Case Report
Austin Oncol Case Rep. 2024; 7(1): 1021.
A Rare Case of Extranodal Primary Gallbladder Lymphoma Presented as Biliary Obstruction and treated with Rituximab and Lenalidomide
Carina Hernandez, MD, PhD¹*; Ghanshyam Ghelani, MBBS²; Ian Pinto, MD²
¹Division of Medicine, State University of New York Upstate Medical University, Syracuse, New York, USA
²Division of Hematology and Oncology, State University of New York Upstate Medical University, Syracuse, NY, USA
*Corresponding author: Carina Hernandez Division of Medicine, State University of New York Upstate Medical University, Syracuse, New York, USA. Email: arina.hernandez3@outlook.com, hernanca@upstate.edu
Received: March 26, 2024 Accepted: April 16, 2024 Published: April 23, 2024
Abstract
Follicular Lymphoma of the gallbladder is a rare occurrence. Many of the patients initially present with symptoms similar to cholecystitis. Rarely does it present with biliary obstruction or imaging finding suggestive of Perihilar Cholangiocarcinoma or Klatskin tumor. Herein, we present a case of a man who presented with right upper quadrant pain and jaundice, his imaging and intraoperative findings were suggestive of Klatskin type cholangiocarcinoma but histopathology report showed grade II extranodal follicular lymphoma. To our knowledge, there have only been a few reports of extranodal follicular lymphoma which had a similar presentation. However, our patient had an unsuccessful resection of an intrahepatic tumor with continued biliary obstruction requiring systemic therapy. The patient was treated with rituximab and lenalidomide with rapid regression of tumor with clinical improvement.
Keywords: Extranodal follicular lymphoma; Lenalidomide; Biliary obstruction
Introduction
Malignant lymphomas are considered tumors of lymph nodes although 40% occur in extranodal tissues, usually from the gastrointestinal tract [1,2]. In a case series of gallbladder lymphoma, common primary lymphoma type was Diffuse Large B-Cell Lymphoma (DLBCL) [3] while follicular lymphoma was rare [4] encompassing only 1-3% of cases [5-7]. There are several case reports of acute cholecystitis as the initial presentation of lymphoma of the gallbladder [8,9]. Follicular lymphoma infiltrating the gallbladder is uncommon. Extranodal lymphoma is defined as being restricted to a solitary site with or without involvement of its contiguous lymph nodes [5]. Follicular lymphoma is an indolent form of Non-Hodgkin’s Lymphoma (NHL) that is characterized by a proliferation of germinal center B-cells of the lymphoid follicle [10,4]. These B-cells express t(14;18)(q32;q21) [10] gene fusion. This in turn results in the BCL2 protein.
Case Presentation
The patient is a male in his mid-seventies with a significant past medical history of hypertension, type 2 diabetes mellitus, hyperlipidemia, adenomatous colon polyp and Barrett’s esophagus diagnosed in 2019. Family history was significant for gastric cancer in his father, history of cerebral vascular accident in his mother, brother and sister. He was a former smoker with a 35-pack year and quit when he was 70 years old. He endorsed alcohol use, about one beer per week. He had no known allergies.
The patient initially presented with lower abdominal pain, decreased appetite and jaundice. He reported that the lower abdominal pain occurred nightly, was consistent, lasting up to four hours and radiating to the epigastrium. He also complained of weight loss, pruritus and dark urine. Initial laboratory results were significant for a CEA of 0.6mg/mL, CA19-9 of 699 U/mL, AST 450 U/L, ALT 574 U/L, ALK 967 U/L, Total bilirubin of 7.90 mg/dL.
Work-up done showed obstructive jaundice. An abdominal ultrasound showed material in the gallbladder lumen likely related to sludge or small stones (Figure 1). There was thickened gallbladder wall with increased echogenicity. The gallbladder wall measured 0.62cm in thickness. The echogenicity in the wall was concerning for porcelain gallbladder. He had mildly dilated intrahepatic ducts with a common bile duct diameter of approximately 6cm. Porcelain gallbladder was confirmed with a CT abdomen and pelvis with IV contrast (Figure 2). It revealed an ill-defined mass (~5cm) involving the neck of the gallbladder, porta hepatis and adjacent liver parenchyma causing an obstruction of the proximal extrahepatic biliary tree. An MRI of the abdomen and pelvis without contrast showed 5.0 x 1.5 cm porta hepatis mass extending along anterior margin of portal vein and causing biliary obstruction (Figure 3). MRI abdomen also revealed a single severe biliary stricture that was found in the upper third of the main bile duct.
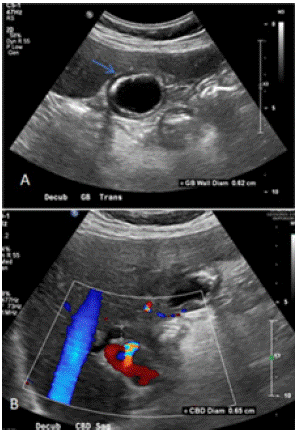
Figure 1: Preoperative Abdominal Ultrasound. (A)There was material in the gallbladder lumen likely sludge. The echogenicity of the wall represented porcelain gallbladder. The gallbladder wall diameter was 0.62cm (blue arrow). (B) There was concern for dilation of the Common Bile duct (CBD) with a diameter of 0.65cm.
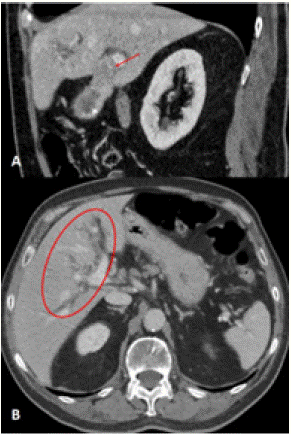
Figure 2: Preoperative CT abdomen and pelvis. (A) Coronal CT of the abdomen and pelvis with intravenous contrast showed intense focal uptake around the gallbladder and hypoattenuation of the liver adjacent to the gallbladder. There was a partially calcified and thickened gallbladder wall. There was an ill-defined mass (red arrow) at the level of the neck of the gallbladder merging with adjacent liver parenchyma which was approximately 5cm in size extends into the porta hepatis. It was associated with obstruction of the proximal extrahepatic biliary tree. (B) Axial CT of the abdomen and pelvis showed significant intrahepatic biliary dilation (red circle). The CBD at level of the pancreatic head was not dilated.
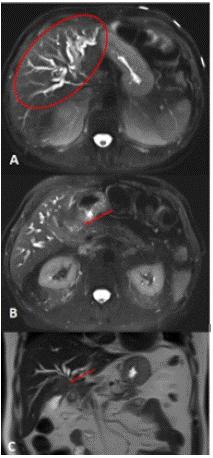
Figure 3: Preoperative MR of the abdomen without contrast. (A) Axial MR abdomen and plevis showing ductal dilation. (B) Axial MR mass (red arrow) and (C) coronal MR mass showed an elongated soft tissue mass approximately 5.0 x 1.5cm (red arrow) which was appreciated along anterior margin of portal vein producing biliary duct obstruction and dilated intrahepatic bile ducts.
Endoscopic Retrograde Cholangiopancreatography (ERCP) was done and a biopsy was performed in the upper third of the main bile duct (Figure 4). One absorbable stent was placed into the porta hepatis. The bile duct brushings cytology revealed rare atypical epithelioid cluster in a scantly cellular background also containing few nucleated and a nucleated squamous cells, epithelioid cells, debris and bacterial aggregates.
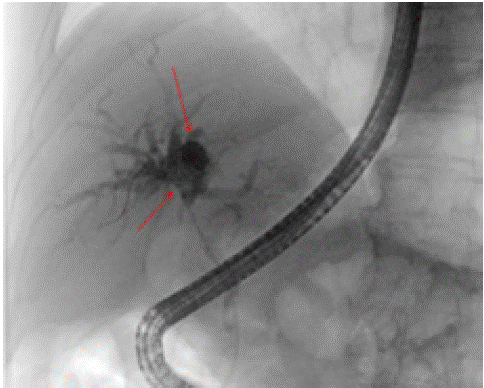
Figure 4: Endoscopic retrograde cholangiopancreatography (ERCP) images showing soft tissue mass measuring 5 cm x 1.5cm (red arrows). There was a severe biliary stricture in the upper third of the main bile duct which was malignant appearing. The left and right main hepatic duct and all intrahepatic branches as well as the common hepatic duct were severely dilated with a mass causing an obstruction.
Subsequently, he was admitted inpatient at an outside hospital for sepsis secondary to stent infection. He required antibiotics for about two weeks. During these admissions he has had around six biopsies which were all indeterminate (IR biliary biopsy brushing). The patient’s course was complicated by biliary stent infection requiring antibiotic and eventually had an IR transhepatic external biliary drains placed that required an upsize a month after placement. The biliary brush biopsies at the time showed atypical cells but were unable to reveal causative etiology.
The patient underwent exploratory laparotomy at an outside hospital three months later with intraoperative ultrasound evaluation, stomach serosal tear repair, attempted resection of the common intrahepatic duct tumor mass, choledochotomy, choledochectomy, and biopsy of the common hepatic duct. They could not resect his biliary obstruction site and thus had cholecystectomy.
The biopsies and gallbladder specimen were sent out to an outside facility for second opinion. Histopathology of the gallbladder tissue revealed follicular lymphoma grade 1-2, Ki-67 variable depending on location 5%-30%. Fish testing for MYC IGH BCl-2, MALT 1, BCL6 negative T-cell receptor gene rearrangement negative. The final report showed a follicular growth pattern and germinal center B-cell immunophenotype with partial CD10 expression which favored an extranodal follicular lymphoma with weak or absent BCL-2 expression representing a primary gallbladder or bile duct lymphoma.
The patient had Grade 2 follicular lymphoma, Lugano Stage IE with a single extra-lymphatic site in the absence of nodal involvement. Considering residual intrahepatic duct tumor, the patient was started on systemic therapy with rituximab weekly. He tolerated six weeks of rituximab. Based on the AUGMENT trial [11], the decision was made to add lenalidomide to the patient's treatment regimen which would be given daily (days 1-28). Concurrent rituximab treatment would be given every four weeks with a plan to complete a total of 18 months of rituximab plus lenalidomide. He would then transition to rituximab only maintenance therapy every 8 weeks for one year.
A repeat CT scan two month after the biopsy showed a 1.2 cm nodule in the right lung base. The internal/external biliary drains were in place. Biliary ductal dilatation with left pneumobilia was seen and prominent porta hepatis and peripancreatic lymph nodes.
Six months later, Magnetic Resonance Cholangiopancreatography (MRCP) (Figure 5) was done once follicular lymphoma was revealed by the biopsy. The MRCP revealed stable position of the internal and external biliary drains with mild prominence of the intrahepatic biliary ductal system, similar to the prior CT in this patient with a history of prior cholecystectomy, a stable ovoid nodular opacity in the posterolateral aspect of the right lower lobe. The hepatic parenchymal signal appeared homogeneous. He had IR Cholangiogram through existing biliary drains and subsequent biliary drain removal.
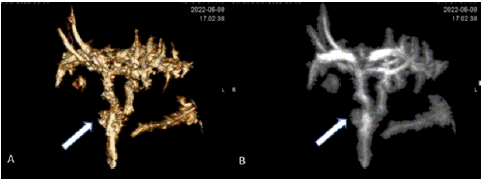
Figure 5: Postoperative MRCP findings shown in (A) Volume Rendering (VR) and (B) Maximum Intensity Projection (MIP). MRCP was performed six months after laparoscopic cholecystectomy and revealed surgically absent gallbladder and mild intrahepatic biliary ductal dilation that was not significantly different from the prior CT. The arrows point to the cystic duct.
Discussion
Primary biliary NHL of the gallbladder is rare and accounts for 0.4% of extranodal NHL which is approximately just 0.1–0.2% of all NHL cases [12,13]. Primary gallbladder lymphoma is defined as an extranodal lymphoma arising and confined to the gallbladder with or without contiguous lymph nodes involvement and distant spread.
A literature review revealed few cases of well-documented primary NHL involvement of the gallbladder. The patients present clinically with symptoms and signs indicating either biliary tract pathology or GI tumor. For example, Mitropoulos et al [14]identified 11 cases of primary Non-Hodgkin’s lymphoma of the gallbladder with a median age of presentation of 68 years of age with complaints of abdominal pain. The majority of patients with gallbladder lymphoma present with symptoms of cholecystitis [6]. Many of these cases of primary Non-Hodgkin’s lymphoma of the gallbladder were diagnosed incidentally in cholecystectomy specimens with very few cases being diagnosed from a fine needle aspirate. According to Mani et al, chronic inflammation acted as a substrate for primary gallbladder and likely caused lymphocytes to migrate to the gallbladder mucosa forming secondary follicles since the gallbladder is normally does not have lymphoid tissue.
In Ayub et al [15] they looked at a population-based analysis and identified 106 cases of Primary Non-Hodgkin’s lymphoma of the gallbladder and evaluated the clinical characteristics and treatments as well as survival outcome based on the SEER (Surveillance, Epidemiology, and End Results) database. They reported that patients with follicular lymphoma were younger at diagnosis (65.7 years) compared to patients with other forms of lymphoma such as Diffuse large B Cell lymphoma or Extranodal Marginal Zone lymphoma (70.3 years). They also saw that it mostly affected white males in their 70s. They observed that patients that had undergone surgical resection survived longer than those that did not. Their results also showed that patients that received adjuvant radiation therapy appeared to survive longer than those that only had surgical intervention.
The largest dataset of patients with primary GI non-Hodgkin’s lymphomas was reported by Shannon et al [16]. They identified 16,129 patients using the SEER database and surgical resection was done in about 46.9% of patients however surgery was not associated with improved survival. Here, we offer a case of follicular lymphoma camouflaged as gallbladder adenocarcinoma. To the best of our knowledge, there have only been a few cases reported of extranodal follicular lymphoma [5,7] which had similar presentation. Due to the rare nature of gallbladder lymphoma, there is currently no standard treatment.
The treatment options for gallbladder lymphoma depend on many factors such staging, prognosis and goals of care. Early gallbladder cancer that is only confined to the gallbladder is treatment with resection. Later stages indicate advanced cancer growth beyond the gallbladder. Limited-stage (Stage I or II) disease can benefit from radiotherapy. Treatment is typically 15-20 fractions over four-week period of time. Prior to initiation of radiotherapy a PET scan and bone marrow biopsy are done. According to Karia et al [1], in cases with urgent cholecystectomy for histological diagnosis is the best practice as well as adjuvant radiation and chemotherapy. Our patient had undergone unsuccessful resection of the mass and so systemic treatment was the next course of action. Of note, one must take into consideration the risk and benefits of attempting to biopsy hilar cholangiocarcinoma since a transperitoneal biopsy could exclude a patient from getting a liver transplant [17].
Systemic treatment with a chemotherapy backbone and a monoclonal antibody targeting CD20 is used such as bendamustine with rituximab. Rituximab is a monoclonal antibody directed against the CD20 antigen on the surface of B-lymphocytes that induces complement-mediated lysis as well as direct cytotoxicity and apoptosis. Bendamustine is an alkylating agent that causes intra and inter strand crosslinks between DNA bases resulting in cell death [18]. A combination of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone also known as R-CHOP is available. A third choice would be to remove the doxorubicin and use R-CVP or rituximab, cyclophosphamide, vincristine, and prednisone. Alternatively, obinutuzumab, which also targets CD20, can be used; CHOP plus obinutuzmab (O-CHOP) or bendamustine plus Obinutuzumab (OB).
The GALLIUM study [19] showed 1202 patients with follicular lymphoma randomized to chemotherapy with G-CHEMO (obinutuzumab plus CHOP, CVP or bendamustine) or R-chemo (rituximab plus CHOP, CVP or bendamustine) and then maintenance for two years. The results showed no difference in overall life expectancy, although more patients were progression-free with obinutuzumab.
The study by Rummel et al. in 2013 [20]compared bendamustine to CHOP as initial therapy for follicular lymphoma, which showed their significant delay in time to next treatment with bendamustine compared to CHOP as well as a smaller side effect profile with bendmustine. Multiple studies have demonstrated improved Progression–Free Survival (PFS) and overall survival for patients with follicular lymphoma and high tumor burden, establishing bendamustine and rituximab as good options for induction treatment [21]. Other treatment options include local radiotherapy or myeloablative therapy followed by stem cell transplant.
In other studies, the treatment regimen includes novel agents; for example, in the ECOG trial [21], bortezomib was added to bendamustine and rituximab with mixed results. The patients were randomized to bendamustine/rituximab induction followed by two-year rituximab maintenance (BR-R) or bendamustine/rituximab with Bortezomib and Rituximab maintenance (BVR-R) or BR followed by lenalidomide (one year) with rituximab maintenance (BR-LR). In the end, their results showed that neither the bortezomib-based induction platform nor the addition of lenalidomide to maintenance rituximab improved survival.
After people achieve remission, maintenance therapy with rituximab for two years is usually done. The PRIMA study [22]showed that two years of rituximab maintenance therapy after first-line treatment for follicular lymphoma significantly improved the PFS; however, it did not improve survival.
Some studies examine non-chemotherapy options, such as randomized trials to rituximab and lenalidomide, an oral agent. Systemic treatment for stage II to IV follicular lymphoma can consist of single agent-rituximab or rituximab with lenalidomide known as R2 [11,23] or RCHOP [24].
Lenalidomide is an immunomodulatory agent that binds the cereblon E3 ubiquitin ligase complex, which results in the recruitment, ubiquitination, and degradation of transcription factors Aiolos and Ikaros [11]. It act?i vates NK cells, which leads to heightened immune surveillance.
Rituximab with lenalidomide (R2) as a first treatment in previously untreated advanced follicular lymphoma was evaluated in the phase 3 RELEVANCE trial. R2 regimen was compared BR, R-CHOP, or R-CVP which is the same as R-CHOP without the doxorubicin. The patients received lenalidomide at a dose of 20mg/day or a dose of 10mg/day for the first six cycles if the creatinine clearance was between 30-59ml/min as in the case with our patient. In the remaining cycles, the patients received lenalidomide at a dose of 10mg/day for a total of 18 cycles as maintenance therapy. The results of this study had similar complete responses in both groups, and interim three-year Progression-Free Survival (PFS) was the same [11].
The AUGMENT trial compared monotherapy rituximab versus R2 which showed an improvement in PFS of 54% with R2 [23]. In the AUGMENT trial, they randomized patients with recurrent follicular lymphoma and marginal zone lymphoma into rituximab/lenalidomide versus rituximab/placebo group. The patients randomized to the R2 branch had more prolonged remission (39 months) than those in the placebo/rituximab group. In the MAGNIFY trial, lenalidomide plus rituximab followed by lenalidomide versus rituximab maintenance for relapsed/refractory follicular, marginal zone or mantle cell lymphoma showed the safety of the R2 regimen. The R2 regimen demonstrated promising clinical activity and is tolerable in untreated and relapsed or refractory patients with indolent NHL [11,23]. Based on this data, the patient was treated with R2, which was tolerated well. He successfully completed 18 cycles of rituximab plus lenalidomide and was started on rituximab maintenance that is planned for 12 months.
Factors that determine the outcome of treatment for lymphoma include the type of lymphoma, age, performance status, stage, bulk of disease, extranodal disease, and treatment. The FLIPI (Follicular Lymphoma International Prognosis Index) [25]estimates the 10-year overall survival based on clinical information. The FLIPI risk factors include: greater than 4 nodal sites, elevated LDH, age greater than 60 years, Stage III or IV and hemoglobin less than 120 g/L or 12 g/dL. The more attributes the patient has at the time of diagnosis, the worse the outcome in overall survival.
The up-front identification of patients at risk of inferior outcomes (prognostic markers) remains an unmet clinical need. Other Clinical indices like FLIPI-2, Follicular Lymphoma Evaluation Index (FLEX) [26, and the Protein-RNA Interaction Mapping Assay-Prognostic Index (PRIMA-PI) [27]are also capable of risk stratifying patients. Follicular lymphoma is the most common form of indolent NHL; however, cases of it primarily affecting the gallbladder are relatively rare in the literature. There is variability in the clinical spectrum, spanning from patients with limited-stage disease to those that follow a more aggressive clinical course demonstrating inferior outcomes [28].
Our patient has undergone treatment for gallbladder follicular lymphoma with biliary obstruction. R2 was well tolerated and is safe treatment for patients presenting with biliary obstruction. He is status post 18 cycles of rituximab and lenalidomide. He is currently on rituximab maintenance every 8 weeks and will continue this regimen for 12 months. Currently there is no clinical evidence of disease progression. He had a recent PET/CT to assess treatment response demonstrating no gross hypermetabolic evidence for malignancy or concerning masses or adenopathy noted.
References
- Karia M, Mitsopoulos G, Patel K, Rafique A, Sheth H. Primary Gallbladder Lymphoma in a Male Patient with No Risk Factors Detected Incidentally by CT Colonography. Case Reports in Surgery. 2015; 2015: 813708.
- Lazaridis C, Pavlidis T, Atmatzidis K, Makris J, Kalaitzis E, Papaziogas T. Primary non-Hodgkin’s lymphoma of the gallbladder. HPB. 1999; 1: 159-162.
- Kanas G, Ge W, Quek RGW, Keeven K, Nersesyan K, Arnason JE. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: population-level projections for 2020-2025. Leuk Lymphoma. 2022; 63: 54-63.
- Shi T, Alomari M, Yang P, Sadikovic B, Sangle N, Howlett CJ. Follicular lymphoma of the gallbladder: Use of immunoglobulin gene clonality studies to facilitate diagnosis of unusual case. AMP Molecular Case Reports. 2017: 1-5.
- Ferluga D, B Luzar, EM Gadzijev. Follicular lymphoma of the gallbladder and extrahepatic bile ducts. Virchows Arch. 2003; 442: 136-40.
- Mani H, Climent F, Colomo L, Pittaluga S, Raffeld M, Jaffe ES. Gall bladder and extrahepatic bile duct lymphomas: clinicopathological observations and biological implications. Am J Surg Pathol. 2010; 34: 1277-86.
- Sugawara G, Nagino M, Oda K, Nishio H, Ebata T, Nimura Y. Follicular lymphoma of the extrahepatic bile duct mimicking cholangiocarcinoma. Journal of Hepato-Biliary-Pancreatic Surgery. 2008; 15: 196-199.
- Yun SP, Seo HI. Diffuse large B-cell lymphoma presenting with cholecystitis-like symptoms. Korean J Clin Oncol. 2018; 14: 48-52.
- Gao F, Zhao H, Chen X, Dong X, Liu T, Fu X. Gallbladder non-Hodgkin’s lymphoma: Case report. International Journal of Surgery Case Reports. 2019; 61: 218-221.
- Khanlari M, Chapman JR. Follicular lymphoma: updates for pathologists. J Pathol Transl Med. 2022; 56: 1-15.
- Morschhauser F, Fowler NH, Feugier P, Bouabdallah R, Tilly H, Palomba ML, et al. Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N Engl J Med. 2018; 379: 934-947.
- Ono A, Tanoue S, Yamada Y, Takaji Y, Okada F, Matsumoto S, et al. Primary malignant lymphoma of the gallbladder: a case report and literature review. Br J Radiol. 2009; 82: e15-9.
- Adachi T, Haraguchi M, Irie J, Yoshimoto T, Uehara R, Ito S, et al. Gallbladder small cell carcinoma: a case report and literature review. Surgical Case Reports. 2016; 2: 71.
- Mitropoulos FA, Angelopoulou MK, Siakantaris MP, Rassidakis G, Vayiopoulos GA, Papalampros E, et al. Primary non-Hodgkin’s lymphoma of the gall bladder. Leuk Lymphoma. 2000; 40: 123-31.
- Ayub A, Rehmani S, Al-Ayoubi A, Lewis E, Santana-Rodrifuez N, Raad W, et al. Primary Non-Hodgkin’s Lymphoma of the Gallbladder: A Population-based Analysis. Anticancer Res. 2017; 37: 2581-2586.
- Shannon EM, MacQueen LT, Miller JM, Maggard-Gibbons M. Management of Primary Gastrointestinal Non-Hodgkin Lymphomas: a Population-Based Survival Analysis. J Gastrointest Surg. 2016; 20: 1141-9.
- Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012; 143: 88-98.e3; quiz e14.
- Plosker GL, Carter NJ. Bendamustine: a review of its use in the management of indolent non-Hodgkin lymphoma. Drugs. 2008; 68: 2645-60.
- Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N Engl J Med. 2017; 377: 1331-1344.
- Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013; 381: 1203-10.
- Evens AM, Hong F, Habermann TM, Advani RH, Gascoyne RD, Witzig TE, et al. A Three-Arm Randomized Phase II Study of Bendamustine/Rituximab with Bortezomib Induction or Lenalidomide Continuation in Untreated Follicular Lymphoma: ECOG-ACRIN E2408. Clin Cancer Res. 2020; 26: 4468-4477.
- Salles G, Seymour JF, Offner F, Lopez-Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011; 377: 42-51.
- Leonard JP, Trneny M, Izutsu K, Fowler NH, Hong X, Zhu J, et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol. 2019; 37: 1188-1199.
- Tomita N, Takasaki H, Fujisawa S, Miyashita K, Ogusa E, Kishimoto K, et al. Standard R-CHOP therapy in follicular lymphoma and diffuse large B-cell lymphoma. J Clin Exp Hematop. 2013; 53: 121-5.
- Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, et al. Follicular lymphoma international prognostic index. Blood. 2004; 104: 1258-65.
- Mir F, Mattiello F, Grigg A, Herold M, Hiddemann W, Marcus R, et al. Follicular Lymphoma Evaluation Index (FLEX): A new clinical prognostic model that is superior to existing risk scores for predicting progression-free survival and early treatment failure after frontline immunochemotherapy. Am J Hematol. 2020; 95: 1503-1510.
- Bachy E, Maurer MJ, Habermann TM, Gelas-Dore B, Maucort-Bulch D, Estell JA, et al. A simplified scoring system in de novo follicular lymphoma treated initially with immunochemotherapy. Blood. 2018; 132: 49-58.
- Kumar E, Pickard L, Okosun J. Pathogenesis of follicular lymphoma: genetics to the microenvironment to clinical translation. British Journal of Haematology. 2021; 194: 810-821.