
Research Article
Austin J Obstet Gynecol. 2022; 9(3): 1207.
Lysosomal Transcription Factor EB (TFEB) in Normal and Gestational Diabetes Mellitus-Complicated Pregnancies
Helman S¹*, Kochan K¹, Michaeli J¹, Chason I¹, Altarescu G², Farkash R¹, Zeevi D², Herskovitz Y², Barenholz-Goultschin O¹ and Grisaru-Granovsky S¹
1Department of Obstetrics and Gynecology, Shaare Zedek Medical Center Affiliated with the Hebrew University Hadassah School of Medicine, Israel
2Department of Human Genetics, Shaare Zedek Medical Center Affiliated with the Hebrew University Hadassah School of Medicine, Israel
*Corresponding author: Sarit Helman, Shaare Zedek Medical Center, Department of Obstetrics and Gynecology, 12 Shmuel Bait St., P.O. Box 3235, Jerusalem 9103102, Israel
Received: October 19, 2022; Accepted: November 11, 2022; Published: November 18, 2022
Abstract
Objective: This study aimed to examine the expression of Lysosomal Transcription Factor EB (TFEB), a lysosomal autophagy regulator, in the peripheral blood cells of women with Gestational Diabetes Mellitus (GDM) at delivery) andtheir neonates.
Study Design: Single-center, prospective, case-control study of mothers with and without GDM. Maternal and neonatal (umbilical cord) paired blood samples, from term, singleton pregnancies were obtained at delivery. TFEB expresison was evaluated by specific RT-PCR.
Results: Forty-three parturients were enrolled; 21 with GDM. Maternal and neonatal characteristics were comparable. Among women with GDM, 16 (76%) had controlled glycemia; 12 (57.1%) with diet alone. TFEB mRNA expression level for mothers with or without GDM and neonates were similar (p=0.776 and p=0.26, respectively). In maternal and neonatal individual paired samples, the mean difference in TFEB mRNA expression levels were wider in the maternalneonatal (venous-umbilical) samples among women with GDM than non-GDM; the major contributor was the higher mean maternal level in this group. Maternal TFEB expression correlated to first trimester maternal BMI (r=0.050), but not with neonatal birth weight. Mean TFEB was higher among female compared to male newborns.
Conclusion: The maternal TFEB mRNA expression is determined by early gestational BMI in all women, and later augmented in women with GDM as compared to their new-borns. We hypothesize that an impaired adaptive autophagy response to metabolic stress among women with GDM may play a role in the pathogenesis of GDM and offspring late morbidities.
Keywords: Gestational diabetes mellitus; Lysosome; Autophagy; TFEB; Pregnancy
Introduction
Gestational Diabetes Mellitus (GDM) is a heterogeneous entity, which includes carbohydrate intolerance of variable severity with onset or first recognition during pregnancy. Women with a history of GDM, have a higher risk of developing type 2 diabetes and metabolic syndrome later in life [1–4].
Robust evidence has accumulated, suggesting that primary adipose tissue dysfunction and dyslipidaemia, enhanced by pregnancy, can precipitate beta-cell dysfunction and insulin resistance [5-7]. Lysosome autophagy processes are critical physiological mechanisms aimed to protect cells during physiological stress and starvation and to maintain homeostasis [8,9]. One of the main lysosomal autophagy regulator genes is transcription factor EB (TFEB) [13]; member of the MiT family, the microphthalmia subfamily of basic helix-loophelix- leucine zipper (bHLH-zip) transcription factors. TFEB is phosphorylated by mTOR and sequestered in the cytoplasm when an organism is sufficiently fed.
TFEB was shown in animal models to participate in early pregnancy establishment and embryo survival [20]. In humans, TFEB was described in placentas from preeclamptic women [21]. Overall, the role of TFEB during pregnancy has not been well-described and requires additional elucidation.
We hypothesize that an impaired adaptive autophagy response to metabolic stress among women with GDM has a role in the pathogenesis of GDM and later in life with the progression to type 2 diabetes and metabolic syndrome as well as in the late morbidities of the diabetic mother offspring’s [22]. As TFEB is a pivotal intersection for many processes that involve autophagy and little is known about serum TFEB levels in human pregnancies, this study was designed to examine TFEB expression in peripheral blood cells of women with or without GDM and their neonates. Thus, we hypothesized that in pregnancy complicated by GDM the maternal serum level will be similar to the uncomplicated pregnancies or lower.
Methods
This prospective, case-control study was conducted in the Department of Obstetrics and Gynecology at a tertiary care, university-affiliated medical center in Israel, from 2015 through 2016. The study protocol was approved by the Institutional Review Board (protocol number 50/14). Informed consent was obtained from women at admission for delivery.
During the study period, two groups of women with singleton pregnancies, at term (37–41 weeks of gestation) were consecutively approached for study enrolment. The study group included women diagnosed with GDM who were admitted for a planned induction of labor to prevent macrosomia or who presented in active labor. GDM diagnosis was based on an abnormal Oral Glucose Tolerance Test (OGTT) defined by the ADA criteria. The women with GDM were further divided into two groups. The GDM A1 group included women who demonstrated carbohydrate intolerance in an OGTT, but whose fasting and postprandial glucose levels were maintained within physiologic ranges by dietary regulation alone. The GDM A2 group included women with GDM who required insulin or oral hypoglycaemic therapy (glipizide or metformin) in response to repeated elevations of fasting or postprandial glucose levels following dietary intervention [23,24].
For the study, satisfactory glycaemic control was defined as daily fasting glucose profile <95 mg%, or <140 mg% 1 hour after a meal and <120 mg% 2 hours after a meal. Unsatisfactory glycaemic control was noted if the above criteria were not met. The control measurements were based on clinic visits records of at least four measurements daily for at least five days/ week from diagnosis to delivery. Women with no antenatal records regarding glycaemic control during pregnancy were excluded from the study.
The control group included pregnant women without GDM, with uncomplicated pregnancies, admitted in labor. Non-GDM was defined as a documented, normal 50 g GCT (<140 mg/dl). Women in the control group were matched for gestational age with women in Groups A1 and A2, at recruitment (before the study blood sample analyses).
Exclusion criteria were women younger than 18 years-of-age, with other background or pregnancy-related complications, a diagnosis of pre-gestational diabetes mellitus type 1 or 2, or metabolic syndrome.
Paired maternal and umbilical cord (venous) blood samples (5- 10 ml) were collected. Maternal blood samples from consecutive parturient were obtained upon enrolment and from the umbilical cord (venous) at delivery, when feasible. All eligible patients were included, irrespective of the mode of delivery.
Data including maternal characteristics, reproductive history, and information about previous complications during pregnancy, delivery, and the neonatal period, were taken from electronic medical records.
Quantitative PCR Analysis of TFEB Gene Expression
TFEB is expressed in human blood at all stages of life, from early infancy through late adulthood (https://www.nextprot.org/entry/ NX_P19484/expression).
Whole blood (5-10 ml) was collected into Tempus™ Blood RNA Tubes (Life Technologies, Thermo Fisher Scientific, Waltham, MA, USA) from all study groups; stored at -20°C until total RNA extraction with the Tempus™ Spin RNA Isolation Kit (Life Technologies) according to the manufacturer’s protocol. Subsequently, RNA was reverse transcribed into cDNA with ImpromII Reverse Transcriptase (Promega Corp., Madison, WI, USA) for quantitative RT-PCR analysis using TaqMan Real-Time PCR (Life Technologies) chemistry on the 7900HT Real-Time PCR System (Applied Biosystems, Inc.). RNA input was normalized to 50ng ahead of reverse transcription. This preliminary normalization step is expected to control for variation in white blood cell count among the various tested samples. Multiplex assay of gene expression in the presence of a housekeeping gene, in the same reaction well, was used to further control technical variation among samples.
TFEB mRNA expression levels, for each participant in the study, were assessed in triplicate by multiplexing a commercial TaqMan TFEB gene expression assay with FAM-labelled probe (Life Technologies; assay ID: Hs00292981_m1) together with a POLR2A housekeeping gene VIC-labelled probe assay (Life Technologies; assay ID: Hs00172187_m1). TFEB Cycle Threshold (Ct) values were normalized to POLR2A expression, within each PCR reaction well, and relative expression units were calculated by comparing GDM vs. non-GDM 2-ΔΔCt values. RNA quality was determined by 260/280 and 260/230 nm absorbance measurements. A second housekeeping gene, TBP, was used for confirmatory normalization in separate experiments. The results with TBP normalization were essentially the same as with POLR2A normalization.
Maternal and umbilical blood samples were coded. The laboratory staff was blinded to the clinical status of the samples.
Statistical Analysis
This study is an exploratory study. At the time of study design, an estimate of the level of TFEB expression in the study groups was not available. Therefore, calculating a sample size a priori based on existing literature would arguably be unreliable, making the study results difficult to replicate. A contribution of the study is providing an effect size specific to the study demographics, for other investigators to pursue.
The independent variable used in the analyses was GDM status (categorically defined as GDM present or absent). The dependent variable was the TFEB level of expression.
Demographic data, reproductive history, and information on complications during pregnancy, delivery, and the neonatal period are presented as mean ± SD and/ or median and interquartile range for continuous variables, depending on their distribution. Categorical characteristics are displayed as numbers and proportions. Relations between categorical variables were evaluated by Chi-square and Fisher’s exact tests; the effect of categorical variables on continuous measurements was tested by t and Mann-Whitney tests. Associations between continuous variables were assessed by Spearman correlations. Wilcoxon signed ranks test was used to assess the relation between two continuous dependent variables. The p-values presented are 2-sided and p-values < 0.05 were considered statistically significant. All analyses were conducted using IBM SPSS Statistics, v22.0.
Results
During the study period, 43 parturients were enrolled; 21 in the GDM group and 22 in the non-GDM group. Maternal and neonatal characteristics in both groups were similar. Although the mean maternal age and the gestational age at delivery differed statistically among the study groups, they were clinically comparable (Table 1). TFEB level of expression was measured in maternal and umbilical cord/neonatal blood samples. In the GDM group, glycaemic status was well-controlled throughout pregnancy in 76%; 57.1% with diet alone, 23.8% with oral medication and 19.0% with insulin. There was no statistically significant difference in the level of TFEB expression among the GDM vs. non-GDM study groups, for the mothers and the neonates (Table 2). Similarly, there was no difference between the mean maternal vs. neonatal level of TFEB expression between the GDM and the non-GDM groups (0.96±0.15 vs. 0.95±0.41 p=0.407, 0.99±0.16 vs. 1.04±0.17 p=0.469 respectively). The level of TFEB expression was negatively correlated to maternal BMI in the first trimester, r = -0.050. No correlation was found between TFEB expression and neonatal birth weight in the separate study groups. The mean difference in TFEB mRNA expression levels were wider in the maternal-neonatal (venous-umbilical) samples among women with GDM than non-GDM; the major contributor was the higher mean maternal level in this group. The mean TFEB level of expression was lower for the male as compared to the female newborns (male 0.87-1.42 mean 1.01, female 0.87-1.34 mean 1.07).
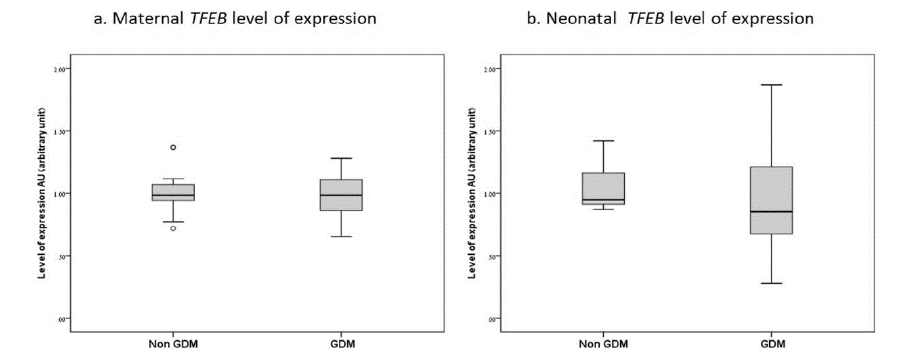
Figure 1:
Characteristics
GDM* (N=21)
Non-GDM (N=22)
P-Value
Maternal
Age, years (mean ± SD)
34.3±6.6
28.6±4.8
0.002
Age > 35 years (%)
42.9%
9.1%
0.11
Education = 12 years (%)
100%
100%
-
Previous miscarriages (% of women in the group)
57.1%
36.4%
0.227
First trimester BMI (mean ± SD)
30±9.3
25.4±3.2
0.500
Third trimester BMI (mean ± SD)
37.8±12
28.7±3.1
0.048
Pregnancy and delivery
Gestational age at delivery, weeks (mean ± SD)
38.7±6.1
39.6±1.1
0.015
Parity (mean ± SD)
5.5±4.4
3.1±2.1
0.132
Spontaneous vaginal delivery (%)
85.7%
100.0%
0.185
Instrumental vaginal delivery (%)
4.8%
0.0%
Ceasrean delivery (%)
9.5%
0.0%
Neonatal
Birth weight, gr (mean ± SD)
3303±522
3364±337
0.653
Macrosomia (> 4,000 gr) (%)
4.8%
0.0%
0.300
Male gender (%)
52.4%
45.5%
0.650
NICU** admission>72 hours (%)
0%
0%
-
5` Apgar score < 7 (%)
0%
0%
-
Shoulder dystocia (%)
0%
0%
-
*GDM-gestational Diabetes Mellitus
**NICU-Neonatal intensive care unit
Table 1: Maternal and neonatal characteristics.
Study group TFEB expression
Mean SD
P-Value
Median [IQR]
Maternal
Non-GDM
0.99±0.16
0.776
0.98 [0.89-1.09]
GDM
0.96±0.15
0.97 [0.84-1.10]
Newborn
Non-GDM
1.04±0.17
0.261
0.94 [0.91-1.17]
GDM
0.95±0.41
0.89 [0.63-1.28]
Table 2: TFEB level of expression in mothers and neonates with/without GDM.
Discussion
The cellular center of autophagy is the lysosome. TFEB, the regulatory master gene of a “lysosomal gene network” mediates the response and adaptation to metabolic challenges [10,11,25]. During late uncomplicated pregnancy, the catabolic state of maternal adipose tissue is characterized by increased triglycerides in plasma, increased production of Very Low-Density Lipoproteins (VLDL) in the liver, combined with reduced adipose tissue Low-Density Lipoproteins (LDL) and decreased hepatic lipase activity [26]. Pregnancies complicated by diabetes are characterized by exaggerated hypertriglyceridemia with a predominance of small dense LDL particles, enhanced levels of non-esterified plasma lipoproteins and ketone bodies. These features are similar to type 2 diabetes and metabolic syndrome [26,27]. Altogether, these findings indicate that altered maternal lipid metabolism, and not only insulin-resistant hyperglycemia might be the pathway to GDM and later to type 2 diabetes and metabolic syndrome.Based on our study we suggest that the lysosmal TFEB similar levels among the study groups may represent impared inducibility of TFEB expression, thus adding to the insulin resistance.
The present study showed that TFEB, peripheral blood lymphocytes expression of mothers with and without GDM in late gestation, and in their neonates, showed a significant positive correlation with the first trimester BMI. High pre-pregnancy body weight and excessive gestational weight-gain are both associated with an increased risk of developing GDM [28].
We did not find a significant correlation between maternal TFEB expression and the GDM women in the third trimester of pregnancy, as compared to the uncomplicated pregnancies. This may be attributed to the limited number of subjects involved in the study, but also to the relatively healthy status of the GDM patient study group, characterized by a combination of high education and lower BMI, together with more than two-thirds of participants with a well-controlled glycaemic state by diet alone. In the present study, we analyzed all GDM patients as a group without correction for therapy covariates; differences due to diet therapy and the drug therapy, the type of oral hypoglycemic drugs and the respective glycemic control may prove important for the TFEB level of expression. This study included the peripheral blood TFEB expression and not the liver, pancreatic or placental tissue expression.
The TFEB expression in umbilical blood between the study groups did not differ or correlate with neonatal birth weights. However, a higher level was observed in the female newborns. This may be a reflection of a sex linked metablic memory.
Future larger studies are required to establish the role of the TFEB-mediated lysosome autophagy in normal and complicated pregnancies, as well as implications for future therapies.
Details of Ethics Approval
The study was approved by the Institutional Review Board for clinical studies of Shaare Zedek Medical Center, Affiliated with the Hebrew University Hadassah School of Medicine, Jerusalem, Israel. Protocol number 50/14.
Author Contributions
KK – Research performance and author of the manuscript
SH – Author of the manuscript, critical review and corresponding author
JM, IC, GA, RF, DZ, YH, OBG – Research performance assistance, manuscript review
SGG – Research concept, manuscript review, and critical appraisal
Conflict of Interest Statement
All authors declare they have no conflict of interest.
Financial Disclosure Statement
No funding or other financial support was received for this work.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- Savona-Ventura C, Chircop M. Birth weight influence on the subsequent development of gestational diabetes mellitus. Acta Diabetol. 2003; 40: 101– 104.
- Claesson R, Åberg A, Maršál K. Abnormal fetal growth is associated with gestational diabetes mellitus later in life: population-based register study. Acta ObstetGynecol Scand. 2007; 86: 652–656.
- Yeung EH, Hu FB, Solomon CG, Chen L, Louis GM, et al. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia. 2010; 53: 668–678.
- Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009; 373: 1773–1779.
- Sartori C, Lazzeroni P, Merli S, Patianna VD, Viaroli F, et al. From Placenta to Polycystic Ovarian Syndrome: The Role of Adipokines. Mediators Inflamm. 2016; 2016: 1–14.
- Moscavitch SD, Kang HC, Filho RAC, Mesquita ET, Neto HCCF, et al. Comparison of adipokines in a cross-sectional study with healthy overweight, insulin-sensitive and healthy lean, insulin-resistant subjects, assisted by a family doctor primary care program. DiabetolMetab Syndr. 2016; 8: 9.
- Kralisch S, Hoffmann A, Lössner U, Stumvoll M, Fasshauer M, et al. Regulation of the novel adipokines/ hepatokines fetuin A and fetuin B in gestational diabetes mellitus. Metabolism. 2017; 68: 88–94.
- Quan W, Jung HS, Lee M-S. Role of autophagy in the progression from obesity to diabetes and in the control of energy balance. Arch Pharm Res. 2013; 36: 223–229.
- Pavel M, Rubinsztein DC. Mammalian autophagy and the plasma membrane. FEBS J. 2017; 284: 672–679.
- Roy S, Leidal AM, Ye J, Ronen SM, Debnath J, et al. Autophagy-Dependent Shuttling of TBC1D5 Controls Plasma Membrane Translocation of GLUT1 and Glucose Uptake. Mol Cell. 2017; 67: 84-95.e5.
- Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013; 14: 283–296.
- Füllgrabe J, Klionsky DJ, Joseph B. The return of the nucleus: transcriptional and epigenetic control of autophagy. Nat Rev Mol Cell Biol. 2014; 15: 65–74.
- Settembre C, Di Malta C, Polito VA, Arencibia MG, Vetrini F, et al. TFEB Links Autophagy to Lysosomal Biogenesis. Science. 2011; 332: 1429–1433.
- Raben N, Puertollano R. TFEB and TFE3: Linking Lysosomes to Cellular Adaptation to Stress. Annu Rev Cell Dev Biol. 2016; 32: 255–278.
- Martina JA, Chen Y, Gucek M, Puertollano R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy. 2012; 8: 903–914.
- Peña-Llopis S, Vega-Rubin-De-Celis S, Schwartz JC, Wolff NC, Tran TAT, et al. Regulation of TFEB and V-ATPases by mTORC1. EMBO J. 2011; 30: 3242–3258.
- Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, et al. A lysosome-tonucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 2012; 31: 1095–1108.
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, et al. Autophagy regulates lipid metabolism. Nature. 2009; 458: 1131–1135.
- Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013; 15: 647–58.
- Steingrímsson E, Tessarollo L, Reid SW, Jenkins NG, Copeland NG. The bHLH-Zip transcription factor Tfeb is essential for placental vascularization. Development. 1998; 125: 4607–16.
- Jebbink JM, Boot RG, Keijser R, Moerland PD, Aten J, et al. Increased glucocerebrosidase expression and activity in preeclamptic placenta. Placenta. 2015; 36: 160–169.
- Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000; 71: 1256S–61S.
- American Diabetes Association AD. Standards of medical care in diabetes--2013. Diabetes Care. 2013; 36: S11-66.
- Gabbe SG, Niebyl JR, Galan HL, Jauniaux ER, Landon MB, Simpson JL D DA. Obstetrics: normal and problem pregnancies. Elsevier Health Sciences, London. 2012.
- Chen L, Wang K, Long A, Jia L, Zhang Y, et al. Fasting-induced hormonal regulation of lysosomal function. Cell Res. 2017; 27: 748–763.
- Herrera E, Ortega-Senovilla H. Disturbances in lipid metabolism in diabetic pregnancy - Are these the cause of the problem? Best Pract Res Clin Endocrinol Metab. 2010; 24: 515–525.
- Rizzo M, Berneis K. Low-density lipoprotein size and cardiovascular risk assessment. QJM An Int J Med. 2006; 99: 1–14.
- Vidanalage CJK, Senarth U, Silva KD, Lekamge U, Liyanage IJ. Effects of initial body mass index on development of gestational diabetes in a rural Sri Lankan population: A case-control study. Diabetes Metab Syndr Clin Res Rev. 2016; 10: S110–S113.