Research Article
Austin Oncol Case Rep. 2018; 3(1): 1010.
The Induction of Immunogenic Cell Death (ICD) During Maintenance Chemotherapy and Subsequent Multimodal Immunotherapy for Glioblastoma (GBM)
Van Gool SW¹*, Makalowski J¹, Feyen O², Prix L³, Schirrmacher V¹ and Stuecker W¹
¹Immuno-Oncological Center Cologne, Hohenstaufenring 30-32, 50674 Cologne, Germany
²Zyagnum, Reißstrasse 1, 64319 Pfungstadt, Germany
³Biofocus, Berghäuser Strasse 295, 45659 Recklinghausen, Germany
*Corresponding author: Van Gool SW, Translational Oncology, IOZK, Hohenstaufenring 30-32, 50674 Köln, Germany
Received: June 01, 2018; Accepted: July 07, 2018; Published: July 18, 2018
Abstract
The prognosis of Glioblastoma multiforme remains poor. Immunotherapy improved survival in a small fraction of patients. We studied the efficiency of multimodal immunotherapy as part of first line treatment for patients with GBM. Immunogenic Cell Death (ICD) was induced with Newcastle Disease Virus (NDV) and Modulated Electrohyperthermia (mEHT), and Dendritic Cell (DC) vaccinations loaded with autologous tumor proteins were performed. In a retrospective analysis of 60 adults, we detected 15 adults in whom NDV/mEHT were added at days 8/9/10 during Temozolomide Maintenance (TMZm) cycles, multimodal immunotherapy with NDV/mEHT/DC vaccinations were administered after TMZm, and further 3-day NDV/mEHT maintenance immunotherapy treatments were given thereafter. Median age was 60 years. Median Karnofsky was 90. There was no added toxicity due to immunotherapy. Median progression-free survival was 13 months (m). With a median follow up of 17m (ranging 4-30m), median overall survival was not reached, and estimated overall survival at 30m was 58% (95%CI: +27, -42). The detection of Apo10 protein epitope (Apo10) and Transketolase-like 1 (TKTL1) in monocytes, the mRNA expression level for PDL1 on circulating tumor cells, and the Th1/Th2 balance in CD4+ T cells showed a dynamic interaction between tumor cells and immune reactivity. The data suggest that the additional induction of ICD via NDV/ mEHT during TMZm is beneficial in improving overall survival. While TMZm only targets dividing tumor cells, ICD targets dividing and non-dividing tumor cells. DC vaccination induces an antitumoral and anti-viral immune response which is maintained by the 3-day NDV/mEHT maintenance immunotherapy treatments.
Keywords: Newcastle disease virus; Modulated electrohyperthermia; Glioblastoma; Immunogenic cell death; chemotherapy
Abbreviations
Apo10: Apo 10 protein epitope; DC: Dendritic Cells; EDIM: Epitope Detection in Monocytes; E:T: Effector:Target;GBM: Glioblastoma Multiforme; ICD: Immunogenic Cell Death; IOZK: Immun-Onkologisches Zentrum Köln; mEHT: modulated Electrohyperthermia; NDV: Newcastle Disease Virus; NK: Natural Killer; OS: Overall Survival; PFS: Progression-Free Survival; POH: Perillyl Alcohol; TEVs: Serum-Derived Tumor Extracellular Vesicles; Th: T helper; TKTL1: Transketolase-Like 1; TMZm: Temozolomide maintenance
Introduction
Diffuse astrocytic tumors are brain tumors occurring in adults and children [1]. The grade IV tumor, called Glioblastoma Multiforme (GBM) is the most frequent brain tumor in adults with an incidence of 3 to 4 per 100000 adults per year [2]. In spite of standard multimodal treatment, consisting of neurosurgery, radiotherapy and chemotherapy, the prognosis is poor with a median Overall Survival (OS) of only 15 months [3]. At time of relapse the Progression-Free Survival (PFS) is 6 months, and the median PFS and OS have not improved over the last decade [4]. In spite of being an orphan disease, GBMs cause the highest number of years of life lost due to cancer [5,6].
Amongst other innovative approaches like anti-angiogenesis and targeted therapy, immunotherapy has been developed as an innovative approach to control GBM [7]. Active specific immunotherapy is based on the injection of autologous mature dendritic cells loaded with tumor antigens derived from different sources. Numerous clinical studies and reviews have been published on the role of immunotherapy for patients with GBM [8,9]. All point to feasibility of the technology without major side effects. Recently a large phase III clinical trial integrating DC vaccination during first line treatment, or in cross-over at time of disease progression, demonstrated improved long-term overall survival [10]. Moreover, meta-analyses pointed out the significant effect of active specific immunotherapy on OS compared to intra-institutional historical control patients [11,12].
Immunotherapy based on immunomodulation with checkpoint blockers like anti-CTLA-4 or anti-PD1 monoclonal antibodies is focus of current clinical research to treat GBM, but did not lead to a break-through like in other tumors [13-15], except for hypermutant GBM [16], presumably because of lack of activated antitumoral immune cells. More recent immunotherapeutic approaches consist of combinations of several treatment modalities of which the antitumoral activities ultimately merges at the effector arm of the immune system.
In this regard, the combination of oncolytic virus therapy and immunotherapy is a promising strategy [17,18]. Virally infected tumor cells can be recognized by NK cells, macrophages, neutrophils and virus-specific T cells. Furthermore, ICD-induced dying tumor cells can lead to an efflux of tumor antigens and damage-associated molecular pattern molecules, which can be taken up by immature dendritic cells for presentation to the T cells in the draining lymph nodes. Similar to virus-mediated ICD of tumor cells, moderate hyperthermia can contribute as immunogenic treatment modality to strengthen antitumoral immune reactivity [19]. The need for rational combinations of immunotherapeutic modalities that work at multiple levels in the cancer immunity cycle in CNS malignancies has recently been reviewed [20]. The combination of Newcastle Disease Virus (NDV), Modulated Electrohyperthermia (mEHT) and DC vaccination has been published as an innovative immunotherapy concept [21].
A further challenge is the integration of the multimodal immunotherapy in the standard antitumoral treatment strategies like surgery, radiochemotherapy and Maintenance Temozolomide (TMZm) chemotherapy. Observations in small cohorts of GBM patients treated with multimodal immunotherapy integrated in the standard Stupp-based treatment might be of help for the scientific community to design proper clinical trials in future.
Patients and Methods
Patients
A retrospective analysis of 133 treated GBM patients was performed at the Immun-Onkologisches Zentrum Köln (IOZK). All patients were treated on an individualized basis outside clinical trial, upon patient request and after extensive explanation of the treatment and signed informed consent. Seventeen patients were IDH mutated or had prior low grade glioma medical history. One patient was classified as Diffuse Midline Glioma. 115 patients were left and classified as primary GBM, 63 of them being treated with multimodal immunotherapy together with standard therapy at primary diagnosis. Fifteen adults were detected in whom three days of NDV/mEHT were associated to TMZm courses. At first contact and during therapy, patient’s blood was investigated for immunologic parameters including PanTum detect Epitope Detection in Monocytes (EDIM) tests [22,23]via Biovis, www.biovis-diagnostik.eu.
Treatment
During the 28-day TMZm cycles, mEHT sessions and NDV injections were scheduled at days 8, 9 and 10. The mEHT was administered with the Oncothermia EHY‑2000 device (Oncotherm GmbH, Troisdorf, Germany) for 50 min at increasing intensity from 40 to 80 Watt. During mEHT, 250 ml NaCl 0.9% infusion supplemented with 7.5g Vitamin C, 40 mg MgCl2, 45 mg CaCl2, 15 mg KCl, 10 ml Magnesiocard containing 737.6 mg Magnesiumaspartahydrochlorid 3H2O with 72.9 mg Mg (Verla-Pharm Arzneimittel GmbH & Co. KG, Tutzing, Germany), and 5 ml Nervoregin comp. H containing 0.1 ml Agaricus (HAB 34) Dil. D 6 (HAB, V. 3a), 0.35 ml Asa foetida Dil. D 5, 2.0 ml Strychnos ignatii Dil. D 6, 0.1 ml Valeriana officinalis Dil. D 3 and 0.65 ml Zincum isovalerianicum Dil. D 8 (Pflüger, Rheda, Germany) was administered, followed by 100 ml NaCl 0.9% infusion, and finally 100 ml NaCl 0.9% infusion with 20 ml Selenase T containing Natriumselenit-Pentahydrat 50 μg/ml Selen (Biosyn GmbH, Fellbach, Germany). At the end of the mEHT session, mesogenic oncolytic MTH-68 strain Newcastle Disease Virus (NDV) was injected at a dose of 10 x 107 infectious particles. Short infusions of NDV with in 100 ml NaCl 0.9% over 20 minutes were switched towards bolus injections of NDV with in 2 ml NaCl 0.9% since September 2017.
After finishing the TMZm cycles, full vaccination cycles were administered with three weeks interval. Each full vaccination cycle consisted of NDV and mEHT administrations at days 1 to 5 and at day 8. An intradermal injection of autologous mature Dendritic Cells (DCs) loaded with autologous tumor antigens was administrated at day 8. Immature DCs were differentiated ex vivo out of adherent peripheral blood monocytes in the presence of 800 U/ml IL-4 and 1000 U/ml GM-CSF. DCs were loaded at day 5 with autologous tumor antigens, obtained via tumor lysate [24,25]or obtained from serum after induction of tumor-derived antigenic extracellular microvesicles [26,27], induced via ICD by mEHT and NDV [19]. DC maturation was induced with NDV (105 infectious particles per 106 DCs) and the cytokine cocktail 1000 U/ml IL-6, 1100 U/ml TNF-a and 1900 U/ml IL-1b. GMP-approved culture medium and cytokines were purchased from Cellgenix (Freiburg, Germany). The vaccine product is an approved medicinal product by the German authorities (DE-NW-04-MIA-2015-0033).
After the vaccination cycles, further maintenance immunotherapy was provided consisting of 3 days NDV/mEHT at intervals of about 6 weeks.
In some patients, immunomodulatory strategies were added. The anti-PD1 mAb Pembrolizumab (Keytruda®, MSD) was infused at 2 mg/kg each 3 weeks according to the instructions of the manufacturer. ATRA (all-trans-retinoic-acid) was used with the aim to deplete myeloid-derived suppressor cells, and was administered for three days at 150 mg/m²/day in three doses with at least 6 hours interval, as published [28].
Progression-Free Survival (PFS) was defined when treatment switch was needed. In case of doubt for pseudoprogression, PFS was eventually retro-actively defined after having the results of the subsequent MRI. All patients were followed further to define the overall survival.
Monitoring
Circulating Tumor Cell (CTC) analysis was performed via Biofocus (www.biofocus.de). Heparinized blood samples of patients were processed as described in detail previously [29]. In brief, CTCs from 30 ml blood were enriched by filtration cytometry [30] using 20 μm polyester filter meshes (Reichelt Chemietechnik, Heidelberg, Germany). RNA was extracted from cells retained on filter meshes with Trizol reagent. For proof of CTCs in these cell preparations, qRT-PCR for relative mRNA expression of a set of four genes (telomerase, ERBB2, c-KIT, EGFR) was performed. Assays were purchased (telomerase: Hs00972649, Applied Biosystems) or designed in-house [29]taking care that fluorescence probes are spanning exon-boundaries. Expression values in the enriched CTC preparation was normalized to the house-keeping gene GAPDH and compared to GAPDH-normalized expression values in mononuclear cells of the patients. Relative expression ratios of >2.0 (telomerase, c-KIT, ERBB2) or >1.0 (EGFR) in enriched CTC preparations were considered overexpressed and CTC-positive. In CTC-positive samples, relative mRNA expression of PD-L1 was subsequently determined by qRT-PCR in a similar manner.
PanTum detect tests were performed at presentation and during treatment. The tests were originally designed as a biologic biopsy (as a special form of liquid biopsy) test exploiting the innate immune system and its interaction with cancer, for early detection of cancerrelated biomarkers like the DNase/Apo10 protein epitope as maker of tumor cells with abnormal apoptosis and proliferation, and the Transketolase-like 1 (TKTL1) epitope as a biomarker for anaerobic glucose metabolism (Warburg effect). More in detail, both biomarkers have been detected intracellularly in monocytes, allowing a sensitive and specific noninvasive detection of cancer patients by blood samples (“biologic biopsy” as a special form of liquid biopsy). This blood test is based on the EDIM technology, which utilizes the fact that activated monocytes phagocytize and present tumor-related material even in the presence of low tumor mass. Those activated monocytes, which contain intracellular tumor epitopes, can be detected by CD14 and CD16 specific antibodies using flow cytometry [22,23,31,32].
Results
Status at start of immunotherapy
GMB patients came to the IOZK with the request to add-on immunotherapeutic strategies during maintenance chemotherapy after neurosurgery and radiochemotherapy, in median 3.38 months after operation (range 1.11-8.72m). Patient characteristics are described in (Table 1). All patients had a Karnofsky score above 60 at presentation. MGMT was methylated in seven of the fifteen patients, four patients had an MGMT not-methylated tumor, while the MGMT status was not defined in another four patients. Four patients were already in the maintenance chemotherapy phase of their treatment, taking TMZm cycles.
Number
Sex
Age
Karnofsky Performance Index
Location
MGMT Methylation status
Extent of resection
TMZm 2
22731
M
54
100
Temporal left
Methylated
R1
0
22752
F
61
70
Temporoparietal right
Methylated
R0
0
22866
F
44
70
Occipital right
Methylated
R0
0
22878
M
67
70
Occipital links
Not methylated
R0
0
23103
M
42
100
Parietal right
Not methylated
S nd 1
0
23260
F
62
70
Parietal left
Methylated
R0
0
23346
M
37
70
Frontal right
Methylated
R1
0
23565
M
57
100
Occipital right
Methylated
R0
5
23579
M
59
80
Frontal right
Not available
R1
0
23623
F
61
100
Frontal right
Not available
S nd 1
3
23696
M
65
90
Temporal left
Not methylated
S nd1
0
23769
M
67
100
Frontal right
Not available
R0
2
23806
M
60
100
Temporal right
Methylated
R1
0
23834
M
60
60
Frontal left
Not available
B
2
23877
M
44
100
Parietal right
Not methylated
R0
0
- S nd: extent of resection not documented.
Number of maintenance TMZ courses prior to combining TMZ + NDV/mEHT
Table 1: GBM patient characteristics.
The blood cell counts of the immune diagnostic blood sample prior to immunotherapy were influenced by the former radio- and chemotherapy. The percentage of monocytes within the white blood cell counts was above the normal value in 9/15 patients (Figure 1A). The absolute number of T cells and B cells were below the normal laboratory limits in 8/15 patients (T cells) respectively 11/15 patients (B cells). Patients had a clear skew towards T helper (Th) 1, as reflected by the % IFN-g- versus IL-4-expressing CD4+ T cells. 60% of the patients had a weak natural killer (NK) cell activity.
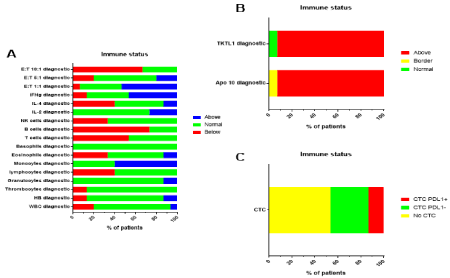
Figure 1: Immune diagnosis at time of presentation.
Before start of modulated electrohyperthermia and NDV injections, a blood sample was taken to evaluate the basic immune data of the patients. A. Data on blood
counts, differential formula of white blood cells, counts of lymphocyte subpopulations, percentage of cytokine expressing fraction within CD4+ T cells, and NK
cytotoxic activity against K562 cells are shown in relation to the normal ranges defined by the respective clinic laboratories. E:T = Effector:Target Ratio of NK cells
against K562 target cells. B. Data on Apo10 and TKTL1 PanTum detect EDIM tests are shown in relation to the normal ranges defined by the clinical laboratory.
C. Data on the mRNA expression for PDL1 (negative or positive means below or above the cut-off of 2) on circulating tumor cells are shown. No CTC means that
no CTC were detected in the blood sample.
All patients had increased scores for the two PanTum detect EDIM test markers TKTL1 and Apo10, except patient 22752 who had borderline increased Apo10 and TKTL1 in the normal range (Figure 1B). All patients were tested for CTCs in the peripheral blood. In 7/15 patients, we found CTCs. In two of these seven patients mRNA expression for PDL1 was increased above the cut-off 2 (Figure 1C).
Immunotherapy
Treatment details are presented in (Table 2). The combination of mEHT and NDV injections started in median 4.13 months after surgery (range 3.41-8.95m). The different modes of immunotherapy including mEHT, NDV injections and DC vaccinations were feasible without major toxicity. Treatment was conducted in an ambulatory fashion. Since September 2017, immunomodulatory strategies were implemented and patients were advised to take short 3-day pulses of high dose ATRA at 150 mg/m² per day in three doses one day before, the day of vaccination and the day after vaccination, as described [28]. A major complaint in some patients, during intake of ATRA was severe headache.
Number
mEHT sessions
NDV injections
DC vaccination
DC cell numbers
Tumor antigens
ATRA
Pembrolizumab
PFS2
Further rescue at progression
OS2
22731
69 (62)
69 (62)
3 (2)
16200000 (7000000)
Tumor-L
+ (-)
-
28,2
R1 + TMZm
+30,36
22752
50 (33)
50 (33)
2
19400000
TEVs7
-
-
16,07
Cyberknife + TMZm
+29,41
22866
43
43
2
14400000
TEVs7
-
-
+27,38
+27,38
22878
44 (6)
44 (6)
23
19820000
Tumor-L
-
+ (-)
6,13
POH + repurposing drugs (63)+ Avastin
18,07
23103
39 (17)
39 (17)
3 (0)
44600000 (0)
Tumor-L
-
-
8,92
R1 + POH + repurposing drugs + Gliovac(64)
22,07
23260
35 (35)
35 (35)
2 (2)
8000000 (8000000)
TEVs
-
-
13,11
Surgery + CCNU + Methadon +Avastin
+20,49
23346
42
42
2
18200000
TEVs
+
+
+17,25
+17,25
23565
21 (9)
21 (9)
2 (1)
15600000 (7800000)
TEVs
+
-
10,59
POH + re-irradiation + PCV 1 cycle
+20,3
23579
25 (25)
25 (25)
2 (2)
26800000 (26800000)
TEVs
-
-
10,46
PCV
+11,97
23623
21 (3)
21 (3)
2 (0)
19500000 (0)
TEVs + Tumor-L
+ (-)
+ (-)
6,89
R1
10,46
23696
12
12
+8,82
+8,82
23769
12
12
+9,15
+9,15
23806
12
12
+6,52
+6,52
23834
9
9
+6,2
+6,2
23877
3
3
+4,46
+4,46
- The data before the brackets are the total treatments the patient received. The data between brackets are the treatments administered till the moment of progression.
- PFS and OS are expressed in months after neurosurgery.
- mEHT/NDV and DC vaccination during TMZm in this particular patient.
- Abbreviations: PCV: Procarbazine + CCNU + Vincristine. POH: perillyl alcohol. R1: Incomplete resection. TMZm: maintenance 5-day cycle of temozolomide. TEVs: Serum-derived Tumor Extracellular Vesicles induced by 5 days of treatment with mEHT/NDV. Tumor-L: Tumor lysate.
Table 2: Immunotherapy details and outcome per patient: total treatment till reporting (treatment till progressive disease)1.
After the 3-day NDV/mEHT treatment integrated in the TMZm cycles, and the subsequent vaccination cycles with interval of 3 weeks, the frequency of the subsequent 3-day maintenance immunotherapy treatments with NDV and mEHT was decided with the patient but sought at about 6 weeks. Once progression was defined, further treatment modalities were administered upon the discretion of the treating physician (Table 2).
Outcome results
The combination of 5 days TMZm and subsequent 3 days NDV and mEHT was very well tolerated, and did not lead to any side effect greater than CTCAE grade II. Patient 23346 had epileptic seizures and showed signs of progression which afterwards became clear to be pseudoprogression. In other patients, clinical symptoms came at time of progression and were considered to be tumor-related. Data on PFS and OS are shown in (Table 2). The median PFS was 13 months. Median OS was not reached with a median follow up of 17 months (rang 4-30 months). Estimated overall survival at 30 months was 58% with CI95% confidence intervals of +27 and -42. In 6/8 progressive patients, patients requested to continue immunotherapy after progression, with further adaptations and combined with other treatments (Table 2). The time between progression and survival for these patients was respectively +2, +13, 12, 13, +7, +10, +1.5 and 4 months.
Treatment-related effects that indirectly reflect tumor biology were assessed during treatment (Figure 2). As shown in (Figure 2A), the mRNA expression for PDL1 in CTCs increased over time in eight out of nine patients. In six patients, the value increased from below to above the cut-off value of 2. The value remained negative for patients 22866 and 23565. Of note, patients who died more rapidly tended to have the highest increase (Patients 22878, 23103 and 23623). Patients who had progressive disease but are still alive, had also a moderate increase in mRNA expression for PDL1 above the cut-off. Patient 22866 did not have circulating tumor cells during maintenance immunotherapy, except in the last sample. PDL1 mRNA expression remained in this sample below the cut-off. Although patient 23565 had CTCs, the expression of mRNA for PDL1 remained negative. Patient 23346 showed a dramatic increase in PDL1 mRNA expression. Due to the increase of PDL-1 expression in the fraction of CTC anti- PD1 mAb pembrolizumab was added as immunomodulatory agent. In both this patient, and patient 22878, mRNA for PDL1 decreased after inclusion of pembrolizumab in the treatment.
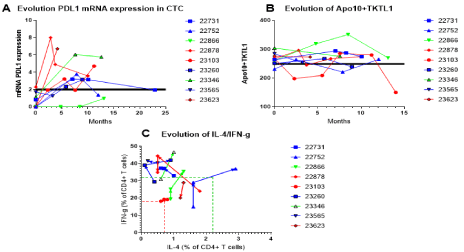
Figure 2: Evolution of A/ mRNA for PDL1 expression on circulating tumor cells, B/ cumulated Apo10 and TKTL1 scores measured by PanTum detect EDIM tests,
and C/ IL-4 and IFN-g expression in CD4+ T cells during combined treatment.
Patients were followed during treatment. Patients in red have died, patients in blue have shown a second event but are alive at time of analyzing the data, patients
in green are in remission. A. The mRNA expression for PDL1 on circulating tumor cells was measured. The cut-off of 2 indicates negative versus positive PDL1
mRNA expression on CTC. B. The sum of both scores for the Apo10 and TKTL1 PanTum detect EDIM tests were measured. The cut-off sum score of 249 is
indicated. C. The evolution of the percentage IL-4 and IFN-g expression within CD4+ T cells over time for individual patients. The dotted lines indicate the minimum
and maximum normal cut-off values as determined by the clinical laboratory.
PanTum detect tests based on the EDIM technology were also performed during treatment (Figure 2B). Because both biomarkers TKTL1 and Apo10 reflect the take-up of dying tumor cell content by innate macrophages, we followed the sum of the two markers during treatment. The cut-off was defined as the sum of the maximal range of normal levels, being a score of 249. Eight out of nine patients, in whom follow up data were available, started above this cut-off. Only the two patients remaining in remission (22866, 23346) reached at least once a value above 300.
Shifts in Th1/Th2 balances over time were measured as % intracellular INF-g or IL-4-expression in circulating CD4+ T cells (Figure 2C). Upon treatment, the cytokine expression increased above the upper limit for IFN-gamma, except in two out of three patients who died (patients 23103 and 23623). Patient 22878 showed first an increased IFN-g production, but dropped again later during his disease course.
For seven patients, follow-up data on low NK cell activity at time of diagnosis were available. NK cell function normalized in three patients (22866, 23260, 23565), while in two patients the NK cell function remained low (22731, 23346). In patients 22752 and 23103, NK cell function normalized but dropped later-on again. Both were treated at that time with corticosteroids. In 5 patients (22731, 22752, 22878, 23375 and 23623) in whom an Elispot test could be performed, testing T cell samples at diagnosis and later-on during treatment in one assay, an increase of IFN-g-producing T cells over time was found when stimulated ex vivowith autologous dendritic cells that were loaded with NDV-lysed tumor cells from GBM or control cell lines (data not shown). We could, however, not observe an increase of T cell reactivity upon ex vivostimulation with DCs loaded with freeze/ thaw lysate of tumor cells from GBM cell lines or available (Patients 22731 and 22878) autologous tumor tissue (data not shown).
Discussion
A retrospective analysis of 15 newly diagnosed GBM patients treated with neurosurgery, radiochemotherapy and maintenance chemotherapy according to the protocol developed by Stupp et al [3] is presented, to which multimodal immunotherapy was added. These patients were treated on individualized basis, after informed consent. The aim was to improve eventually the outcome of their disease at least by slowing down disease progression via stimulation of antitumoral immune reactivity. Both radiotherapy and temozolomide target the genetic structure of the GBM cells [33,34]. NDV kills GBM tumor cells over the ICD pathway, with increase of ectocalreticulin expression and tumor antigen expression on the surface of the tumor cells and release of HMGB1 as danger signal [35]. Efficacy of NDV against human GBM tumor cells has been shown [36]. The use of NDV in clinical trials for patients with GBM have been reported demonstrating feasibility without toxicity and suggesting tumor control [37-40]. Hyperthermia is a long-established treatment strategy against gliomas [41] with a current “revival” [42]. Modulated electrohyperthermia provokes the expression of heat shock proteins in tumor cells which play an important role as an immunological danger signal in GBM [43]. Recent work demonstrated that locoregional mEHT not only induced antitumor activity but depicted also an abscopal effect for schrinking of tumors outside the scope of the localized treatment [44]. Tumor responses induced by electrohyperthermia could be demonstrated in clinical trials for patients with GBM [45-47]. The concept of ICD as a welldefined cell death pathway entity has been recently reviewed in the hallmark paper by Galluzzi et al [48]. Our paper describes experiences in a small group of patients treated with the combination of genetic and immunogenic mechanisms for induction of tumor cell death in order to improve tumor control. ICD has been induced after clearance of temozolomide out of the body and still in time distance to the next chemotherapy cycle in order to allow ICD-induced immunity.
DC vaccinations have been given after the chemotherapy. The obvious reason is that temozolomide might affect T cell proliferation and hence the anti-tumoral immune response upon DC vaccination [49]. Albeit at different doses and schedules, TMZ has been shown to affect CD4 T cells [50]and regulatory T cells [51], while dendritic cells and CD8+ T cells are less affected [52]. Similar to previous work [24], DCs were loaded with tumor antigens obtained from lysates from fresh frozen tumor tissue. In several patients, however, no or not sufficient amount of tumor tissue with required quality was available. Therefore mEHT/NDV-induced serum-derived antigenic extracellular vesicles (EVs, in particular microvesicles [53]) were used to load the DCs. EVs are in low number present in normal human, but are increased in patients with brain cancer [26]. The presence of tumor rejection antigens on tumor-derived EVs is known [54]. Immunotherapy with tumor-derived EVs together with an adjuvant for the induction of tumor-specific antitumor cytotoxic T cell response has been demonstrated [55]. Loading DCs with tumorderived EVs as effective anti-cancer vaccine has been demonstrated [56].
During treatment we observed in most patients an increase in mRNA expression for PDL1 in CTC. Such type of kinetic data during immunotherapy are novel and suggest a dynamic interplay between the induced antitumoral immune system by the multimodal immunotherapy, and the development of immune escape mechanisms at the side of the tumor cells. The data are compatible with and can in part explain the disappointing results of PD1-based checkpoint inhibitors for malignant glioma as single “immunotherapy” agent [14,57,58], except when hypermutation is in place and an occurring antitumoral immune response is presumed [16].
The PanTum detect EDIM tests for Apo10 and TKTL1 were performed for the first time before implementation of immunotherapy in the standard treatment. The PanTum detect tests reflect the interaction between monocytes/macrophages and tumor cells. These particular tests were originally developed in the context of cancer diagnostics [22,23,31,32]. Only one study pointed to the evolution of the PanTum detect tests after complete surgery [59]. In this study, 8.7 to 10.8 months after complete resection, the test showed negative values for patients in remission. TheTKTL1 score, however, just can slightly be increased after any event on tissue (e.g. after surgery) resulting in tissue regeneration, whereas no abnormal Apo10 increase could be observed due to any biological side effect. Remarkably, all patients described in our study had at least one positive score at time of first blood test, except one patient who scored borderline for Apo10 expression only. Knowing the life span of monocytes and macrophages going up to two weeks, the data suggest that the positive EDIM test at first presentation reflect mainly therapy-induced effects including clearance of residual tumor cells by macrophages. This concept is further supported by the fact that the highest combined score apo10/TKTL1 was observed in those patients with the best disease control. The latter combined score was also used in the manuscript of Grimm et al. [59]and depicts a useful tool to assess tumor control activity at the level of tumor-immunity interaction.
In all 5 patients tested, we could clearly demonstrate with ELISPOT an increase in IFN-g producing T cells over time upon treatment when lysate of NDV-lysed GBM cell lines were presented by the autologous DCs. The index reached the level above 2 as defined by Banchereau et al. [60]. Because the values were also positive when NDV-lysed irrelevant tumor cell lines were used, the T cell response measured is interpreted to be NDV antigen-mediated. The anti-viral immune reactivity induced supports the concept of maintenance immunotherapy with NDV/mEHT alone, in which the repetitive viral infection of residual tumor cells can maintain the antitumoral immune response via the viral antigens. We did not find an increased T cell response upon DCs loaded with freeze/thaw lysate of GBM cell lines nor autologous tumor lysate. Epigenetic profiling of the GBM tumor cell lines cultured in 2 dimensions showed no compatibility with the known GBM profiles [61]. The Elispot assay for quantification of antitumoral immune responsiveness has very weak sensitivity, and is ultimately not correlated with the outcome of the patients [62].
Although this retrospective analysis of 15 treated GBM patients holds some weaknesses, the data provide some novel tools for monitoring the tumor-host interaction during therapy. The described treatment concept can be of value when designing new clinical trial protocols on complex combinations of oncolytic virus therapy, modulated electrohyperthermia, DC vaccination and immunomodulatory strategies together with chemotherapy. The combination of alkylating agents with ICD-inducers and followed by full immunization and immunomodulation strategies might improve the prognosis of patients with GBM treated at time of primary event.
Acknowledgement
The authors thank the technical staff from the GMP laboratory for their dedication in the production of the dendritic cell vaccinations as approved medicinal product.
References
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131: 803-20.
- Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17: iv1- iv62.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10: 459-66.
- Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro Oncol. 2013; 15: 4-27.
- Rouse C, Gittleman H, Ostrom QT, Kruchko C, Barnholtz-Sloan JS. Years of potential life lost for brain and CNS tumors relative to other cancers in adults in the United States, 2010. Neuro Oncol. 2016; 18:70-77.
- Burnet NG, Jefferies SJ, Benson RJ, Hunt DP, Treasure FP. Years of life Lost (YLL) from cancer is an important measure of population burden--and should be considered when allocating research funds. Br J Cancer. 2005; 92:241-5.
- McGranahan T, Li G, Nagpal S. History and current state of immunotherapy in glioma and brain metastasis. Ther Adv Med Oncol. 2017; 9:347-68.
- Srinivasan VM, Ferguson SD, Lee S, Weathers SP, Kerrigan BCP, Heimberger AB. Tumor Vaccines for Malignant Gliomas. Neurotherapeutics. 2017; 14:345-57.
- Tivnan A, Heilinger T, Lavelle EC, Prehn JH. Advances in immunotherapy for the treatment of glioblastoma. J Neurooncol. 2017; 131:1-9.
- Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018; 16:142.
- Wang X, Zhao HY, Zhang FC, Sun Y, Xiong ZY, Jiang XB. Dendritic cellbased vaccine for the treatment of malignant glioma: a systematic review. Cancer Invest. 2014; 32:451-7.
- Cao JX, Zhang XY, Liu JL, Li D, Li JL, Liu YS, et al. Clinical efficacy of tumor antigen-pulsed DC treatment for high-grade glioma patients: evidence from a meta-analysis. PLoS ONE. 2014;9:e107173.
- Mirzaei R, Sarkar S, Yong VW. T Cell Exhaustion in Glioblastoma: Intricacies of Immune Checkpoints. Trends Immunol. 2017; 38:104-15.
- Huang J, Liu F, Liu Z, Tang H, Wu H, Gong Q, et al. Immune Checkpoint in Glioblastoma: Promising and Challenging. Front Pharmacol. 2017;8:242.
- Huang B, Zhang H, Gu L, Ye B, Jian Z, Stary C, et al. Advances in Immunotherapy for Glioblastoma Multiforme. J Immunol Res. 2017;2017:3597613.
- Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol. 2016; 34:2206-11.
- Saha D, Martuza RL, Rabkin SD. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell. 2017; 32:253-67 e5.
- Desai R, Suryadevara CM, Batich KA, Farber SH, Sanchez-Perez L, Sampson JH. Emerging immunotherapies for glioblastoma. Expert Opin Emerg Drugs. 2016; 21:133-45.
- Vandenberk L, Belmans J, Van Woensel M, Riva M, Van Gool SW. Exploiting the Immunogenic Potential of Cancer Cells for Improved Dendritic Cell Vaccines. Front Immunol. 2015;6:663.
- Ameratunga M, Coleman N, Welsh L, Saran F, Lopez J. CNS cancer immunity cycle and strategies to target this for glioblastoma. Oncotarget. 2018; 9:22802-16.
- Schirrmacher V, Lorenzen D, Van Gool SW, Stuecker W. A new strategy of cancer immunotherapy combining Hyperthermia/Oncolytic Virus Pretreatment with Specific Autologous Anti-Tumor Vaccination - A Review. Austin Oncol Case Rep. 2017; 2.
- Feyen O, Coy JF, Prasad V, Schierl R, Saenger J, Baum RP. EDIM-TKTL1 blood test: a noninvasive method to detect upregulated glucose metabolism in patients with malignancies. Future Oncol. 2012;8:1349-59.
- Coy JF. EDIM-TKTL1/Apo10 Blood Test: An Innate Immune System Based Liquid Biopsy for the Early Detection, Characterization and Targeted Treatment of Cancer. Int J Mol Sci. 2017; 18.
- Van Gool SW. Brain Tumor Immunotherapy: What have We Learned so Far? Front Oncol. 2015;5:98.
- Vandenberk L, Garg AD, Verschuere T, Koks C, Belmans J, Beullens M, et al. Irradiation of necrotic cancer cells, employed for pulsing Dendritic Cells (DCs), potentiates DC vaccine-induced antitumor immunity against highgrade glioma. Oncoimmunology. 2016;5:e1083669.
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008; 10:1470-6.
- Schindler SM, Little JP, Klegeris A. Microparticles: a new perspective in central nervous system disorders. Biomed Res Int. 2014: 756327.
- Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013; 62:909-18.
- Austrup F, Uciechowski P, Eder C, Bockmann B, Suchy B, Driesel G, et al. Prognostic value of genomic alterations in minimal residual cancer cells purified from the blood of breast cancer patients. Br J Cancer. 2000; 83:1664- 73.
- Truchaud C, Caldani C, Bisconte JC, Bergogne-Berezin E, Buisson Y. Filtration cytometry: parallel real-time analysis for bacteria, cells, and particles. Clin Chem. 1992;38:1650-951.
- Grimm M, Schmitt S, Teriete P, Biegner T, Stenzl A, Hennenlotter J, et al. A biomarker based detection and characterization of carcinomas exploiting two fundamental biophysical mechanisms in mammalian cells. BMC Cancer. 2013;13:569.
- Grimm M, Feyen O, Coy JF, Hofmann H, Teriete P, Reinert S. Analysis of circulating CD14+/CD16+ Monocyte-Derived Macrophages (MDMs) in the peripheral blood of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016; 121:301-6.
- Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000; 6:2585-2597.
- Koukourakis GV, Kouloulias V, Zacharias G, Papadimitriou C, Pantelakos P, Maravelis G, et al. Temozolomide with radiation therapy in high grade brain gliomas: pharmaceuticals considerations and efficacy; a review article. Molecules. 2009; 14:1561-77.
- Koks CAE, Garg AD, Ehrhardt M, Riva M, De Vleeschouwer S, Agostinis P, et al. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer. 2014; 136:e313-e25.
- Alkassar M, Gartner B, Roemer K, Graesser F, Rommelaere J, Kaestner L, et al. The combined effects of oncolytic reovirus plus Newcastle disease virus and reovirus plus parvovirus on U87 and U373 cellsin vitro and in vivo. J Neurooncol. 2011; 104:715-27.
- Csatary LK, Bakacs T. Use of Newcastle Disease Virus Vaccine (MTH-68/H) in a Patient With High-grade Glioblastoma. JAMA. 1999; 281:1588-9.
- Csatary LK, Gosztonyi G, Szeberenyi J, Fabian Z, Liszka V, Bodey B, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol. 2004; 67:83-93.
- Wagner S, Csatary CM, Gosztonyi G, Koch HC, Hartmann C, Peters O, et al. Combined treatment of pediatric high-grade glioma with the oncolytic viral strain MTH-68/H and oral valproic acid. APMIS. 2006; 114:731-43.
- Freeman AI, Zakay-Rones Z, Gomori JM, Linetsky E, Rasooly L, Greenbaum E, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006; 13:221-8.
- Tanaka R. [Radiofrequency hyperthermia in malignant brain tumors: clinical trials]. Gan To Kagaku Ryoho. 1988; 15:1370-5.
- Lee Titsworth W, Murad GJ, Hoh BL, Rahman M. Fighting fire with fire: the revival of thermotherapy for gliomas. Anticancer Res. 2014; 34:565-74.
- Yang I, Han S, Parsa AT. Heat-shock protein vaccines as active immunotherapy against human gliomas. Expert Rev Anticancer Ther. 2009; 9:1577-82.
- Vancsik T, Kovago C, Kiss E, Papp E, Forika G, Benyo Z, et al. Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts. J Cancer. 2018; 9:41-53.
- Fiorentini G, Giovanis P, Rossi S, Dentico P, Paola R, Turrisi G, et al. A phase II clinical study on relapsed malignant gliomas treated with electrohyperthermia. In Vivo. 2006; 20:721-4.
- Tanaka R, Takahashi H, Uzuka T, Kakinuma K. [Current status of hyperthermia for therapy of brain tumor]. Nihon Rinsho. 2005; 63:483-9.
- Wismeth C, Dudel C, Pascher C, Ramm P, Pietsch T, Hirschmann B, et al. Transcranial electro-hyperthermia combined with alkylating chemotherapy in patients with relapsed high-grade gliomas: phase I clinical results. J Neurooncol. 2010; 98:395-405.
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018.
- Pellegatta S, Eoli M, Cuccarini V, Anghileri E, Pollo B, Pessina S, et al. Survival gain in glioblastoma patients treated with dendritic cell immunotherapy is associated with increased NK but not CD8(+) T cell activation in the presence of adjuvant temozolomide. Oncoimmunology. 2018; 7:e1412901.
- Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, et al. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004; 22:610-616.
- Ridolfi L, Petrini M, Granato AM, Gentilcore G, Simeone E, Ascierto PA, et al. Low-dose temozolomide before dendritic-cell vaccination reduces (specifically) CD4+CD25++Foxp3+ regulatory T-cells in advanced melanoma patients. J Transl Med. 2013;11:135.
- Xu X, Stockhammer F, Schmitt A, Casalegno-Garduno R, Enders A, Mani J, et al. Therapeutical doses of temozolomide do not impair the function of dendritic cells and CD8+ T cells. Int J Oncol. 2012; 40:764-72.
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006; 20:1487-95.
- Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001; 7:297-303.
- Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008; 16:782-90.
- Gu X, Erb U, Buchler MW, Zoller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer. 2015; 136:E74-E84.
- Reardon DA, Wen PY, Wucherpfennig KW, Sampson JH. Immunomodulation for glioblastoma. Curr Opin Neurol. 2017; 30:361-369.
- Lieberman NAP, Vitanza NA, Crane CA. Immunotherapy for brain tumors: understanding early successes and limitations. Expert Rev Neurother. 2018:1-9.
- Grimm M, Kraut W, Hoefert S, Krimmel M, Biegner T, Teriete P, et al. Evaluation of a biomarker based blood test for monitoring surgical resection of oral squamous cell carcinomas. Clin Oral Investig. 2016; 20:329-38.
- Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451-6458.
- Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, et al. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014; 14:92-107.
- Clay TM, Hobeika AC, Mosca PJ, Lyerly HK, Morse MA. Assays for monitoring cellular immune responses to active immunotherapy of cancer. Clin Cancer Res. 2001; 7:1127-1135.
- Kast RE, Karpel-Massler G, Halatsch ME. CUSP9* treatment protocol for recurrent glioblastoma: aprepitant, artesunate, auranofin, captopril, celecoxib, disulfiram, itraconazole, ritonavir, sertraline augmenting continuous low dose temozolomide. Oncotarget. 2014; 5:8052-8082.
- Bota DA, Alexandru-Abrams D, Pretto C, Hofman FM, Chen TC, Fu B, et al. Use of ERC-1671 Vaccine in a Patient with Recurrent Glioblastoma Multiforme after Progression during Bevacizumab Therapy: First Published Report. Perm J. 2015; 19:41-6.