
Case Report
Austin Oncol. 2016; 1(1): 1001.
Brain Metastasis and Intracranial Hemorrhage Following Bevacizumab Therapy for Rhabdomyosarcoma
Caitlin M¹, Jaszianne T2,3 and Kathleen AN4*
¹Children’s Mercy Hospitals and Clinics, USA
²Division of Clinical Pharmacology, Children’s Mercy Hospital and Clinics, USA
³Division of Pediatric Hematology/Oncology, Children’s Mercy Hospitals and Clinics, USA
*Corresponding author: Kathleen A Neville, Section of Clinical Pharmacology and Toxicology, University of Arkansas for Medical Sciences, USA
Received: November 24, 2015; Accepted: January 27, 2016; Published: January 29, 2016
Abstract
Brain metastases in childhood solid tumors are rare and portend a poor prognosis. We report a patient with relapsed rhabdomyosarcoma who developed brain metastases and intracranial hemorrhage following treatment with the antiangiogenic agent bevacizumab. Current reports identify the potential of antiangiogenic therapies to promote methods for cancer to metastasize, perhaps explaining this patient’s uncommon presentation of metastatic brain lesions. Reports have also established an increased risk of bleeding with bevacizumab in adults, however, this has not been clearly defined in the pediatric population. This lack of information demonstrates a critical need for further research to evaluate the safety of bevacizumab in children.
Keywords: Bevacizumab; Children; Intracranial Hemorrhage; Metastasis
Introduction
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma of childhood, accounting for 40% of all pediatric soft tissue sarcomas, with more than half of cases presenting in children younger than age 10 [1]. While RMS is known for its aggressive nature and potential for disseminated disease, metastases to the brain are considered rare in children [2-4]. In a retrospective study [2], 10 of 419 (2.4%) pediatric patients with RMS developed brain metastasis. The present case describes a teenaged male with relapsed RMS who was treated with bevacizumab according to the Children’s Oncology Group (COG) ARST0921 protocol. Following six cycles of treatment the patient rapidly developed central nervous system symptoms and Magnetic Resonance Imaging (MRI) revealed multiple metastatic brain lesions complicated by intracranial hemorrhage. This case demonstrates the atypical finding of metastasis to the brain in RMS and the unique manifestation of severe intracranial hemorrhage after bevacizumab therapy.
Case Presentation
We present a 17 year-old male with relapsed alveolar rhabdomyosarcoma, first diagnosed at age 15 with localized disease in the Hypothenar eminence of his left hand. Staging revealed metastasis to both the epitrochlear and axillary nodes. Initial treatment included surgical resection, radiation therapy (4140cGY), and chemotherapy according to the ARST0531 protocol, consisting of vincristine, dactinomycin, cyclophosphamide, and irinotecan. The patient had an excellent response, achieving complete remission within 13 months.
The patient remained off therapy and in good health until 17 months after diagnosis when he developed a palpable mass near the inferior border of his xyphoid process. Pathology of the 4.1cm x 3.8cm x 5.2 cm suprahepatic mass was consistent with relapsed alveolar rhabdomyosarcoma. Despite gross total resection, the mass recurred one month later with two adjacent satellite nodules in the right juxtacardiac region. At this time, the patient was started on salvage therapy per the COG Phase II protocol ARST0921, which included vinorelbine, cyclophosphamide, and bevacizumab (15mg/ kg intravenously on day 1 of each cycle per protocol Study Arm A).The patient tolerated the first five cycles of chemotherapy without any neurologic changes or extenuating chemotherapy side effects. A Computed Tomography (CT) scan of the chest, abdomen, and pelvis prior to the second cycle of chemotherapy revealed improvement in the suprahepatic mass size with a decrease in tumor volume by >20% and no evidence of new nodules or disease progression. Prior to the sixth cycle of chemotherapy, the patient reported having symptoms of intermittent blurred vision. He was referred to an ophthalmologist who reported a normal exam. Laboratory evaluation prior to cycle 6 revealed an elevated D-dimer of 6.72 (normal <0.50 mcg/mL), an increased fibrinogen of 499 (normal 150-400 mg/dL) and elevated PTT of 40.3 (normal 25-35 seconds). All other exam and laboratory findings were not clinically significant to inhibit study participation thus the patient continued on the protocol.
Routine imaging performed after the completion of cycle 6 therapy demonstrated an increase in size of the previously noted suprahepatic mass (4.2cm x 9.2cm x 11.3cm), with mass effect on the liver and invasion of the two right cardiophrenic angle nodules. An MRI and MRA were subsequently scheduled due to continued complaints of blurred vision and diplopia. The imaging revealed multiple round lesions in the bilateral frontal lobes and cerebellar regions, as well as expansive hemorrhages throughout the parenchyma. These findings warranted removing the patient from the study.
Approximately 4 days after the intracranial bleeding was identified, the patient presented to the emergency department with severe headache, blurry vision, vomiting, and body aches. He had last received chemotherapy three weeks prior to presentation in the emergency department. His D-dimer was noted to be >20 mcg/ mL. A repeat MRI and MRA of the brain were obtained and redemonstrated areas of supratentorial and infratentorial hemorrhage with vasogenic edema and a significant increase in size of all lesions. These hemorrhagic lesions were present in the patient’s medial right frontal lobe, left midfrontal lobes, bilateral cerebellar hemispheres, and left pons (Figure 1). The hemorrhages correlated with the patient’s hypocoagulable state, but it was uncertain how these lesions related to his history of metastatic RMS. A normal echocardiogram with good biventricular function and no evidence of vegetations alleviated the suspicion of embolic phenomena. The patient was admitted to the hospital for further management. He was discharged home shortly after admission with hospice care and died 15 days later.
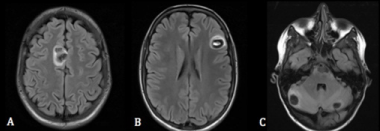
Figure 1: T2 weighted MRI of the brain without contrast demonstrating
hemorrhagic, edematous lesions within A) right superior frontal gyrus, B) left
midfrontal lobe, C) ventral pons and peripheral cerebellum.
Discussion
The brain is an uncommon site of metastasis in pediatric solid tumors [3,4]. Several reports suggest, however, that with improvements in long-term survival and imaging techniques more children are being diagnosed with brain metastases [3-5]. Unfortunately, treatment outcomes in these children are dismal with most patients surviving less than 6 months after brain metastases are reported [4]. Due to this patient’s abrupt onset of neurological symptoms prior to MRI detection of intracranial metastasis and ensuing rapid demise, this led to the consideration of other contributing factors to explain the patient’s rapid progression.
Bevacizumab is a humanized monoclonal antibody that disrupts tumor angiogenesis by inhibiting Vascular Endothelial Growth Factor (VEGF) [6]. It is currently approved in adults for treatment of various malignancies and is used in the pediatric population as an alternative therapy for otherwise unresponsive tumors [7-8]. While the hematologic toxicities of bevacizumab are well known in adults, such a relationship has not been clearly established in children. A phase I trial of bevacizumab in pediatric patients with refractory solid tumors found that treatment was well tolerated without observed hemorrhage or thrombosis, and no dose-limiting toxicities were identified [8]. In contrast, a separate prospective cohort study evaluated the safety of bevacizumab in pediatric populations and reported overall incidences of serious adverse events as high as 17% [6]. These results indicate a critical need for further studies to evaluate the safety profile of bevacizumab in children.
Understanding bevacizumab’s inhibitory effect on VEGF signaling may help explain its association with bleeding. Carden et al. studied the risk of intracranial bleeding during anti-VEGF therapy and postulated that VEGF antagonism adversely affects endothelial cell proliferation, survival, and blood vessel integrity leading to increased fragility of the vasculature [9]. Additionally, these agents may lead to reductions in nitric oxide and prostacyclin, creating a predisposition to hypertension and thromboembolic events [10].
VEGF blockade may also increase the potential for metastasis in childhood solid tumors. Bergers and Hanahan [11] found that hypoxic conditions caused by anti-angiogenic treatment led to recruitment of vascular progenitor cells that assist the tumor in re-initiating angiogenesis. Additionally, when angiogenesis is suppressed by agents like bevacizumab, disruption of the remaining tumor vasculature architecture may promote other methods for tumor cells to survive, proliferate, and invade into existing blood vessels – thus potentially promoting hematologic metastasis [11]. Fischer et al. [12] reported rare extra neural ependymoma metastases following bevacizumab and suggested the means highlighted above as a possible mechanism.
In summary, brain metastases are rare in children with RMS. The patient in this case had an unusual presentation of multiple metastatic brain lesions complicated by severe intracranial hemorrhage. The patient’s abrupt presentation; elevations in D-dimer, PTT, and fibrinogen; and prominent MRI findings after receiving bevacizumab; along with the scientific findings surrounding anti-VEGF therapy, suggest a possible correlation between anti-angiogenic therapy and the development of distant metastasis and bleeding. Additional research is warranted to investigate the means of cerebral metastasis in RMS and any correlations with anti-angiogenic agents.
Conflict of Interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper, nor do they have any commercial or proprietary interest in the drugs, materials, and/or devices discussed in this report.
References
- Ognjanovic S, Linabery AM, Charbonneau B, Ross JA. Trends in childhood rhabdomyosarcoma incidence and survival in the United States, 1975-2005. Cancer. 2009; 115: 4218-4226.
- Parasuraman S, Langston J, Rao BN, Poquette CA, Jenkins JJ, Merchant T, et al. Brain metastases in pediatric Ewing sarcoma and rhabdomyosarcoma: the St. Jude Children’s Research Hospital experience. J Pediatr Hematol Oncol. 1999; 21: 370-377.
- Bouffet E, Doumi N, Thiesse P, Mottolese C, Jouvet A, Lacroze M,et al. Brain metastases in children with solid tumors. Cancer. 1997; 79: 403-410.
- Kebudi R, Ayan I, Görgün O, Agaoglu FY, Vural S, Darendeliler E. Brain metastasis in pediatric extracranial solid tumors: survey and literature review. J Neurooncol. 2005; 71: 43-48.
- Osawa S, Kumabe T, Saito R, Sonoda Y, Niizuma H, Watanabe M, et al. Infratentorial brain metastases of pediatric non-epithelial malignant tumors: three case reports. Brain Tumor Pathol. 2011; 28: 167-174.
- de Pasquale MD, Castellano A, de Sio L, de Laurentis C, Mastronuzzi A, Serra A, et al. Bevacizumab in pediatric patients: how safe is it? Anticancer Res. 2011; 11: 3953-3957.
- Besse B, Lasserre SF, Compton P, Huang J, Augustus S, Ulrich-Peter R. Bevacizumab Safety in Patients with Central Nervous System Metastases. Clin Cancer Res. 2010; 16: 269-278.
- Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, et al. Phase I Trial and Pharmacokinetic Study of Bevacizumab in Pediatric Patients With Refractory Solid Tumors: A Children’s Oncology Group Study. J Clin Oncol. 2008; 26: 399-405.
- Carden CP, Larkin J MG, Rosenthal MA. What is the risk of intracranial bleeding during anti-VEGF therapy? Neuro Oncol. 2008; 10: 624–630.
- Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007; 96: 1788-1795.
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008; 8: 592-603.
- Fischer C, Hague SS, Huse JT, Blochin E, Souweidane MM, Lis E, et al. Extraneural ependymoma: Distant bone, lung, liver, and lymph node metastases following bevacizumab. Pediatric Blood Cancer. 2013; 60: 143- 145.