit, between February 2 and April 29, 2021, after vaccination of the general population began. Medical records of patients who had received two doses of the BNT162b2 vaccine were retrospectively reviewed (PRE-vaccine), using the hospital’s electronic database, up to January 15, 2017. They were compared to prospectively collected data of those patients (POST-vaccine).
To minimize bias, each woman served as self-control before and after vaccination. Women previously infected by COVID-19 were excluded. Additionally, to neutralize the effect of sperm on fertilization, only Intracytoplasmic Sperm Injection (ICSI) patients who were currently being treated with an ICSI cycle and had an earlier ICSI cycle available were included in the study.
Data obtained included: patient demographics (age and Body Mass Index (BMI)); indication for IVF treatment (i.e., female/male factor, unknown infertility, and need for Pre-implantation Genetic Diagnosis (PGD) or fertility preservation); Follicle Stimulating Hormone (FSH) value; data regarding the IVF cycle (length of Gonadotropin (GT) stimulation and total GT dose, estrogen level on the day before Ovum Pick Up (OPU), the number of oocytes retrieved, the number of mature oocytes, the number of fertilized oocytes, the number and quality of embryos at day 3); and the time since the first dose of the vaccine. The second vaccine dose was given as recommended, 21 days after the first dose [5,7]. The embryos’ quality at day 3 were determined by cell number, symmetry and fragmentation. According to the Society for Assisted Reproductive Technology (SART) grading guidelines, they were graded as good, fair or poor [23]. Clinical pregnancy based on the first hCG value was reported if the data were available for both cycles. Some patients didn’t proceed to embryo transfer, aiming for fertility preservation or PGD testing later on. The pregnancy rate was calculated for the total paired cycle with hCG value available for both cycles (n=15).
The primary outcomes were compared between the PRE and POST vaccination groups and consisted of: the IVF cycle outcomes, including the number of oocytes retrieved; the number of matured oocytes; the fertilization rate; and the number and quality of embryos at day 3.
Statistical Analysis
IVF treatment parameters are presented as median, interquartile range, mean, or percentage. Comparisons between PRE and POSTvaccine values were conducted with Mann Whitney test. A P-value of 0.05 or less was considered significant. The significance of pregnancy rate before and after vaccination was assessed by McNemar’s test. Statistical analyses were carried out using Excel 2013. A sample size of 32 women (in the entire cohort) was required to detect a significant difference of 30% in the number of oocytes retrieved (probability of Type 1 error of 0.05 and 80% power).
Ethical Approval
Approval was obtained from the Institutional Review Board (IRB) of the Hadassah-Hebrew University Medical Center. The requirement for written informed consent was waived by the IRB.
Results
During the study period, 297 women were treated in the IVF unit. More than half (n= 56%) had completed the two doses of the BNT162b2 vaccine. Thirteen percent (n=39) of the women had a prior history of SARS-CoV-2 infection, while 87% (n=258) were eligible for vaccination. In this subgroup of eligible women for vaccination, 64% had completed the two vaccine doses, 2% had received only one dose, and 34% chose not to be vaccinated for COVID-19 (Figure 1).
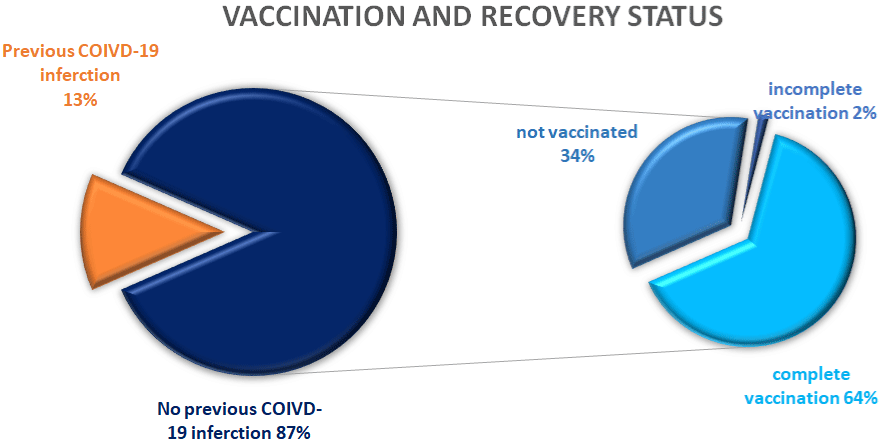
Figure 1: Vaccination and recovery status of women treated in the In Vitro
Fertilization clinic.
The figure shows(left side) that 13% of the women referred to the IVF clinic
during the study period were previously infected with COVID-19 and were
ineligible for vaccination. In the remaining 87% of women (right side): 64%
were fully vaccinated, 2% had completed a single dose of the vaccine, and
34% were unvaccinated.
A flow chart describing patient inclusion in this study is shown in (Figure 2). Women with a previous infection of COVID-19 and those who had not completed their vaccination were excluded from the study. The final cohort included 47 women for whom both ICSI cycle data were available before and after the vaccination. Their demographic data and indications for undergoing IVF are shown in (Table 1). These women had a mean interval of 362 ± 368 days between the two OPU. The characteristics of their IVF cycles before and after vaccination were similar for all the parameters (Table 2).
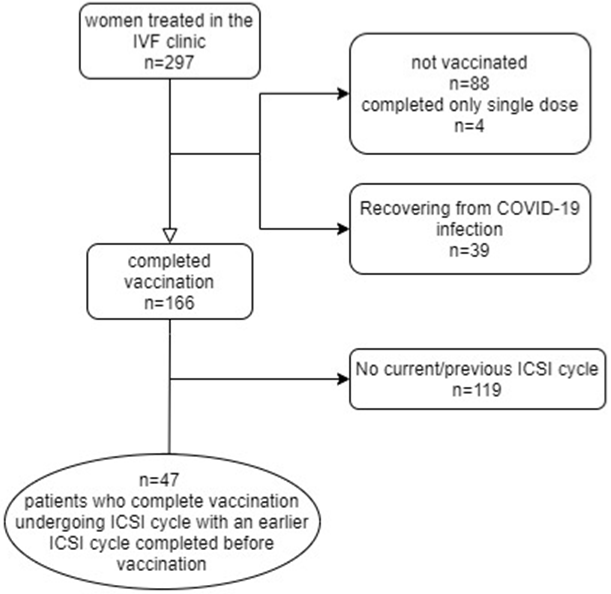
Figure 2: Study design.
IVF – In Vitro Fertilization, ICSI - Intracytoplasmic Sperm Injection.
Age (years)
37.36 ± 7.5
Body Mass Index (BMI)
27.5±6.2
Time from the first vaccine dose (days)
57.3 ± 24.7
FSH prior to IVF (IU/L)
9.27 ± 4.9
Interval between both OPU (days)
362.7 ± 386.4
Indication for IVF (%)
Female
49
Male
19
Unexplained
21
PGD
11
All continuous variables are expressed as mean ± Standard Deviation (SD); OPU – Ovum pick up, PGD - pre-implantation genetic diagnosis
Table 1: Demographics of patient sample and indication for IVF.
Various outcomes of the IVF cycles were found to be not significantly different before and after vaccination, such as the mean number of oocytes retrieved (5.0 (3.0-7.0) vs. 6.0 (3.0-10.0), p=0.73), the number of mature oocytes (4.0 (3.0-9.0) vs. 5.0 (2.0-9.5), p=0.89), and the fertilization rate (66.7 (41.4-98.4) vs. 60.0 (42.2- 71.4), p=0.23). In addition, embryo parameters were assessed, and no significant differences were found between the number of cleavage embryos (2.0 (1.0-6.0) vs. 2.0 (1.0-5.5), p=0.95), and the number of good and fair embryos (1.0 (1.0-3.0) vs. 2.0 (0.0-4.0), p=0.73) before and after vaccination.
An assessment of the pregnancy rate was implemented on 15 women from the sample. The number and percentage of clinical pregnancies did not significantly differ between PRE- and POSTvaccination groups (Table 2). Three women were pregnant in both cycles. One woman was pregnant only in her earlier cycle, and two women were pregnant after their vaccination. Nine women did not conceive in any of their cycles, resulting in a non-significant difference of p=0.56.
IVF cycle characteristics (n=47)
PRE vaccination
POST vaccination
P-value
Length of GT stimulation (days)
11.0 [9.0-12.0] (10.7)
10.0 [9.5-12.0] (10.8)
0.95
Total GT dose (IU)
2811 [2023.54050.0] (3769.2)
3236.0 [2322.5-4612.5] (3535.5)
0.17
Estrogen on the day before OPU (pmol/L)
6972.0 [3826.5-8813.5] (6754.6)
7265.0 [3930.0-9520.0] (7009.4)
0.67
Number of oocytes picked up
5.0 [3.0-10.0] (7.1)
6.0 [3.0-10.0] (8.0)
0.73
Number of matured oocytes
4.0 [3.0-9.0] (6.0)
5.0 [2.0-9.5] (6.6)
0.89
Percentage of fertilized oocytes (%)
66.7 [41.4-89.4] (63.9)
60.0 [42.2-71.4] (54.6)
0.23
Number of cleavage embryo
2.0 [1.0-6.0] (3.4)
2.0 [1.0-5.5] (3.8)
0.95
Number of good and fair embryo
1.0 [1.0-3.0] (2.5)
2.0 [0.0-4.0] (3.1)
0.73
Pregnancy assessment (n=15)
Number of clinical pregnancy
4
6
0.56
Percentage of clinical pregnancy
27
40
All continuous variables are expressed as median [interquartile range] (mean);
GT: Gonadotropin; IU: International Unite; OPU: Ovum Pick Up
All continuous variables are expressed as mean ± Standard Deviation (SD) or percent; IU – International Unites, OPU – Ovum Pick Up
Table 2: Patients’ IVF cycle parameters and outcomes before and after BNT162b2 vaccination.
Discussion
This study evaluate the impact of the BNT162b2 vaccine on women’s fertility. From our findings, the vaccine did not affect women’s fertility. Specifically, no differences were found between the ICSI cycles that each patient underwent before and after vaccination. The ICSI outcomes included: the number of oocytes retrieved, the number of matured oocytes and the percentage of fertilized oocytes were similar in the PRE- and POST- vaccination groups. Moreover, we continued the follow-up for 3 additional days and assessed the quantity and quality of cleavage embryos, and found no changes in any of the assessment parameters or embryo quality. Lastly, a subgroup from our sample showed that the pregnancy rate was also similar in the PRE- and POST- vaccination groups. Therefore, these findings are important in showing that the BNT162b2 vaccine does not affect IVF treatment parameters or the pregnancy rate. Our study results thus support the statement from the American College of Obstetricians and Gynecologists, the American Society for Reproductive Medicine, and the Society for Maternal-Fetal Medicine that “no loss of fertility has been reported among trial participants or among the millions who have received the vaccines since their authorization, and no signs of infertility appeared in animal studies. Loss of fertility is scientifically unlikely” [24]. Our result aligns with previous research assessing the impact of COVID vaccine on different levels of women’s fertility. Mohr-Sasson et al. Demonstrate that the same vaccine doesn’t impact AMH (anti-Mullerian hormone) level [22]. Others demonstrate that the vaccine doesn’t compromise follicular function [25]. From a clinical aspect, it has been shown that this vaccine doesn’t affect performance and implantation in assisted reproductive cycles following vaccination [26,27]. However, despite those studies, low compliance to the vaccine peruse [10,28-30]. The impetus for this study came about due to the persistent misinformation and rumors widely disseminated on social media about the effects of the BNT162b2 vaccine on women’s fertility. This misinformation affects vaccination compliance. Of the 297 women treated in the IVF unit during the study period, only 56% had completed vaccination, i.e., two doses of the BNT162b2 vaccine, a much lower rate than Israel’s national vaccination average of 71-80% in the general population of those aged 20-49 years [31]. Such fears were be based on various non-scientifically sound claims [9], reinforcing the usual uneasiness felt in response to a new vaccine. One of the baseless arguments for the BNT162b2 vaccine negatively impacting women’s fertility was that the vaccine contains a spike protein called syncytin-1, which is vital for forming the placenta. Antibodies produced against this protein may attack the placenta too, leading to abortions. However, these claims have since been revoked, as the vaccine contains neither syncytin-1 nor the mRNA sequence for syncytin-1 [20].
Another possible claim for the BNT162b2 vaccine causing female infertility is that the functional receptor for SARS-CoV-2 is ACE2 (angiotensin converting enzyme-2), which modulates the cleavage of angiotensin II in the renin-angiotensin system. COVID-19 invades host cells and down regulates ACE2 expression, causing increased pro-inflammatory response by angiotensin II. Since ACE2 and angiotensin II regulate essential reproductive functions, such as folliculogenesis, steroidogenesis, oocyte maturation and ovulation, there was the concern that the vaccine--which mimics the virus, could also reduce fertility by the same mechanism [24]. However, this has not been proven, and moreover, the BNT162b2 vaccine does not have the ACE2 receptor to cause such infertility. Our results also refute this claim since we found no negative impact on folliculogenesis and embryogenesis, as the number of oocytes and their maturation was not impaired.
Our study has several limitations. The main one is the retrospective nature of the analysis of the PRE vaccine group. Our study carried an inherent selection and information bias due to the medical record coding. Another potential limitation is the relatively small sample size of the study population. Nevertheless, the sound methodology where each patient served as their own self-control strengthens the results and allows us to provide reliable answers to the questions raised regarding the effects of BNT162b2 vaccination on fertility is a positive aspect of the study. Finally, we included data on the pregnancy rate for 15 women with data in the PRE- and POST-vaccination groups. This small analysis strengthens our findings, showing no difference in IVF treatment parameters before and after vaccination, and directly analyzes the effect of vaccination on women’s fertility. There is an inherent bias in considering the pregnancy rate: the results showed that couples whose previous ICSI cycle ended in pregnancy are less likely to return for another cycle. The mean interval between both OPU was 362 days. The impact of such time on fertility differs, depending on the patient’s age: namely, the passing of time is more significant in older women. However, no such fertility differences were seen in our study. If any such differences had been shown, we would expect reduced fertility of the POST- group. More extensive research is warranted to validate these initial findings, demonstrating that the BNT162b2 vaccination does not impact women’s fertility.
In conclusion, our study assesses the impact of the BNT162b2 COVID-19 vaccine on women’s fertility and provides encouraging data showing that this vaccine likely does not impair women’s fertility. The extended follow-up demonstrating no effect from folliculogenesis and embryo quality until the pregnancy rate helps abolish misinformation from unreliable sources, reassuring patients to improve compliance and promote COVID-19 eradication.
Author Contributions
M Safrai: Design study, Data collection, Manuscript writing.
A Rottenstreich Statistical analysis, Editing.
S Herzberg: Data collection, Editing.
T Imbar: Project development, Editing.
B Reubinoff: Protocol, Editing.
A Ben-Meir: Design study, management, Editing.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed consent was waived by the IRB.
References
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Medica: Atenei Parmensis. 2020; 91: 157-160.
- COVID Live Update: 163,728,900 Cases and 3,393,648 Deaths from the Coronavirus - Worldometer. 2021.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New England Journal of Medicine. 2020; 383: 2603–2615.
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021; 397: 1819–1829.
- Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. New England Journal of Medicine. 2021; 384: 1412-1423.
- Pfizer/BioNTech: BNT162b2 – COVID19 Vaccine Tracker. 2021.
- Walsh EE, Frenck Jr RW, Falsey AR, Kitchin N, Absalon J, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. New England Journal of Medicine. 2020; 383: 2439–2450.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine. 2021; 384: 403–416.
- Rasmussen SA, Kelley CF, Horton JP, Jamieson DJ. Coronavirus Disease 2019 (COVID-19) Vaccines and Pregnancy: What Obstetricians Need to Know. Obstet Gynecol. 2021; 137: 408–414.
- Sallam M, Dababseh D, Eid H, Al-Mahzoum K, Al-Haidar A, et al. High Rates of COVID-19 Vaccine Hesitancy and Its Association with Conspiracy Beliefs: A Study in Jordan and Kuwait among Other Arab Countries. 2021; 9: 42.
- Blake Evans, Carolyn Alexander, Emily Barnard, M Max Ezzati, Micah J Hill, et al. COVID-19 vaccine and infertility: baseless claims and unfounded social media panic | Fertility and Sterility Dialog. 2021.
- Sajjadi NB, Nowlin W, Nowlin R, Wenger D, Martin Beal J, et al. United States internet searches for “infertility” following COVID-19 vaccine misinformation. Journal of Osteopathic Medicine. 2021.
- Berry SD, Johnson KS, Myles L, Herndon L, Montoya A, et al. Lessons learned from frontline skilled nursing facility staff regarding COVID-19 vaccine hesitancy. J Am Geriatr Soc. 2021.
- Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, et al. Update: characteristics of symptomatic women of reproductive age with laboratoryconfirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. Morbidity and Mortality Weekly Report. 2020; 69: 1641.
- Jering KS, Claggett BL, Cunningham JW, Rosenthal N, Vardey O, et al Clinical Characteristics and Outcomes of Hospitalized Women Giving Birth With and Without COVID-19. JAMA Internal Medicine. 2021; 181: 714-717.
- Lokken EM, Taylor GG, Huebner EM, Vanderhoeven J, Hendrickson S, et al. Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients. Am J Obstet Gynecol. 2021; 225: e1-75.
- Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2021; 137: 571-580.
- Woodworth KR, Olsen EO, Neelam V, Lewis EL, Galang RR, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 jurisdictions, March 29–October 14, 2020. Morbidity and Mortality Weekly Report. 2020; 69: 1635.
- Gotluru C, Roach A, Cherry SH, Runowicz CD. Carolyn D. Sex, Hormones, Immune Functions, and Susceptibility to Coronavirus Disease 2019 (COVID- 19)-Related Morbidity. Obstet Gynecol. 2021; 137: 423–429.
- ht tps : / /www.as rm.org/globalas set s /as rm/as rm- content /news - and-publ i cat ions / cov id-19/ cov idtas k for ceupdate11.pdf?utm_ source=Informz&utm_medium=email&utm_campaign=EmailBlast. Accessed 20 Mar 2021
- Aizer A, Noach-Hirsh M, Dratviman-Storobinsky O, Yung Y, Haas J, et al. The effect of coronavirus disease 2019 immunity on frozen-thawed embryo transfer cycles outcome. Fertil Steril. 2022; 117: 974–979.
- Mohr-Sasson A, Haas J, Abuhasira S, Sivan M, Amdurski HD, et al. The effect of Covid-19 mRNA vaccine on serum anti-Müllerian hormone levels. Hum Reprod. 2022; 37: 534–541.
- Racowsky C, Vernon M, Mayer J, Ball GD, Behr B, et al. Standardization of grading embryo morphology. 2010; 27: 437-9.
- Medical Experts Continue to Assert that COVID Vaccines Do Not Impact Fertility. ACOG. 2021.
- Bentov Y, Beharier O, Moav-Zafrir A, Kabessa M, Godin M, et al. Ovarian follicular function is not altered by SARS-CoV-2 infection or BNT162b2 mRNA Covid-19 vaccination. medRxiv. 2021.
- Orvieto R, Noach-Hirsh M, Segev-Zahav A, Haas, J, Nahum R, et al. Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle?. Reprod Biol Endocrinol. 2021; 19: 69.
- Aizer A, Noach-Hirsh M, Dratviman-Storobinsky O, Nahum R, Machtinger R, et al. The effect of coronavirus disease 2019 immunity on frozen-thawed embryo transfer cycles outcome. Fertil Steril. 2022; 117: 974–979.
- Ajana B, Engstler E, Anas Ismail, Kousta M. Perceptions and attitudes towards Covid-19 vaccines: narratives from members of the UK public. Journal of Public Health. 2022.
- Gbeasor-Komlanvi FA, Afanvi KA, Konu YR, Agbobli Y, Sadio AJ, et al Prevalence and factors associated with COVID-19 vaccine hesitancy in health professionals in Togo, 2021. Public Health Pract (Oxf). 2021; 2: 100220.
- Baklouti M, ben Ayed H, Maamri H, Ketata N, Yaich S, et al. Prevalence and Factors Affecting Willingness to Accept or Refuse Vaccination against COVID-19 among Healthcare Professionals in Southern Tunisia. Hosp Top. 2022; 1–10.
- https://datadashboard.health.gov.il/COVID-19/general. Accessed 22 May 2021