
Review Article
Austin Oncol. 2016; 1(1): 1002.
Proton Mr Spectroscopy, Fundamental Physics and Clinical Applications
Kemal Arda¹* and Hasan Aydin²
¹Ankara Ataturk Rearch Hospital / Radiology Department, Turkey
²Dr. Abdurrahman Yurtaslan Oncology Training and Research Hospital/ Radiology Department, Turkey
*Corresponding author: Kemal Arda, 3281 Sk. 10/47 Yasamkent/Ankara, Turkey
Received: December 09, 2015; Accepted: January 27, 2016; Published: January 29, 2016
Abstract
Proton MR spectroscopy (H-MRS) is one of the ultrahigh MR imaging technique that has recently been used to evaluate the metabolic alterations of tissues, a molecular imaging approach, mostly depend upon the evaluation of in vivo molecular cells and tissues which allows in vivo molecular studies, inappropriate spatial resolution is one of the major shortcoming of this technique in the clinical practice.
The routine metabolites which are identified with short and long TE are: N-Acetyl Aspartate (NAA), Creatine (Cr), Choline (Cho), Lactate (Lac). Using short TE, some additional metabolites are identified such as; Lipids (lip), Glutamine and glutamate (Glx), Myo-Inositol (mI).
H-MRS was mostly performed for brain tumors, sleep apnea, epilepsy,breast lesions, thyroid nodules, prostatic tumors in order to aid and assist for diagnosis and planning of treatment.
Proton MR Spectroscopy can be combined to routine MR imaging in variable conditions by detecting metabolite alterations and measuring their resonance peak levels.
Keywords: MRI; Spectroscopy; Physics; Tumor; Nodule; Carcinoma
Introduction
Proton MR spectroscopy (H-MRS) is one of the ultrahigh MR imaging technique that has recently been used to evaluate the metabolic alterations of tissues, at first, this technique is used for the assessment and interpretation of metabolic changes in the central nervous system, mainly for brain that has been used to observe metabolite changes for different intracranial pathologies such as tumors, multiple sclerosis, stroke, tuberculomas, epilepsy, metabolic and inherited brain disorders, and traumatic injuries, nowadays its clinical use and applications are widened, evolving thyroid, breast, prostate etc [1-4].
H-MRS is believed to be a molecular imaging approach, mostly depend upon the evaluation of in vivo molecular cells and tissues which allows in vivo molecular studies, inappropriate spatial resolution is one of the major short coming of this technique in the clinical practice [2-4]. H-MRS can be acquired as an additional pulse sequence to routine MRI sequences and contribute to a multimodality study of functional and metabolic information rather than morphologic tissue properties. It aids in the diagnosis, treatment, follow up and therapy response of patients especially against brain tumors, helps to achieve the best outcome of patients [2,3,5,6].
MR spectroscopy is an unlimited technique which indicates some certain metabolites in the metabolic spectrum and one has to know the normal metabolic position of a tissue before the interpretation of metabolic alterations inside it [5-6].
Fundamental Physical Principles of H-MRS
The physical principles of H-MRS are similar to conventional MRI, the magnetic properties of atomic nucleus are the fundamentals for both methods. Various matters which have different electrical charges will have different velocities in a certain magnetic field which may provide the measurement of various metabolites [7-8].
The strength of the MR signal is directly proportional to the number of protons of that frequency in spectroscopy, spectroscopy can be described in the time domain, whereas MRS data is usually displayed in the frequency domain, area under a specific peak in the frequency domain is proportional to the number of protons, resonating at that certain frequency [8-9].
The frequency axis is proportional to the magnetic field strength so the peak locations on the axis will depend on the B0, due to the lack of natural tissue that shows zero frequency, substances are mixed to be measured with a reference but these reference materials are toxic and cannot be used in in-vivo spectroscopy. By this method, scientists express the frequency difference between the substance (that will be measured) and the reference as a non-dimension quality, quality value is represented by dcs (in parts per million) [9].Then this equation is used as follows;
dcs = f s / (f transmitter x 10-6 ) + offset
In this equation, f s represents the frequency of the sample, f transmitter shows the frequency of the transmitter and offset is the constant that references the ppm scale (in in-vivo standards). This standard is usually N-acetyl aspartate (NAA) (that has a chemical shift value of 2,01 ppm) peak for H-MRS [2-4,7-8]. MR spectrum (Figure 1) is obtained by using water supression and spectroscopic sequences as Point-Resolved Spectroscopy (PRESS), Stimulated Echo Acquisition Mode (STEAM) etc. In order to get optimized water saturation with consistent water resonance and water suppression pulses, automatic shimming of the linear x, y, z gradients have to be used for optimization of Field Of View (FOV) homogeneity, time domain data is multiplied with a Gaussian function of 90 (Centre 0, half width 256 ms), 2D Fourier transformed phase and with corrected base-line, quantified by means of frequency domain curve fitting with the assumption of a Gaussian line shape’’ by using system manufacturers [1,3,7,8]. Due to the magnetic field in homogeneities, shimming is required for H-MRS spectral data and the acquisition in which B0 field has to be performed as homogeneous as possible, use of high-channel shim coils and shimming of linear gradient coils aid in improved outcomes, particularly important at higher magnetic field strengths due to increase of induced susceptibility shifts with increased Tesla gradients [2,3,5,8-10].
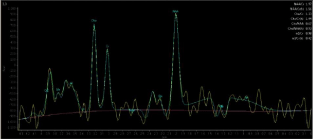
Figure 1: Normal H-MRS Spectrum obtained with 144 ms time of echo.
Free flowing water molecule resonances have to be suppressed in order to obtain peak metabolite resonances, at first, water suppression involves a sum of three water-selective Radiofrequency (RF) pulses application, then acquisition of dephasing gradient pulses which inhibit and null the water peak resonance, water suppression is achieved by using 110°-120° RF pulses with selective inversion recovery and starting the measurement at zero point crossing of the water signal [3,8,9,11].
STEAM is composed of three 90-degree pulses to obtain a stimulated echo, whereas PRESS technique uses one 90-degree pulse and two 180-degree pulses for obtaining an echo [9-11].
STEAM can be performed with a very short echo time (TE), A precise voxel is created with this method and there is an incomplete recovery of signal, STEAM users aim to minimize power deposition via using high field (>3T) systems which can generate ultra short time of echo (<20 mseconds) [8-10].
PRESS can be performed with a long or a short TE which induce the complete recovery of signals. This technique provides double signal to noise ratio (SNR) when compared to the STEAM.
Single Voxel (SV) or Multi Voxel Techniques (MV) can be applied with either PRESS or STEAM for localization which is acquired in 3-5 minutes. Voxel positions are extremely important, some parts containing lipids- bone and air has to be avoided when choosing a place to locate the voxel. The main limitation for single voxel technique is the assessment of only one single area during an acquisition [9].
Multivoxel technique enables to re-position of a number of voxels simultaneously, MV technique can be performed as a 2D or 3D, by using MV technique, the contra lateral side and extension of lesions can be assessed more accurately.
Metabolites in H-MRS
The routine metabolites which are identified with short and long TE are: N-Acetyl Aspartate (NAA), Creatine (Cr), Choline (Cho), Lactate (Lac). Using short TE, some additional metabolites are identified such as; Lipids (lip), Glutamine and glutamate (Glx), Myo- Inositol (mI) (1,8,11-14).
For MR spectrum, 0 to 4.35 ppm is analysed. Metabolite signal peaks are observed as follows; N-Acetyl Aspartate (NAA) at 2 ppm, Choline (Cho) at 3.2 ppm, Glutamate (Glt) and Glutamine (Glx) at 2.45 ppm, Creatine (Cr) at 3-3.1 ppm, Phosphocreatine (Cr2) at 3.8- 3.9 ppm, , Glycine and or Myo-Inositol (Gly-MI) at 3.6-3.75 ppm, Lipids (Lip) at 0.9-1.3 ppm, Lactate (Lac) at 1.3-1.4 ppm [1,6,12-14].
NAA is the marker of neuronal viability, Cho is a component of phospholipid metabolism that shows cellular membrane turnover and reflects cellular proliferation, Cr is the marker of cerebral metabolism and is used as an internal reference, mI is a very important osmolyte and plays a role in the regulation of cell volume, this metabolite is also a glial marker of astrocytes and a product of myelin degredation [4,9,14]. Lac shows anaerobic glycolysis and lip indicates disruption or necrosis of myelin sheath, In addition, some other metabolites as alanine (Ala) (especially in the diagnosis of menengiomas), Glx (for depiction of chronic hepatic encephalopathy and hypoxic encephalopathy), succinate (used in the diagnosis of abcess) etc. may aid the clinical diagnosis [14].
In the former studies, Cho/NAA and Cho/Cr ratios are mostly used to distinguish brain tumors from non-neoplastic disorders. As a general belief; Cho resonance is the best discriminator in the grading of cerebral gliomas, Cho peak obviously increases from low-grade to Glioblastome Multiforme (GBM) [1,6,7,14-18]. The other important index of distinguishing low-grade glial tumors from malignant gliomas, is the amount of lipids, resonance changes of NAA and Cr peaks have less potential in the determination of malignancy of cerebral tumors [1,14,15,17]. However, in addition to high-grade gliomas, metastases also yield extremely elevated Cho and significantly reduced NAA and Cr, Cho/ Cr and Cho/NAA ratios are also valuable in determining the malignancy of metastatic lesions [5- 12, 13, 16-18].
Brain Tumours and H-MRS
Various types of non-neoplastic brain lesions: Infections, demyelinating lesions, ischemia etc. can be misdiagnosed as brain tumors, H-MRS may provide important data in the diagnosis of miscellaneous brain lesions which provides important information related to the cell proliferation, neuronal integrity, energy metabolism and necrotic transformation of brain or neoplastic tissues [1,8, 12-21]. This technique is especially used for the routine brain MR imaging in order to solve diagnostic problems such as differentiation of low and high grade tumors, neoplastic and non-neoplastic lesions, ischemia from low grade gliomas or discriminating the metastases from primary brain tumors, H-MRS has been used to study tumor biology, grade gliomas, biopsy procedures and plan treatment [14-21].
Aydin et al. (1) evaluated 62 patients (33 patients with intracranial mass and 29 patients with non-neoplastic brain lesions) by H-MRS. They reported that NAA/Cr ratio was extremely higher than tumor group, higher Cho/NAA and Cho/mI ratios with lower NAA/Cr ratios were most likely to be malignant. Besides, additional lipid and lactate peaks were generally seen in malignant group in this research. In the previous literature, they tried to analyze the efficacy of H-MRS in brain tumors and most of the authors used Cho/NAA, Cho/Cr ratios to differentiate brain tumors from non-neoplastic disorders [16,19-21]. Cho peak was extremely high in GBM group (Figure 2), in addition to high-grade gliomas, metastases were also assumed to have extremely increased Cho levels, significantly reduced NAA and Cr levels [1,14-21].
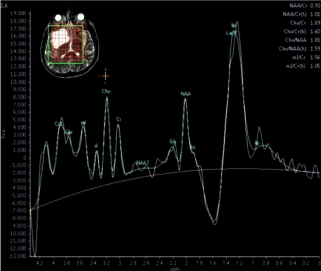
Figure 2: Metabolite peaks in high grade glial tumour.
MR-Spectroscopy may also reveal reduced NAA without increased Cho and H-MRS findings may spare the patient from unnecessary biopsies [16,18-21]. Most of the authors indicated that Cho/NAA, Cho/Cr ratios were the valuable tools in the evaluation of malignancy of cranial neoplasms. Most of the former researches in the literature stated that Cho/NAA and Cho/Cr ratios increased from low grade to high-grade gliomas and these ratios were also extremely higher in metastases and primitive neuro-ectodermal tumors (PNET) [19-22].
In conclusion, H-MRS was a very important diagnostic tool, providing many important datas in assessment of brain lesions and researchers supply important informations to the relevant literature by using this advanced MRI technique.
H-MRS in Thyroid Nodules
Majority of thyroid nodules are benign but have to be distinguished from rare malignant nodules, clinical examination, ultrasonography (US), radionuclide scintigraphy and fine needle aspiration biopsy (FNAB) are the common methods in the assessment of thyroid nodules [23-28]. US has been used as a first step for the evaluation of thyroid nodules, hypofunctional nodules diagnosed via scintigraphy may aid in the diagnosis of malignant thyroid nodules with a very low specificity [25]. Cytologic evaluation after FNAB is a reliable approach in order to distinguish malignant and benign nodules, reduces the number of diagnostic thyroidectomies performed for uncharacterized thyroid lesions and increases the complete initial surgical treatment for malignant thyroid nodules [25-26]. However, the results of FNAB in multinodular goiter may not be as reliable as in solitary nodules, up to 80% of thyroid carcinomas may not be detected by FNAB in most patients with multinodular goiter and also false-negative results cannot be avoided totally [25,26,29,30]. Routine MRI sequences (T1 and T2 weighted) has also a limited role in the assesment of thyroid nodules [25, 30-31].
H-MRS has been used in various regions of the body, but unfortunately neck region may cause technical difficulties such as shimming and subject motion for in vivo spectroscopy. Literature about H-MRS of thyroid lesions is very limited due to the technical difficulties of spectroscopic acquisitions of the thyroid and neck, Swallowing and breathing may cause motion artefacts, shimming difficulties may arise from large magnetic susceptibility differences between air in the trachea and the neck, contamination of spectra by adjacent fat is possible [32,33].
King et al. [27] showed Cho peaks in 87% of thyroid carcinomas, reported mean Cho/Cr ratio of about 4.3- 5.4, they also showed that no Cho and Cr peaks were detected in the normal thyroid tissue at 1.5 T in their research. Gupta et al. [30] presented 88.8% sensitivity and 100% specificity of H-MRS with correspondence to the Cho peak in eight malignant nodules, but in two benign colloidal nodules, they also indicated significant Cho peak (80% positive predictive value and 100% negative predictive value).
We stated that malignant nodules had significantly higher Cho/ Cr ratios than the benign nodules, revealed 92.3% sensitivity and 100% specificity of Cho/Cr ratio for the depiction of malignancy [34].
As a result, H-MRS has technical difficulties like motion artifacts, shimming difficulties and contamination of spectra by adjacent fat but technical developments of H-MRS sequences with advanced coils in the field of =3T MRI can overcome these disadvantages and provide really important information for thyroid nodules’ malignancy [27,28,30,34].
H-MRS in Breast Imaging
H-MRS can be performed as a complementary technique to breast MR imaging and usually performed with single-voxel technique [35,38]. Breast MRI has an increasing role in the identifications of lesions and determine the extent of disease [39-41]. The architectural assessment and dynamic contrast uptake are the pathognomonic measures for breast MR interpretation, additional metabolic measures may be efficient with MR Spectroscopy and it provides biochemical measuremants of tumor metabolism [37,38,39,41].
Previous researchers had reported sensitivities of 70-100% and specificities of 67-100% for H-MRS in the application upon breast lesions. They all suggested that H-MRS might supplement breast MR imaging, reducing the number of biopsies especially in the diagnosis of benign ones [36].
Aydin et al. used single voxel spectroscopic technique for lesion characterization to confirm benign and malignant BI-RADS 3 and 4 lesions, predicted 89% sensitivity and 60% specificity for all BI-RADS 3 and 4 masses with H-MRS in terms of presence of choline in the lesions [87].
In the previous reports, many authors presented that the voxel size differences among tumors could contribute and influence the results of H-MRS as they could not use the voxel sizes lower than 10 mm3 due to technical problems [36,38,42]. The elevation of composite choline levels about the threshold of choline signal-to noise ratio 2 or more, was used to discriminate between benign and malignant breast masses, Elevated levels of choline metabolites had been reported in many studies of excised human breast tumors [37-39].
The use of long echo times (=135 msec.) led to an improved visibility of the choline compounds in the spectra because of a decreased overlap with the lipid signal that had no diagnostic value in breast tumours. Thus, breast H-MRS examination should be performed with a long echo time (135-270 msec.) to increase the visibility of composite choline signal [41-42].
Joe et al showed that there were changes in the linewidth, increase of 15-21% and area and decrease of 11-18% of the Cho peak in the same subjects with pre and postinjection of gadodiamide [42]. Lenkinski et al [43] reported that negatively charged chelates; gadopentetate dimeglumine, gadobenat dimeglumine, gadoterate meglumine broadened the Cho peak and reduced the area of Cho peak in vivo by an average of 40%, recommended to use neutral chelates such as gadodiamide, gadoversetamid, gadoteridol and gadobutrol in MRI/ MRS studies of the breast, as they had smaller changes in the Cho peak after the administration of contrast agent in vivo studies and the effect of such gadolinium-based neutral contrast agents on the MRspectra was negligible.
H-MRS can be performed after unhanced and contrast-enhanced breast MRI with entire examination not more than 40 min, can allow higher sensitivity than the routine breast MR imaging, further training of radiologists to interpret breast MR spectroscopy may potentially increase the diagnostic accuracy of breast diseases.
H-MRS in Prostate Gland
Clnical examination and Prostate Spesific Antigen (PSA) levels provide important diagnostic data for the depiction of prostate cancer in elderly men, cancer foci should be localised by imaging tools and after localization of the malignant prostate nodule, biopsy is needed confirm the diagnosis, cancer focus in the prostate gland generally appear hypoechoic on Transrectal Ultrasonography (TRUS), but many small malignant nodules are isoechoic and not easy to localize them by US [44-46]. Moreover, many benign conditions may mimic cancer focus which may lower the specificity of TRUS and cause elevation of false-negative rates up to 40% [45-47]. An additional diagnostic approach is needed because of lower sensitivity and specificity of TRUS, it has already been known that up to 25% of cancers have a normal PSA level and greater than 50% of cancer patients have a normal digital rectal examination results [45-49].
MRI provides the best depiction of prostate contours and internal zonal anatomy, MRI also allows functional gland assessment with dynamic contrast-enhanced MR imaging (DCE-MRI), Diffusion Weighted Imaging (DWI) and H-MRS. MRI is highly sensitive and specific for detection, staging and localization of prostate cancer, also indicates the extra capsular spread due to its high soft tissue resolution [45,50].
Futterer et al [48] regarded 77% to 80% sensitivity and 84% to 87% specificity for H-MRS and indicated that H-MRS and dynamic MRI were significantly more accurate than the T2W sequence for prostate cancer localization.
Wetter et al [49] reported 75% sensitivity and 87% specificity for MRI and 88% sensitivity to 70% specificity for H-MRS, concluded that combined MRI and H-MRS of the prostate had no diagnostic advantage in the staging of cancer over routine MRI alone.
Reinsberg et al [50], Kumar et al [51] combined DWI and H-MRS for detecting prostate cancers in their investigations and both of them regarded significantly higher Cho/Cit ratios and lower ADC values in tumor containing voxels with 80% to 90% sensitivities and specificities.
According to our experience, The specificity was 49% for both Cho/Cit and Cho+Cre/Cit ratios, 69% sensitivity for Cho/Cit ratio, and 70% sensitivity for Cho+Cre/Cit ratio with 3D-H-MRS and it was presented the H-MRS as the most efficient sequence in detecting the cancer foci, in terms of Cho+Cre/Cit and Cho/Cit ratios (Figure 3). To our belief, however, multiparametric MRI which is composed of T2W imaging, DCE-MRI, DWI and H-MRS, rather than use of either sequences alone, can accurately improve the detection, localization and staging of prostate cancers.
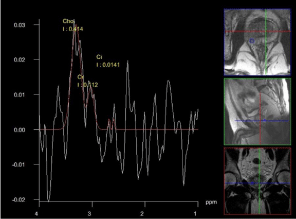
Figure 3: 3D H-MRS in prostate gland showing the suspicious area for
cancer.
Conclusion
Proton MR Spectroscopy can be combined to routine MR imaging in variable conditions by detecting metabolite alterations and measuring their resonance peak levels. This technique can be routinely performed under elective situations on clinical 1.0 T and more strength magnets with suitable acquisition times. It can also be used in clinical practice to guide the clinicians and surgeons in the detection of most aggressive parts of tumor, may avoid unnecessary surgeries which may reduce the morbidity and mortality due to iatrogenic effects and may guide them to a better management of patients.
With further development of coils and softwares, moreover an increase in magnetic field strengths and advanced technology, there is going to be an improved sensitivity with increasing signal to noise ratios which will result in high-quality spectroscopy and increased spectral resolution. New advanced pulses may allow more fast data acquisitions in the future and 3D-HMRS may easily achieve spatial distribution of spectroscopic data.
References
- Aydin H, Sipahioğlu S, Oktay NA, Altin E, KizilgÃz V, Hekimoglu B. The value of proton MR-spectroscopy in the differentiation of brain tumours from non-neoplastic brain lesions. JBR-BTR. 2011; 94: 1-10
- Ross B, Michaelis T. Clinical applications of magnetic resonance spectroscopy. Magn Reson Q. 1994; 10: 191-247.
- Cousins JP. Clinical MR spectroscopy: fundamentals, current applications, and future potential. AJR Am J Roentgenol. 1995; 164: 1337-1347.
- Barker PB (2004) Fundamentals of MR spectroscopy. In: Clinical MR Neuroimaging. Diffusion, Perfusion and Spectroscopy. Gillard JH, Waldman AD, Barker PB (Eds.), Cambridge University Press, UK.
- Parmar H, Lim TC, Yin H, Chua V, Khin LW, Raidy T, Hui F. Multi-voxel MR spectroscopic imaging of the brain: utility in clinical setting-initial results. Eur J Radiol. 2005; 55: 401-408.
- Sibtain NA, Howe FA, Saunders DE. The clinical value of proton magnetic resonance spectroscopy in adult brain tumours. Clin Radiol. 2007; 62: 109- 119.
- Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol. 2009; 64: 12-21.
- Castillo M, Kwock L, Mukherji SK. Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol. 1996; 17: 1-15.
- Fayed N, Olmos S, Morales H, Modrego PJ (2006) Physical basis of magnetic resonance spectroscopy and its application to central nervous system diseases. Am J Applied Sci 3:1836-1845.
- Mullins ME. MR spectroscopy: truly molecular imaging; past, present and future. Neuroimaging Clin N Am. 2006; 16: 605-618, viii.
- Arslanoglu A, Bonekamp D, Barker PB, Horská A. Quantitative proton MR spectroscopic imaging of the mesial temporal lobe. J Magn Reson Imaging. 2004; 20: 772-778.
- Möller-Hartmann W, Herminghaus S, Krings T, Marquardt G, Lanfermann H, Pilatus U, Zanella FE. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002; 44: 371-381.
- Callot V, Galanaud D, Le Fur Y, Confort-Gouny S, Ranjeva JP, Cozzone PJ. (1)H MR spectroscopy of human brain tumours: a practical approach. Eur J Radiol. 2008; 67: 268-274.
- Hasan A, Volkan K, Gunes TI et al. (2013) Clinical Proton-Mr-Spectroscopy, Past-Present and Future Applications-An Update. J Med Diagn Meth S8: 001. doi:10.4172/2168-9784.S8-001
- Aydin H, Oktay NA, Sipahioglu S et al.(2010) The Efficacy and Value of Proton MR Spectroscopy in Evaluating the Brain Tumours. The New Journal of Medicine 27: 37-42.
- Hourani R, Brant LJ, Rizk T, Weingart JD, Barker PB, Horská A. Can proton MR spectroscopic and perfusion imaging differentiate between neoplastic and nonneoplastic brain lesions in adults? AJNR Am J Neuroradiol. 2008; 29: 366-372.
- Majós C, Aguilera C, Alonso J, Julià-Sapé M, Castañer S, Sánchez JJ, Samitier A. Proton MR spectroscopy improves discrimination between tumor and pseudotumoral lesion in solid brain masses. AJNR Am J Neuroradiol. 2009; 30: 544-551.
- Poptani H, Gupta RK, Roy R, Pandey R, Jain VK, Chhabra DK. Characterization of intracranial mass lesions with in vivo proton MR spectroscopy. AJNR Am J Neuroradiol. 1995; 16: 1593-1603.
- Majós C, Aguilera C, Alonso J, Julià-Sapé M, Castañer S, Sánchez JJ, Samitier A. Proton MR spectroscopy improves discrimination between tumor and pseudotumoral lesion in solid brain masses. AJNR Am J Neuroradiol. 2009; 30: 544-551.
- Möller-Hartmann W, Herminghaus S, Krings T, Marquardt G, Lanfermann H, Pilatus U, Zanella FE. Clinical application of proton magnetic resonance spectroscopy in the diagnosis of intracranial mass lesions. Neuroradiology. 2002; 44: 371-381.
- Majós C, Alonso J, Aguilera C, Serrallonga M, Pérez-Martín J, Acebes JJ, Arús C. Proton magnetic resonance spectroscopy ((1)H MRS) of human brain tumours: assessment of differences between tumour types and its applicability in brain tumour categorization. Eur Radiol. 2003; 13: 582-591.
- Parmar H, Lim TC, Yin H, Chua V, Khin LW, Raidy T, Hui F. Multi-voxel MR spectroscopic imaging of the brain: utility in clinical setting-initial results. Eur J Radiol. 2005; 55: 401-408.
- Razek AA, Sadek AG, Kombar OR, Elmahdy TE, Nada N. Role of apparent diffusion coefficient values in differentiation between malignant and benign solitary thyroid nodules. AJNR Am J Neuroradiol. 2008; 29: 563-568.
- Tunca F, Giles Y, Salmaslioglu A et al. The pre-operative exclusion of thyroid carcinoma in multi-nodular goiter: dynamic contrast-enhanced magnetic resonance imaging versus ultrasonography-guided fine needle aspiration biopsy. Surgery 2007; 142: 992–1002.
- Bartolotta TV, Midiri M, Galia M, Runza G, Attard M, Savoia G, Lagalla R. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound: initial results. Eur Radiol. 2006; 16: 2234- 2241.
- Reading CC, Charboneau JW, Hay ID, Sebo TJ. Sonography of thyroid nodules: a “classic pattern” diagnostic approach. Ultrasound Q. 2005; 21: 157-165.
- King AD, Yeung DK, Ahuja AT, Tse GM, Chan AB, Lam SS, van Hasselt AC. In vivo 1H MR spectroscopy of thyroid carcinoma. Eur J Radiol. 2005; 54: 112-117.
- Schueller-Weidekamm C, Kaserer K, Schueller G, Scheuba C, Ringl H, Weber M, Czerny C. Can quantitative diffusion-weighted MR imaging differentiate benign and malignant cold thyroid nodules? Initial results in 25 patients. AJNR Am J Neuroradiol. 2009; 30: 417-422.
- Gotway MB, Higgins CB. MR imaging of the thyroid and parathyroid glands. Magn Reson Imaging Clin N Am. 2000; 8: 163-182, ix.
- Gupta N, Kakar AK, Chowdhury V, Gulati P, Shankar LR, Vindal A. Magnetic resonance spectroscopy as a diagnostic modality for carcinoma thyroid. Eur J Radiol. 2007; 64: 414-418.
- Lean CL, Delbridge L, Russell P, May GL, Mackinnon WB, Roman S, Fahey TJ 3rd. Diagnosis of follicular thyroid lesions by proton magnetic resonance on fine needle biopsy. J Clin Endocrinol Metab. 1995; 80: 1306-1311.
- Russell P, Lean CL, Delbridge L, May GL, Dowd S, Mountford CE. Proton magnetic resonance and human thyroid neoplasia. I: Discrimination between benign and malignant neoplasms. Am J Med. 1994; 96: 383-388.
- Delbridge L, Lean CL, Russell P, May GL, Roman S, Dowd S, Reeve TS. Proton magnetic resonance and human thyroid neoplasia. II: Potential avoidance of surgery for benign follicular neoplasms. World J Surg. 1994; 18: 512-516.
- Aydin H, Kizilgöz V, Tatar I et al. The role of proton MR spectroscopy and apparent diffusion coefficient values in the diagnosis of malignant thyroid nodules: preliminary results. Clin Imaging. 2012; 36: 323-33.
- Bartella L, Morris E, Dershaw D.D et al. Proton MR Spectroscopy with choline peak as malignancy marker improves positive predictive value for Breast Cancer diagnosis: Preliminary Study. Radiology 2006: 239; 686-692.
- Sardanelli F, Fausto A, Di Leo G, de Nijs R, Vorbuchner M, Podo F. In vivo proton MR spectroscopy of the breast using the total choline peak integral as a marker of malignancy. AJR Am J Roentgenol. 2009; 192: 1608-1617.
- Bartella L, Thakur SB, Morris EA, Dershaw DD, Huang W, Chough E, Cruz MC. Enhancing nonmass lesions in the breast: evaluation with proton (1H) MR spectroscopy. Radiology. 2007; 245: 80-87.
- Baek HM, Yu HJ, Chen JH, Nalcioglu O, Su MY. Quantitative correlation between (1)H MRS and dynamic contrast-enhanced MRI of human breast cancer. Magn Reson Imaging. 2008; 26: 523-531.
- Sadowski EA, Kelcz F. Frequency of malignancy in lesions classified as probably benign after dynamic contrast-enhanced breast MRI examination. J Magn Reson Imaging. 2005; 21: 556-564.
- Tse GM, Cheung HS, Pang LM, Chu WC, Law BK, Kung FY, Yeung DK. Characterization of lesions of the breast with proton MR spectroscopy: comparison of carcinomas, benign lesions, and phyllodes tumors. AJR Am J Roentgenol. 2003; 181: 1267-1272.
- Stanwell P, Mountford C. In vivo proton MR spectroscopy of the breast. Radiographics. 2007; 27 Suppl 1: S253-266.
- Joe BN, Chen VY, Salibi N, Fuangtharntip P, Hildebolt CF, Bae KT. Evaluation of 1H-magnetic resonance spectroscopy of breast cancer pre- and postgadolinium administration. Invest Radiol. 2005; 40: 405-411.
- Lenkinski RE, Wang X, Elian M, Goldberg SN. Interaction of gadoliniumbased MR contrast agents with choline: implications for MR spectroscopy (MRS) of the breast. Magn Reson Med. 2009; 61: 1286-1292.
- Turkbey B, Albert PS, Kurdziel K, Choyke PL. Imaging localized prostate cancer: current approaches and new developments. AJR Am J Roentgenol. 2009; 192: 1471-1480.
- Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007; 243: 28- 53.
- Levine MA, Ittman M, Melamed J, Lepor H. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol. 1998; 159: 471-475.
- Mazaheri Y, Shukla-Dave A, Hricak H, et al. Prostate cancer: identification with combined diffusion-weighted MR imaging and 3D-HMR spectroscopic imaging-correlation with pathologic findings. Radiology. 2008; 246: 480-488.
- Fütterer JJ, Heijmink SW, Scheenen TW, Veltman J, Huisman HJ, Vos P, Hulsbergen-Van de Kaa CA. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology. 2006; 241: 449-458.
- Wetter A, Engl TA, Nadjmabadi D, Fliessbach K, Lehnert T, Gurung J, Beecken WD. Combined MRI and MR spectroscopy of the prostate before radical prostatectomy. AJR Am J Roentgenol. 2006; 187: 724-730.
- Reinsberg SA, Payne GS, Riches SF, Ashley S, Brewster JM, Morgan VA, deSouza NM. Combined use of diffusion-weighted MRI and 1H MR spectroscopy to increase accuracy in prostate cancer detection. AJR Am J Roentgenol. 2007; 188: 91-98.
- Kumar V, Jagannathan NR, Kumar R, Das SC, Jindal L, Thulkar S, Gupta SD. Correlation between metabolite ratios and ADC values of prostate in men with increased PSA level. Magn Reson Imaging. 2006; 24: 541-548.