Special Issue - Cancer Vaccines
Austin Oncol Case Rep. 2017; 2(1): 1006.
A New Strategy of Cancer Immunotherapy Combining Hyperthermia/Oncolytic Virus Pretreatment with Specific Autologous Anti-Tumor Vaccination – A Review
Schirrmacher V*, Lorenzen D, Van Gool SW and Stuecker W
Immunological and Oncological Center Cologne (IOZK), Germany
*Corresponding author: Schirrmacher Volker, Immunological and Oncological Center Cologne (IOZK), Cologne, Germany
Received: February 02, 2017; Accepted: February 14, 2017; Published: February 17, 2017
Abstract
This review describes and explains a specific immunotherapy strategy that has been developed at the Immunological and Oncological Center Cologne in Germany. The strategy is based on many years of basic and translational research. It is a highly individualized approach to activate and target the patient’s immune system against the patient’s own tumor. In a first step, the patient’s immune system is conditioned by pretreatment with oncolytic virus in combination with hyperthermia. The second step consists of active-specific autologous anti-tumor vaccination. The vaccine, VOL-DC, consists of autologous Dendritic Cells (DCs) which are loaded with Viral Oncolysate (VOL) from the patient’s tumor cells. VOL transfers to the DCs information about tumor-associated antigens from the patient’s tumor and Pathogen-Associated Molecular Patterns (PAMPs, danger signals) from the virus. The product VOL-DC, which involves Newcastle Disease Virus, has been approved in Germany as an Advanced Therapeutic Medicinal Product for individual treatment by the institution which received this permit. This review includes promising results from case-series studies of glioblastoma patients. It also discusses future strategies for treatment of late-stage disease including combination of this immunotherapy with immune checkpoint blocking antibodies and/or with costimulatory bispecific antibodies.
Keywords: Cancer vaccine; Dendritic cell; Oncolytic virus; Newcastle disease virus; Immunogenic cell death; Memory T cell
Abbreviations
APC: Antigen-Presenting Cell; ATMP: Advanced Therapeutic Medicinal Product; ATV-NDV: Autologous Tumor cell Vaccine modified by infection with NDV; BM: Bone Marrow; Ca: Carcinoma; COMP: Committee for Orphan Medical Products; CRC: Colorectal Carcinoma; CTL: Cytotoxic T Lymphocyte; DAMP: Damage- Associated Molecular Pattern; DC: Dendritic Cell; DFS: Disease- Free Survival; ELISPOT: Enzyme-Linked Immuno-Spot; EMA: European Medicines Agency; ER: Endoplasmic Reticulum; GBM: Glioblastoma Multiforme; GMP: Good Manufacturing Practice; HLA: Human Lymphocyte Antigen; ICD: Immunogenic Cell Death; IFN: Interferon; IFNRA: α chain of the type I Interferon Receptor; IOZK: Immunological and Oncological Center Cologne; IPS: IFN-ß Promoter Stimulator; IRF: Interferon Regulatory Factor; ISG: IFNStimulated Gene; ISRE: IFN-Stimulated Response Element; mEHT: modulated Electrohyperthermia; MHC: Major Histocompatibility Complex; MTC: Memory T Cell; NDV: Newcastle Disease Virus; NK cell: Natural Killer cell; OS: Overall Survival; OV: Oncolytic Virus; PAMP: Pathogen-Associated Molecular Pattern; PFS: Progression-Free Survival; RIG: Retinoic acid Inducible Gene; STAT: Signal Transducers and Activators of Transcription; TAA: Tumor Associated Antigen; TIL: Tumor-Infiltrating Lymphocyte; TLR: Toll- Like Receptor; TF: Transcription Factor; Th: T-helper cell; Treg: T regulatory cell; VOL: Viral Oncolysate; VOL-DC: Dendritic Cell pulsed with VOL
Introduction
Immunotherapy of cancer holds promise for cancer patients as a new biological treatment procedure. In contrast to surgery, chemoand radiotherapy - present-day standard therapies - which focus only on the tumor, immunotherapy is concerned with both, the tumor and the tumor-bearing host. Tumor-host interactions are particularly relevant in the fight against metastatic disease. Because the immune system is systemic it has the potential to protect against systemic disease such as metastasis.
Results of recent clinical studies involving novel immunotherapy strategies such as immune checkpoint blockade and adoptive T-cell transfer approaches have clearly established immunotherapy as an important modality for the treatment of cancer [1]. Immunotherapy can complement the traditional approaches of standard therapy or small molecule targeted therapy or it can be employed as monotherapy. To date, immunotherapy has been shown to be capable of inducing durable clinical benefit, although only in a fraction of the patients. The challenge, therefore, is how to increase the fraction of responding patients. It is generally accepted that the ultimate goal and read-out of immunotherapy is the increase in median Overall Survival (OS) and particularly in the percentage of patients with longterm survival. There is no doubt that this necessitates new strategies for personalization and combinatorial approaches [2,3].
A personalized immunotherapy approach is what the Immunological and Oncological Center (IOZK) in Cologne, Germany, is aiming at. It has developed a treatment technology involving the patient’s tumor cells, the patient’s Dendritic Cells (DCs) and an Oncolytic Virus (OV) [4,5] to produce a vaccine called VOLDC. VOL stands for Viral Oncolysate and the virus used is the bird virus Newcastle Disease Virus (NDV). Treatment of cancer patients by NDV oncolysate vaccines without DCs, performed in the 1970s, had already given interesting results [6,7] but for decades thereafter there had been only little interest in OVs. A revival of interest occurred luckily in the 2000s with more knowledge about OVs and new technologies, such as DC culture [8].
VOL-DC is a DC1 polarized vaccine which presents Tumor- Associated Antigens (TAAs) from the patient’s tumor to the patient’s T cells and activates the latter to TAA-specific T helper cells and Cytotoxic T Lymphocytes (CTLs). After several such vaccinations, the patient’s immune system is enriched with TAA-specific Memory T Cells (MTCs) which help to protect against possibly disseminated tumor cells and micro-metastases. It is this immunological memory which distinguishes immunotherapy from conventional therapies and provides a basis for improvements in long-term survival.
VOL-DC is a licensed Advanced Therapeutic Medicinal Product (ATMP) in Northern-Westfalia, Germany, and applied to patients at IOZK on a compassionate use basis.
Immune modulation by hyperthermia/oncolytic virus pretreatment
Active specific immunization of cancer patients requires an immune system which is competent and not dysregulated. Therefore, before patients at IOZK receive vaccinations, their immune system is assessed in depth. Immune modulation/conditioning of the immune system 1 week before vaccination is done by modulated Electrohyperthermia (mEHT) combined with systemic (i.v.) application of NDV [5].
Modulated electrohyperthermia: Hyperthermia with a tissue temperature of 38,5 to 40,5°C can activate the immune system [9,10]. mEHT is combined with systemic oncolytic NDV virotherapy based on the observations that mEHT can enhance virus tumor targeting [10] and virus replication [11]. mEHT is applied either locally or systemically, depending on the clinical situation. Viral infection of tumor cells and hyperthermia with a radiofrequency of 13 MHz cause an Endoplasmic Reticulum (ER) stress response, modify the surface properties of tumor cells and induce Immunogenic tumor Cell Death (ICD) mechanisms [4,5,12].
Systemic NDV application: It is the high safety profile which allows the avian paramyxovirus NDV to be applied to cancer patients [5]. The reported side effects after experience with clinical application for 50 years [7] are grade 1 and 2. Special characteristics of NDV include:
i) Lack of gene exchange via recombination,
ii) Lack of interaction with host cell DNA,
iii) Viral replication in the cytoplasm of cells being independent of host cell proliferation and
iv) Tumor selectivity of virus replication and of host cell lysis [5,7].
Identified mechanisms that can explain tumor selectivity of virus replication and oncolysis in tumor cells of non-permissive hosts such as man involve:
i) Defects in activation of anti-viral signaling pathways,
ii) Defects in type I IFN signaling pathways and
iii) Defects in apoptotic pathways [5].
Systemic NDV application has the following positive effects:
i) Induction of type I Interferons (IFNs) [13] which inhibit secretion of Th2 cytokines (IL-4 and IL-5), stimulate Th1 cells and counter-act Treg cells,
ii) Induction of ICD in virus-infected tumor cells [4,5,7,14] and
iii) Priming of NDV Viral Oncolysate (VOL) - reactive T cells which can be monitored by an in vitro ELISPOT assay [15].
Immune modulation prior to specific vaccination has a preconditioning effect on the patient’s immune system. The induction of VOL-reactive T cells will create systemic recall responses at the site of VOL-DC vaccination and thereby enhance DC migration to the draining lymph nodes with improved antitumor efficacy [16].
Concept of the DC vaccine VOL-DC
Tumorlysate-pulsed DCs as APCs for TAAs: Human TAAs were first described in the early 1990s. It formed the basis for a new era of molecularly defined tumor immunology. In the following years, numerous TAAs, either uniquely expressed or more common, have been characterized and respective HLA-restricted epitopes or neo-epitopes that are capable of triggering CD8+ and CD4+ T cell responses have been identified.
With the introduction of dendritic cell culture and the demonstration of DC’s T cell stimulatory capacity [8], methods have been developed to load DCs with TAAs or tumorlysate [17] and to use them as professional Antigen-Presenting Cells (APCs) for the de novo induction of TAA-specific T cell responses in cancer patients.
We decided to use autologous tumorlysate instead of defined TAAs to produce APCs based on the following findings:
1. Upon loading with crude tumor lysate proteins, human DCs cross-present CD8+ T cell epitopes in the context of MHC class I and present CD4+ T cell epitopes in the context of MHC class II. This was shown to be an active metabolic process leading to functional DCs able to induce TAA-specific CD8+ CTL activity [18].
2. Using a short-term IFN- γ ELISPOT assay, cancer-reactive MTCs from patient’s Bone Marrow (BM) were analyzed by stimulation with tumorlysate-pulsed DCs. Altogether, T cells reactive against the entirety of TAAs could be detected in about 40% of primary operated breast cancer patients. These existed at frequencies of 1:200 to 1:10,000 of total T cells and included CD8+ CTL and CD4+ Th cells [19].
3. The memory T-cell repertoire of the BM of primary operated breast cancer patients was analyzed in more detail and compared to that of healthy female donors. This was done by tumorlysate-pulsed DCs and DCs pulsed with HLA-A2-peptides from 10 different breast TAAs and a variety of normal breast tissue-associated antigens. The T-cell repertoire was highly polyvalent and exhibited pronounced inter-individual differences in the pattern of TAAs recognized by each patient [20].
4. In comparison to reactivity towards TAAs, reactivity to normal breast tissue-associated antigens was much lower. Healthy individuals also contained TAA-reactive T cells but this repertoire was more restricted and the frequencies were in the same low range as T cells reacting to normal breast tissue-associated antigens [20]. Since there was hardly a repertoire of MTCs reacting to normal tissue antigens, it does not seem necessary to purify single TAAs from tumor cells for vaccination purposes to avoid reactivity to tumor-derived normal tissue antigens.
Taken together, the analysis revealed that the BM appears as a lymphoid organ particularly involved in the induction and/or maintenance of natural T cell immunity against tumor antigens [19]. The spontaneous generation of cancer-reactive MTCs appears as a physiological process, also independent of TAA purification. The analyses involving tumorlysate-pulsed DCs revealed
i) That tumorlysate can serve as a source of TAAs,
ii) That tumorlysate-pulsed DCs as APCs do not induce autoimmune reactivity and
iii) That tumorlysate makes it unnecessary to purify TAAs.
Tumorlysate obtained from heat-stressed tumor cells was found to generate more potent DC vaccines than other antigen-loading strategies [21].
Viral oncolysate for DC programming, polarization and TAA information transfer: To create an effective DC vaccine it is important to avoid that the antigen-loaded cells induce tolerance instead of an immune responses. DCs are not only activators but also modulators of immune responses. Depending on the context in which DCs interact with antigens they will affect T helper precursor cells in different ways, for instance to differentiate into Th1, Th2, Th17 or Treg.
To avoid a wrong polarization of T helper cells we decided to introduce so called “danger signals” into the DC vaccine by virus infection. Foreign viral non-capped RNA in the cytoplasm of a cell infected by an RNA virus is a Pathogen-Associated Molecular Pattern (PAMP) that stimulates innate immunity [5]. Thus, VOL-DCs consist of three components: autologous tumor cells as a source of tumorcharacteristic TAAs, patient-derived DCs as professional APCs and the oncolytic virus NDV do deliver PAMPs. In addition, the emission of Damage-Associated Molecular Patterns (DAMPs) following ICD favors the establishment of a productive interface with the immune system [5,22]. Further features of NDV relate to the immune response:
i) Up-regulation of MHC I,
ii) Activation of NK cells,
iii) Activation of monocytes,
iv) Activation of DCs,
v) Costimulation of CD4+ and CD8+ T-cells [5].
This results in the elicitation of tumor-targeting immune responses associated with the potential to eliminate residual, treatment-resistant cancer cells and to establish tumor-reactive immunological memory.
NDV viral oncolysate (VOL) from autologous tumor cells is used to load patient-derived immature DCs. The material is taken up by macropinocytosis and processed along endogenous professional antigen-presentation pathways for generation of HLA-class II peptide complexes and along cross-presentation pathways to generate HLAclass I peptide complexes. VOL also contains viable NDV.
With the viral oncolysate the DCs receive information about the patient’s own tumor, in particular about its individually specific and common TAAs. The rationale for this autologous approach has been summarized [23]. New findings about the individuality of genetic and immunobiological changes in human tumor cells support this rationale. For instance, a dominance of mutation-derived unique tumor neo-antigens was described in an analysis of 598 human colorectal carcinomas indicating that cancer vaccination requires individualized strategies [24].
Importance of NDV-induced signaling through RIG-I and type I interferon receptor: The bird virus NDV induces in DCs and other normal cells from non-permissive hosts such as man a strong type I interferon response. This response which occurs within 18 hrs after infection consists of an early phase and a late phase [25].
The early phase is initiated through the recognition of viral RNA by two types of pathogen recognition receptors:
i) Endosomal Toll-Like Receptors (TLRs), particularly TLR3,7 and 8 and
ii) Cytoplasmic Retinoic acid Inducible Gene I (RIG-I) receptors. RIG-I binds specifically to RNA containing 5`-phosphate, such as viral RNA. Mammalian RNA is either capped or contains base modifications. RIG-I triggering involves the upregulation of RIG-I RNA copies. Once activated, RIG-I binds to the adaptor protein IFN-ß Promoter Stimulator-1 (IPS-1) which, after a further signaling cascade, activates the IFN regulatory factor IRF-3. This Transcription Factor (TF) is then phosphorylated, translocates to the nucleus and induces the IFN response [25].
During the late phase of the IFN response, the type I IFN molecules secreted during the early phase (IFN- α and IFN-ß) interact with the cell surface expressed α-chain of the type I Interferon Receptor (IFNRA). This initiates an amplification loop of the IFN response, which involves Signal Transducers and Activators of Transcription (STAT) proteins and the IFN regulatory factor IRF-7. Upon ligand binding, receptor associated Tyk2 and JAK1 become activated by transphosphorylation. These in turn phosphorylate STAT1 and STAT2. These STAT proteins then heterodimerize and form a complex with the IFN regulatory factor IRF-9 known as ISGF3. It translocates to the nucleus to bind to the IFN-Stimulated Response Element (ISRE) and directs the expression of IFN-Stimulated Genes (ISGs) that create the antiviral state in the target cells which blocks viral replication [25].
The effect of NDV on human DCs has been studied in a very sophisticated way using technologies developed by systems biology [26]. NDV was considered a prototype avian virus to study an uninhibited cellular response to virus infection. The analysis revealed that the genetic program underlying the anti-viral cell-state transition during the first 18 h post-infection can be explained by a single convergent regulatory network involving 24 critical TFs. These factors regulated 779 of the 1351 up-regulated genes. Many of these genes were associated with polarization of the DCs towards DC1 and for induction of Th1 T-cell mediated immune responses.
Such programming and polarization of human DCs by NDV to DC1 was confirmed in functional studies [27].
Signaling through RIG-I and IFNRA plays an important role for immune activation by the avian virus NDV in man. They also play an important role for immune evasion by the primate virus Ebola. The latter does not induce type I IFN responses in man as it has viral proteins that specifically and strongly interfere with RIG-I and IFNRA signaling [28].
Preparation of VOL-DCs and functional tests
Preparation: In 2015, IOZK succeeded in producing NDV from cell culture at high titers and with high purity according to GMP guidelines. This is worldwide achieved for the first time. Also, the methodology to produce VOL-DC has meanwhile been established and quality certified as an ATMP at the IOZK in Germany.
Figure 1 illustrates the main features for the preparation of the three-component vaccine, exemplified for Glio Blastoma Multiforme (GBM). Following consent between the patient, the operating institution and IOZK, a sample of freshly operated tumor is received in IOZKs GMP facility for further processing via cell separation, cell culture, cell characterization and virus infection. The viral oncolysate is freeze-thawed to become devoid of viable tumor cells and then co-incubated with immature DCs from a short-term culture of a sample of the patient’s white blood cells. After a further maturation step, the vaccine VOL-DC is ready for intradermal application to the patient. The differentiation process from adherent monocytes (CD14++,CD86+,CD209-,CD83-) via semiadherent immature DCs (CD14+,CD86+,CD209++,CD83-) to floating mature DCs (CD14- ,CD86++,CD209+,CD83++) is followed by flow cytometry.
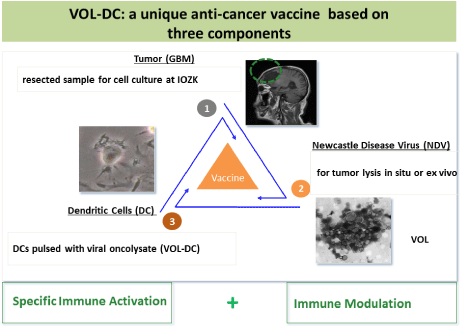
Figure 1: Main features for the preparation of the three-component vaccine
VOL-DC.
Functional tests: A study with memory T cells from breast cancer patients compared the stimulatory capacity of VOL-DCs to that of tumorlysate-pulsed DCs. Stimulation with VOL-DCs showed increased expression of costimulatory molecules and induced higher IFN-γ ELISPOT responses. Supernatants from co-cultures of MTCs and VOL-DCs contained increased titers of IFN-α and IL-15 [29].
The study revealed that VOL-DCs are superior to tumorlysatepulsed DCs and potently stimulate MTCs from cancer patients.
Clinical studies
Pre-studies with ATV-NDV: The development of the threecomponent vaccine VOL-DC is an extension from the twocomponent vaccine ATV-NDV. This term stands for Autologous Tumor Vaccine modified by infection with NDV. The rationale for using autologous cancer vaccines is based on experience from animal tumor studies. Pre-clinical results from post-operative vaccination studies in mouse and guinea pig models of metastasizing cancers revealed that protective anti-tumor immunity could be induced by autologous but not allogeneic vaccines. The induced protective immunity was highly specific for the autologous tumor [23].
Table 1 lists examples of clinical vaccination trials employing the whole cell tumor vaccine ATV-NDV [30-36]. Here we want to comment only two of these studies. In Study 6, 23 patients suffering from GBM were vaccinated with ATV-NDV from cell culture to assess feasibility, safety and clinical benefit [35]. The median Progression-Free Survival (PFS) of vaccinated patients was 40 weeks (versus 26 weeks in 87 non-vaccinated control subjects from the same time period and the same clinic). The median Overall Survival (OS) was 100 weeks (25 months) (versus 49 weeks (12 months) in control subjects; p<0.001). In the vaccinated group, immune monitoring revealed significant increases of skin DTH reactivity, of the number of tumor-reactive MTCs in the blood and of the number of CD8+ Tumor-Infiltrating T-cells (TILs) in frozen tissue slices from GBM recurrences. There was one complete remission of non-resectable remaining tumor [35].
Study-Disease
Study Type
Clinical Outcome
Ref.
1. Breast Ca (locally advanced)
Phase II (n = 32)
Improved OS and DFS
30
2. CRC (locally advanced) (stage II & III)
Phase II (n = 57)
Improved OS and DFS
31
3. CRC (R0 res liver mets) (stage IV)
Phase II (n = 23)
Improved OS and DFS
32
4. Pancreatic Ca (G3)
Phase II (n = 53)
Improved OS and DFS
33
5. Head and Neck Ca (stage III + IV)
Phase II (n = 18)
Improved OS and DFS
34
6. Glioblastoma multiforme (GBM)
Phase II (n = 23)
Improved OS and PFS
35
Phase II/III (n = 51)
36
A prospective randomized-controlled study, stratified for :
Colon Ca (R0 res liver mets)
Improved OS (p = 0.01)
Rectum Ca (R0 res liver mets)
Not significant
All studies were performed post-operatively in the adjuvant situation as prophylaxis against metastases; they were approved by local ethic committees. All autologous NDV virus modified tumor cell vaccines were applied intradermally. CRC: Colorectal Carcinoma; DFS: Disease-Free Survival; OS: Overall Survival; PFS: Progression-Free Survival.
Table 1: Clinical pre-studies with the vaccine ATV-NDV.
Study 7 (Table 1), a prospectively randomized Phase II/III trial, investigated the efficiency of ATV-NDV after liver resection for hepatic metastases of Colorectal Carcinoma (CRC) as a tertiary prevention method [36]. 25 of such stage IV CRC patients were vaccinated and compared with a similar number of non-vaccinated otherwise comparable patients. After an exceptionally long follow-up period of 9 to10 years, there was no significant difference between the vaccinated and the control arm when comparing all patients. However, when stratified for tumor location, there were significant differences between colon and rectum. While there was no significant difference for rectal cancer, a significant benefit was seen in the colon cancer subgroup in terms of long-term metastasis-free survival and OS. In the control arm, 78.6% had died, in the vaccinated arm only 30.8% [36].
The trial is a good example of Evidence Based Medicine. It provides clinical evidence for the value and potential of the cancer vaccine ATV-NDV.
New results from case series studies with VOL-DC at IOZK: IOZK exists since more than 10 years as a private praxis in Cologne. During this time more than 1200 cancer patients were treated with biological therapies. Among them were more than 70 different types of cancer. Since this treatment is highly individual the results can only be evaluated as single case or as case-series studies. Two remarkable single cases were recently published. One describes long-term remission of prostate cancer with extensive bone metastases [37], the other reports long-term survival of a breast cancer patient with extensive liver metastases [15].
Now we summarize new results from a retrospective case-series of GBM patients treated at IOZK between 2006 and 2010. Figure 2 shows the results of a Kaplan-Meier survival analysis of 10 newly diagnosed operated patients. Median OS was 30 months in comparison to 14.6 months after standard radio/chemotherapy according to the Stupp protocol [38]. The 5-year survival of primary operated GBM in the current series with combinatorial immunotherapy was almost 20%.
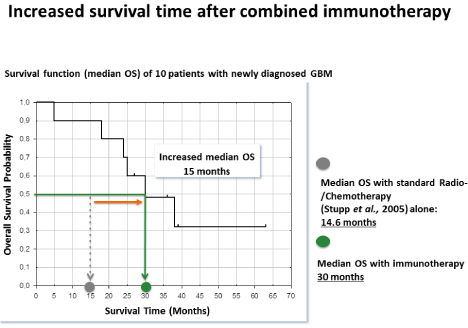
Figure 2: The first Kaplan-Meier analysis of OS probability versus survival
time of n = 10 newly diagnosed GBM patients in comparison to standard
radio/chemotherapy. These data derive from patients treated before the time
of the GMP certificate.
Of importance is also the clinical safety profile. Most side effects related to immunotherapy were moderate (common cytotoxicity criteria grade 1-2) short term flu-like symptoms (fever, headaches and chill). There was no negative impact on quality of life.
Future phase II/III clinical study design from Deltavir
Delta-Vir GmbH is a start-up company from IOZK at the BIOCITY Leipzig (Germany). Its aim is to expand the immunotherapy developed at IOZK and to evaluate the treatment concept in clinical studies. Deltavir has produced a dossier for the European Medicines Agency (EMA) which was accepted by the Scientific Advice Procedure. Deltavirs product for the treatment of GBM has also been presented to the Committee for Orphan Medical Products (COMP).
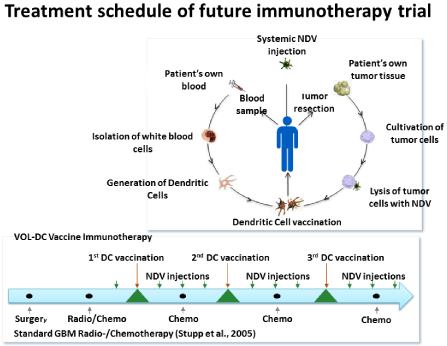
Figure 3 illustrates the study design for a randomized 2 arm study of GBM comparing standard therapy against standard treatment plus treatment with NDV and VOL-DC. The primary endpoint is OS after 2 years and the secondary endpoints are PFS and quality of life.

Figure 3: Study design for a future randomized 2 arm study by Delta-Vir
GmbH. Comparison of combinatorial immunotherapy with NDV and VOL-DC
plus standard treatment versus standard treatment alone.
Counteracting tumor-induced resistance
Breaking tumor resistance by oncolytic NDV: Many characteristics of oncolytic NDV suggest that this biological agent has the potential to break resistance of cancer cells to a variety of therapies. Some of these are summarized in Table 2. Since this virus replicates in the cytoplasm of tumor cells, it is independent of DNA replication. The virus replicates perfectly well in X-irradiated tumor cells, for instance in cells of the vaccine ATV-NDV. Many tumor cells in a resting state such as tumor stem cells or cells from tumor dormancy are not affected by chemo- or radio-therapy, but are still targeted by oncolytic NDV.
1. Oncolysis by NDV is independent of cell proliferation. This is in contrast to chemo- and radio- therapy.
2. Oncolytic NDV has the potential to target cancer stem cells which are in a resting state of the cell cycle.
3. Oncolytic NDV shows selectivity for apoptosis-resistant cells [39].
4. Oncolytic NDV can exert activity against hypoxic cancer cells [40].
5. Oncolysis by NDV is supported by tumor resistance to type I interferon [25].
6. GBM is characterized by Rac1 protein expression and aberrant signaling which facilitates tumor cell motility and invasion. Interestingly, Rac1 is targeted by NDV infection [41].
7. T-cell tolerance to tumor-associated antigens could be broken by tumor cell infection with NDV [42].
8. Systemic tumor resistance to immune checkpoint blockade immunotherapy could be overcome by oncolytic virotherapy with NDV [43].
Table 2: Breaking tumor therapy resistance by oncolytic NDV.
Of special interest are also recent findings demonstrating that oncolytic NDV selectively infects apoptosis-resistant [39], hypoxic [40] or type I interferon-resistant tumor cells [25]. This oncolytic virus could therefore very well complement conventional therapies which lack these properties. NDV seems particularly suited to treat GBM tumors. GBM is characterized by Rac1 protein expression and aberrant signaling which facilitates tumor cell motility and invasion. It is exactly this Rac1 protein which is targeted by NDV infection [41].
NDV may even break resistance mechanisms to immunotherapy. T-cell tolerance (non-responsiveness) to melanoma-associated TAAs could be overcome by melanoma cell infection with NDV [42]. Furthermore, localized oncolytic virotherapy with NDV was demonstrated to overcome even systemic tumor resistance to immune checkpoint blockade [43].
These findings suggest combining hyperthermia/NDV pretreatment and VOL-DC with immune checkpoint blocking antibodies. The former procedure induces TILs and the latter prevents negative influences from tumor cells against the TILs.
Increasing T cell costimulatory signals: T cell costimulation (via signal 2) is necessary to induce a response of naïve T cells. When costimulatory signals are lacking, for instance on a TAA-presenting tumor cell, interactions with TAA-specific T cells (signal 1 only) render the T cell anergic: the T cell becomes refractory to signals even when the antigen-specific T-cell receptor interacts with TAA. This situation has been described in cancer patients, especially in chronic situations of advanced disease [44]. T cell anergy [45] may exist in different states, not all of them being irreversible. For instance, tumor-reactive MTCs from draining lymph nodes of carcinoma patients could be re-activated in a short-term ELISPOT assay using as vaccine ATV-NDV with optimized signals 1 and 2, but not with a similarly modified vaccine from an unrelated tumor cell line [46].
We selected the viral Hemagglutinin-Neuraminidase (HN) protein of NDV as a universal anchor molecule of a vaccine to attach costimulatory molecules. This has the advantage that any NDV-infected tumor vaccine could be further modified by such HN binding costimulatory molecules. The design and production of a bispecific single-chain anti-HN-anti-CD28 fusion protein (bsHNCD28) which can easily attach to the vaccine ATV-NDV has been described [47].
Whether strong T cell costimulation can cause re-activation of unreactive, possibly anergized memory T cells from late-stage cancer patients has been investigated in CRC patients [48]. 14 CRC patients with late-stage disease which could not be operated anymore with curative intent were treated with the newly modified vaccine ATVNDV- bsHN-CD28. No severe adverse events were observed. While before vaccination, none of the patients showed immunological responsiveness of blood-derived T cells in ELISPOT assays, after vaccinations all patients showed an immunological response of bloodderived T cells, at least once during the course of five vaccinations.
Also, we demonstrated a dose-response relationship with the purified costimulatory molecule bsHN-CD28 when added to the vaccine. Within the first 3 days after the first vaccination there was a strong and significant increasing of blood-circulatory TAA-peptidespecific T cells. Such responses were observed in ELISPOT assays to DCs pulsed either with autologous tumorlysate or with 20mer peptides from defined common TAAs such as p53, CEA, Her-2/ neu and Mage-3 [48]. A partial response (>30% decrease, RECIST criteria) of metastases was documented in four patients.
The study suggests that the three-component vaccine is safe and can re-activate possibly anergized T cells in advanced-stage cancer [48].
Summary and conclusions
This review is based on more than three decades of pre-clinical and translational research. The overall goal is a contribution to establishing immunotherapy as a new standard treatment of cancer. Although this may still be a long way to go, it will be to the benefit of cancer patients. This conviction is based on the fact that the immune system functions in a systemic way, is capable of distinguishing tumor from normal tissue and is equipped with a specific memory function.
Following a description of our rationale, we present results from clinical studies. These include pre-studies with the autologous virusmodified tumor cell vaccine ATV-NDV, developed and tested in Heidelberg, Germany, as well as new results from case-series studies with the three-component dendritic cell vaccine VOL-DC, developed and tested in Cologne, Germany.
The experience obtained at IOZK with the described combinatorial immunotherapy involving hyperthermia, oncolytic virus and autologous VOL-DC vaccines is promising. There were surprising responses in cases in which conventional therapies had failed. The evaluation of the case-series from GBM suggests clearcut prolongation of median OS. Of further importance is that the immunotherapy is well tolerated and does not have a negative impact on the patient’s life style.
Concerning future developments, we propose two further strategies:
i) To combine such immunotherapy with procedures to counteract tumor-induced breaks and
ii) To provide additional T-cell co-stimulatory signals to overcome T-cell anergy. Immune checkpoint blockade will reduce negative signals while costimulation will provide positive signals to TILs. The outcome in the tumor microenvironment will likely depend on the ratio of positive to negative signals. Both strategies appear relevant, in particular in stages of late-stage disease in which other types of therapies have failed.
References
- Huber C, Rammensee HG, Wölfel T, Britten CM. Krebs-Immuntherapien: Standards und Innovationen. Deutscher Ärzteverlag. 2008.
- Sathyanarayanan V, Neelapu SS. Cancer immunotherapy: Strategies for personalization and combinatorial approaches. Mol Oncol. 2015; 9: 2043-53.
- Reardon DA, Gilbert MR, Wick W, Liau L. Immunotherapy for neuro-oncology: the critical rationale for combinatorial therapy. Neuro Oncol. 2015.
- Fournier P, Schirrmacher V. Oncolytic Newcastle Disease Virus as Cutting Edge between Tumor and Host. Biology (Basel). 2013; 2: 936-975.
- Schirrmacher V. Oncolytic Newcastle disease virus as a prospective anti-cancer therapy. A biological agent with potential to break therapy resistance. Expert Opin Biol Ther. 2015; 15: 1757-1771.
- Murray DR, Cassel WA, Torbin AH, Olkowski ZL, Moore ME. Viral oncolysate in the management of malignant melanoma. II. Clinical studies. Cancer. 1977; 40: 680-686.
- Schirrmacher V. Fifty Years of Clinical Application of Newcastle Disease Virus: Time to celebrate! Biomedicines. 2016.
- Steinmann RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006; 311:17-58.
- Weigelin B, Krause M, Friedl P. Cytotoxic T lymphocyte migration and effector function in the tumor microenvironment. Immunol Lett. 2011; 138: 19-21.
- Chang E, Chalikonda S, Friedl J, Xu H, Phan GQ, Marincola FM, et al. Targeting vaccinia to solid tumors with local hyperthermia. Hum Gene Ther. 2005; 16: 435-444.
- Eisenberg DP, Carpenter SG, Adusumilli PS, Chan MK, Hendershott KJ, Yu Z, et al. Hyperthermia potentiates oncolytic herpes virus killing of pancreatic cancer through a heat shock protein pathway. Surgery. 2010; 148: 325-334.
- Kroemer G, Galuzzi I, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Ann Rev Immunol. 2013; 31: 51-72.
- Fournier P, Arnold A, Wilden H, Schirrmacher V. Newcastle disease virus induces pro-inflammatory conditions and type I interferon for counter-acting Treg activity. Int J Oncol. 2012; 40: 840-850.
- Koks CA, Garg AD, Ehrhardt M, Riva M, Vandenberk L, Boon L, et al. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer. 2015; 136: E313-325.
- Schirrmacher V, Stücker W, Lulei M, Bihari AS, Sprenger T. Long-term survival of a breast cancer patient with extensive liver metastases upon immune and virotherapy: a case report. Immunotherapy. 2015; 7: 855-860.
- Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015; 519: 366-369.
- Nava S, Lisini D, Pogliani S, Dossena M, Bersano A, Pellegatta S, et al. Safe and Reproducible Preparation of Functional Dendritic Cells for Immunotherapy in Glioblastoma Patients. Stem Cells Transl Med. 2015; 4: 1164-1172.
- De Vleeschouwer S, Arredouani M, Adé M, Cadot P, Vermassen E, Ceuppens JL, et al. Uptake and presentation of malignant glioma tumor cell lysates by monocyte-derived dendritic cells. Cancer Immunol Immunother. 2005; 54: 372-382.
- Schirrmacher V. Cancer-reactive memory T cells from bone marrow: Spontaneous induction and therapeutic potential(Review). Int J Oncol. 2015; 47: 2005-2016.
- Sommerfeld N, Schütz F, Sohn C, Forster J, Schirrmacher V, Beckhove P. The shaping of a polyvalent and highly individual T-cell repertoire in the bone marrow of breast cancer patients. Cancer Res. 2006; 66: 8258-8265.
- Wei FQ, Sun W, Wong TS, Gao W, Wen YH, Wei JW, et al. Eliciting cytotoxic T lymphocytes against human laryngeal cancer-derived antigens: evaluation of dendritic cells pulsed with a heat-treated tumor lysate and other antigen-loading strategies for dendritic-cell-based vaccination. J Exp Clin Cancer Res. 2016; 35: 18.
- Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San Pedro JM, et al. Molecular and Translational Classifications of DAMPs in Immunogenic Cell Death. Front Immunol. 2015; 6: 588.
- Schirrmacher V, Fournier P, Schlag P. Autologous tumor cell vaccines for post-operative active-specific immunotherapy of colorectal carcinoma: long-term patient survival and mechanism of function. Expert Rev Vaccines. 2014; 13: 117-130.
- Angelova M, Charoentong P, Hackl H, Fischer ML, Snajder R, Krogsdam AM, et al. Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biol. 2015; 16: 64.
- Fournier P, Wilden H, Schirrmacher V. Importance of retinoic-acid-inducible gene I and of receptor for type I interferon for cellular resistance to infection by Newcastle disease virus. Int J Oncol. 2012; 40: 287-298.
- Zaslavsky E, Hershberg U, Seto J, Pham AM, Marquez S, Duke JL, et al. Antiviral response dictated by choreographed cascade of transcription factors. J Immunol. 2010; 184: 2908-2917.
- Fournier P, Arnold A, Schirrmacher V. Polarization of human monocyte-derived dendritic cells to DC1 by in vitro stimulation with Newcastle Disease Virus. J BUON. 2009.
- Schirrmacher V. Signaling through RIG-I and type I interferon receptor: Immune activation by Newcastle disease virus in man versus immune evasion by Ebola virus (Review). Int J Mol Med. 2015; 36: 3-10.
- Bai L, Koopmann J, Fiola C, Fournier P, Schirrmacher V. Dendritic cells pulsed with viral oncolysate potently stimulate autologous T cells from cancer patients. Int J Oncol. 2002; 21: 685-694.
- Ahlert T, Sauerbrei W, Bastert G, Ruhland S, Bartik B, Simiantonaki N, et al. Tumor cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J Clin Oncol. 1997; 15: 1354-1366.
- Ockert D, Schirrmacher V, Beck N, Stoelben E, Ahlert T, Flechtenmacher J, et al. Newcastle disease virus-infected intact autologous tumor cell vaccine for adjuvant active specific immunotherapy of resected colorectal carcinoma. Clin Cancer Res. 1996; 2: 21-28.
- Schlag P, Manasterski M, Gerneth T, Hohenberger P, Dueck M, Herfarth C, et al. Active specific immunotherapy with Newcastle-disease-virus-modified autologous tumor cells following resection of liver metastases in colorectal cancer. Cancer Immunol Immunother. 1992; 35: 325-330.
- Schirrmacher V, Ahlert T, Pröbstle T, Steiner HH, Herold-Mende C, Gerhards R, et al. Immunization with virus-modified tumor cells. Semin Oncol. 1998; 25: 677-696.
- Karcher J, Dyckhoff G, Beckhove P, Reisser C, Brysch M, Ziouta Y, et al. Antitumor vaccination in patients with head and neck squamous carcinomas with autologous virus-modified tumor cells. Cancer Res. 2004; 64: 8057-8061.
- Steiner HH, Bonsanto MM, Beckhove P, Brysch M, Geletneky K, Ahmadi R, et al. Anti-tumor vaccination of patients with glioblastoma multiforme: a pilot study to assess feasibility, safety, and clinical benefit. J Clin Oncol. 2004; 22: 4272-4281.
- Schulze T, Kemmner W, Weitz J, Wernecke KD, Schirrmacher V, Schlag PM. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother. 2009; 58: 61-69.
- Schirrmacher V, Bihari AS, Stücker W, Sprenger T. Long-term remission of prostate cancer with extensive bone metastases upon immuno- and virotherapy: A case report. Oncol Lett. 2014; 8: 2403-2406.
- Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA. 2015; 314: 2535-2543.
- Mansour M, Palese P, Zamarin D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J Virol. 2011; 85: 6015-6023.
- Chang WC, Stanbridge EJ, Yusoff K, Shafee N. The Oncolytic Activity of Newcastle Disease Virus in Clear Cell Renal Carcinoma Cells in Normoxic and Hypoxic Conditions: The Interplay Between von Hippel-Lindau and Interferon-beta Signaling. J Interferon Cytokine Res. 2013; 33: 346-354.
- Puhlmann J, Puehler F, Mumberg D, Boukamp P, Beier R. Rac 1 is required for oncolytic NDV replication in human cancer cells and establishes a link between tumorigenesis and sensitivity to oncolytic virus. Oncogene. 2010; 29: 2205-2216.
- Termeer CC, Schirrmacher V, Brocker EB, Becker JC. Newcastle disease virus infection induces B7-1/B7-2-independent T-cell costimulatory activity in human melanoma cells. Cancer Gene Ther. 2000; 7: 316-323.
- Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014.
- Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006; 6: 715-727.
- Schwartz RH. T cell anergy. Annu Rev Immunol. 2003; 21: 305-334.
- Aigner M, Janke M, Lulei M, Beckhove P, Fournier P, Schirrmacher V. An effective tumor vaccine optimized for costimulation via bispecific and trispecific fusion proteins. Int J Oncol. 2008; 32: 777-789.
- Haas C, Lulei M, Fournier P, Arnold A, Schirrmacher V. T-cell triggering by CD3- and CD28-binding molecules linked to a human virus-modified tumor cell vaccine. Vaccine. 2005; 23: 2439-2453.
- Schirrmacher V, Schlude C, Weitz J, Beckhove P. Strong T-cell costimulation can reactivate tumor antigen-specific T cells in late-stage metastasized colorectal carcinoma patients: results from a phase I clinical study. Int J Oncol. 2014; 46: 71-77.