
Case Report
Austin Oncol. 2016; 1(1): 1004.
Meningeal Anaplastic Hemangiopericytoma
de Julian Campayo M¹*, Gaona-Morales J², Broseta-Torres R² and De las Peñas R¹
¹Department of Neuro-Oncology, Consorcio Hospitalario Provincial de Castellon, Castellon, Spain
²Department of Neuro-Pathology, H General Universitario de Castellón, Castellón, Spain
*Corresponding author: María De Julian Campayo, Department of Neuro-Oncology, Consorcio Hospitalario Provincial de Castellon, Orphan and Rare Tumors Spanish Group (GETHI), Castellon, Spain
Received: December 10, 2015; Accepted: February 08, 2016; Published: February 10, 2016
Abstract
Hemangiopericytoma (HPC) is a rare tumor with high risk of recurrence at neural and metastatic potential level. We report the case of a woman with a primary Meningeal Anaplastic Hemangiopericytoma (MHPC) at right temporal lobe. In the same way, given the low frequency of this tumor, we have reviewed the available data on its characteristics, as well as its therapeutic management.
Keywords: Hemangiopericytoma; Intracranial meningeal neoplasm
Case Presentation
The case features a 66 year old woman with a history of high blood pressure during pharmacological treatment. She had no other pathological history. She presented with a right hemicranial headache which had become resistant to regular analgesics. The physical exam, including neurological and eye fundus tests, showed no abnormalities.
The Computerized Tomography (CT) scan showed a right temporal lesion which was hyperdense, with hypodense centre and marked perilesional edema, compression of the right lateral and third ventricles, as well as subfalcine herniation with intense enhancement after administration of contrast. In the Magnetic Resonance Scan (MRI), we saw a lesion of 55x45x45mm, with deformity of the Silvian fissure. This was an isointense T1 lesion, with the central area being of less intensity, markedly heterogenous in T2 Flair and spin echo sequences, leaving the hyper intense central areas – which describe a variegated morphology – visible. This coincided with the areas of greater magnetic susceptibility in T2 sequences, attributable to the presence of necrosis and vascular structures. In the periphery of the lesion, we could see a displacement of the vessels, with no signs of infiltration. This was surrounded by a digit form vasogenic edema, which causes displacement of the parenchymatous structures of the basal ganglions and of the middle line, with the ipsilateral ventricle being collapsed. After the intravenous administration of gadolinium, the lesion was intensely enhanced, leaving a non-captant central area, with no clear “dural tail”, although it appeared to be in contact with the lesion at its posterior part (Figure 1). The perfusion sequence reveal great lesion vascularization, with a CBV always greater than x4, at some points even reaching x12. Spectroscopy didn’t offer any specific defining characteristics.
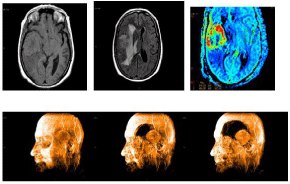
Figure 1: Pre-operative magnetic resonance images show a extra-axial mass
in the temporal lobe (A) T1- weighted, isointense multilobulated margin, and
deformity of the Silvian fissure, (B) T2 –weighted, compressing of the right
lateral and third ventricles with surrounding edema. (C): Perfusion sequence
reveal great lesion vascularization
(D). 3D magnetic resonance scan.
Prior to the surgical resection we carried out tumor embolization. We could see arterial irrigation in the tumor, both through the ICA and in the branches of the external CA on the right side (mainly via the ECA), with venous draining towards the sigmoid sinus.
The patient underwent a complete microsurgical in bloc resection of the extra-axial lesion, via the arachnoid plane. There were no complications during the procedure. The postoperative MRI scan carried out after surgery didn’t show any tumor remains. The symptoms passed after surgery.
The hystopathological examination revealed hyper cellular tumors composed of fusiform, pleomorphic cells, arranged in all directions. They featured focus of ischemic necrosis and 6x10 mitotic activity, fields of great increase. Irregular vessels could be seen, focally appearing to be of “staghorn” nature. Some vessels were of great size and still bore this appearance, with chemo-embolization material being noticed, together with the main ischemic foci. Tumor cellularity was surrounded by a web of abundant reticulin (Figure 2).
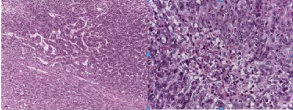
Figure 2: Hematoxylin and eosine stain reveals the pathology of the tumor
is a diagnosis os meningeal hemangyoperiitoma. hypercellular tumor
composed of pleomorphic cells, Irregular vessels focally appearing to be of
“staghorn” nature.
Immunohistochemical staining revealed positive staining for CD34 (focal), vimentin, reticulin and bcl-2, and negative for Epithelial Membrane Antigen (EMA), Glial Fibrillary Acidic Protein (GFAP), CK AE1 / AE3 and S-100. The proliferation index, examined via the Ki67 antibody, reached 8-10% (Figure 3).

Figure 3: Inmunohistochemistry. Tumor cells strongly expresssing vimentin
(A), focal CD 34 (B), and reticulin (C).
After the procedure, the patient underwent Volumetric Modulated Arc Therapy (VMAT technique) for three months on the surgical bed. This was finalized by May 2014, with the patient receiving a total doses of 58Gy. Twelve months after the surgical resection, no clinical-radiological evidence of local recurrence existed (Figure 4), nor did any evidence of distant metastases.
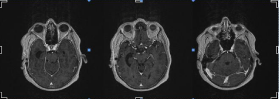
Figure 4: Post-operative magnetic resonance images taken within 12 month
of surgery show no evidence of local recurrence.
Discussion
The Hemangiopericytoma are uncommon hypervascular neoplasms, of mesenchymal lineage. They arise from the malignant transformation of Zimmerman pericytes – contractile spindle cells surrounding capillaries and post capillary venules. HPCs may occur anywhere in the body; usually the most commonly reported locations are lower extremities and retroperitoneum. Intracranial HPC constitute 2-4% of meningeal tumors and less than 1% of the tumors in the central nervous system [1-5].
The hemangiopericytoma was first reported by Stout and Murray in 1942 [6], and the first reported case of hemangiopericytoma originating in the meninges was described by Begg and Garret in 1954 [7]. They were originally considered a variation of meningioma, and they were given the name of angioblastic meningioma. More recently, thanks to its special clinical behavior, together with its immunohistochemical, structural and genetic characteristics, it has been recognised as a different entity altogether [8-12]. Since 1993, the World Health Organization’s (WHO) [13] classification of tumors in the nervous system recognizes them as being nonmeningothelial mesenchymal tumors.
The interest in differentiating meningiomas from hemangiopericytomas – with which they share similar clinical and radiological characteristics – resides in the fact that hemangiopericytomas are characterized by their propensity to local recurrence and the potential to metastasize in extraneural areas [2,4,14]. Thus, given their aggressiveness, they need a selection of optimal treatment.
The chosen treatment for intracranial hemangiopericytomas is of a surgical nature. A total resection of the lesion is recommended whenever possible, due to the fact that the resection has correlated with the free interval of recurrence and overall survival [1,3-5,14,15]. Given that they are very vascularized tumors, with predisposition to bleed heavily during operation, pre-surgical embolization must be considered [3,4]. Nevertheless, a complete surgical resection does not eliminate the high risk of recurrence and in the majority of cases, recurrences that occur in the central nervous system do so in the place where the original tumor appeared [2,16]. The most important factor which determines the recurrence and prognosis in these patients is the extension of the surgical resection [1,2,15]. There are studies which show that a total resection of the lesion prolongs the moment of recurrence for an average of 65 months [2,17]. Furthermore, a meta-analysis has demonstrated a survival ratio of 10 years through 69% of those who underwent a gross total resection, compared to 44% of those who obtained a subtotal resection [18].
Treatment with adjuvant radiotherapy is controversial, since the data we have available originate in small samples of retrospective studies which have conflicting results. Studies do exist with postoperative radiotherapy, radiotherapy with doses of at least 45- 50 Gy (since the HPC sensitivity is dose-dependent) [19,20] even in cases of complete resection, which has proven useful in increasing recurrence-free interval [12,21], although this therapy has not shown to bear effect on recurrences along the neuraxis or against distant metastases [12,21]. On the other hand, there are studies which show no benefit in the addition of radiotherapy after a total resection [18]. However, it seems logical to suggest the use of adjuvant radiotherapy and cases of incomplete resection – either in lesions that are technically unresectable, or as an exclusive palliative treatment. Radio surgery seems to be an effective treatment with favorable results for recurrent or residual MHPC that are well-defined and small in size [1,2]. As well as having a tendency for local recurrence and recurrence along the neuraxis, intracranial hemangiopericytomas also tend to metastasize in other areas outside of the nervous system, and they are able to appear several years after the treatment of the primary tumor [22,23], which makes it necessary to have follow up monitoring lasting as long as possible. Bone, liver and lung are the most commonly reported sites of metastasis in HPC. Additional resections must be considered in these circumstances, although this is not always possible [24]. A strategy of optimal treatment of advanced HPC has not yet been identified [14,24-26].
The role of chemotherapy based on anthracyclines is still controversial, with modest efficacy in the treatment of MHPC [27,28]. Recent studies have identified the fusion gene NAB2-STAT6, both in HPC and in solitary fibrous tumors [29,30]. Although this hasn’t yet been translated into therapeutic targets, it certainly does suggest a new line of research. Hypervascularization of these tumors, and their immunohistochemistry (IHC) expression of the Vascular Endothelial Growth Factor Receptor (VEGFR) and Platelet-Derived Growth Factor Receptor (PDGFR) [19,31-33,34,35] is the rational basis for encouraging the study of targeted therapies with antiangiogenic drugs. Until now, only small experimental studies and a series of reported cases have been published. Some of these propose the possible role of IFN-a [22,36] in the stabilization of the disease. Others suggest that imatinib [34,35], sorafenib34 and sunitinib [37,38] can achieve stable, sustained responses. There have also been cases reported with pazopanib [39], a multi-targeted tyrosine-kinase inhibitor [40]. This showed efficacy in patients who had a metastatic hemangiopericytoma, with longer-term recurrence free survival and overall survival [41,42]. There is a retrospective study in which the combination of temozolomide and bevacizumab has a high rate of partial Choi responses, making it a promising therapy [28]. Despite these results, further research is needed for validation in other prospective studies.
Conclusion
Given the high risk of recurrence, the MHPC must be completely resected, as long as it is technically possible. Even still, when a complete resection is performed, adjuvant RT should be considered, as this appears to reduce the rate of local recurrence. Long-term monitoring is essential to detect recurrences or distant metastases, which can appear decades after the correct handling of an initial primary tumor.
References
- Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T, et al. Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012; 118: 1628-1636.
- Kim JH, Jung HW, Kim YS, Kim CJ, Hwang SK, Paek SH, et al. Meningeal hemangiopericytomas: long-term outcome and biological behavior. See comment in PubMed Commons below Surg Neurol. 2003; 59: 47-53.
- Barba I, Moreno A, Martinez-Pérez I, Tate AR, Cabañas ME, Baquero M, et al. Magnetic resonance spectroscopy of brain hemangiopericytomas: high myoinositol concentrations and discrimination from meningiomas. See comment in PubMed Commons below J Neurosurg. 2001; 94: 55-60.
- Chiechi MV, Smirniotopoulos JG, Mena H. Intracranial hemangiopericytomas: MR and CT features. See comment in PubMed Commons below AJNR Am J Neuroradiol. 1996; 17: 1365-1371.
- Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma: long-term outcome revisited. Clinical article. See comment in PubMed Commons below J Neurosurg. 2011; 114: 747-755.
- Stout AP, Murray MR. HEMANGIOPERICYTOMA: A VASCULAR TUMOR FEATURING ZIMMERMANN'S PERICYTES. See comment in PubMed Commons below Ann Surg. 1942; 116: 26-33.
- BEGG CF, GARRET R. Hemangiopericytoma occurring in the meninges: case report. See comment in PubMed Commons below Cancer. 1954; 7: 602-606.
- Coffey RJ, Cascino TL, Shaw EG. Radiosurgical treatment of recurrent hemangiopericytomas of the meninges: preliminary results. See comment in PubMed Commons below J Neurosurg. 1993; 78: 903-908.
- Joseph JT, Lisle DK, Jacoby LB, Paulus W, Barone R, Cohen ML, et al. NF2 gene analysis distinguishes hemangiopericytoma from meningioma. See comment in PubMed Commons below Am J Pathol. 1995; 147: 1450-1455.
- Mena H, Ribas JL, Pezeshkpour GH, Cowan DN, Parisi JE . Hemangiopericytoma of the central nervous system: a review of 94 cases. See comment in PubMed Commons below Hum Pathol. 1991; 22: 84-91.
- Ramsey HJ . Fine structure of hemangiopericytoma and hemangio-endothelioma. See comment in PubMed Commons below Cancer. 1966; 19: 2005-2018.
- Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma: long-term outcome revisited. Clinical article. See comment in PubMed Commons below J Neurosurg. 2011; 114: 747-755.
- Klehiues P, Cavenee WK.WHO Classification of tumours of the nervous system. Pathology and genetics of tumours of the nervous system. Lyon; International Agency for Research of Cancer. 2000; 6-7.
- Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG. Meningeal hemangiopericytoma: histopathological features, treatment, and long term follow up of 44 cases. Neurosurgery. 1989; 25: 514-522.
- Spitz FR, Bouvet M, Pisters PW, Pollock RE, Feig BW. Hemangiopericytoma: a 20-year single-institution experience. See comment in PubMed Commons below Ann Surg Oncol. 1998; 5: 350-355.
- Espat NJ, Lewis JJ, Leung D, Woodruff JM, Antonescu CR, Shia J, et al. Conventional hemangiopericytoma: modern analysis of outcome. See comment in PubMed Commons below Cancer. 2002; 95: 1746-1751.
- Magdeleinat P, Alifano M, Petino A, Le Rochais JP, Dulmet E, Galateau F, et al. Solitary fibrous tumors of the pleura: clinical characteristics, surgical treatment and outcome. See comment in PubMed Commons below Eur J Cardiothorac Surg. 2002; 21: 1087-1093.
- Chamberlain MC, Glantz MJ. Sequential salvage chemotherapy for recurrent intracranial hemangiopericytoma. Neurosurgery 2008; 63: 720–726.
- Beadle GF, Hillcoat BL . Treatment of advanced malignant hemangiopericytoma with combination adriamycin and DTIC: a report of four cases. See comment in PubMed Commons below J Surg Oncol. 1983; 22: 167-170.
- Park MS, Patel SR, Ludwig JA, Trent JC, Conrad CA, Lazar AJ, et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer. 2011; 117: 4939- 4947.
- Delgado M1, Pérez-Ruiz E, Alcalde J, Pérez D, Villatoro R, Rueda A . Anti-angiogenic treatment (sunitinib) for disseminated malignant haemangiopericytoma: a case study and review of the literature. See comment in PubMed Commons below Case Rep Oncol. 2011; 4: 55-59.
- Kirn DH, Kramer A . Long-term freedom from disease progression with interferon alfa therapy in two patients with malignant hemangiopericytoma. See comment in PubMed Commons below J Natl Cancer Inst. 1996; 88: 764-765.
- Domont J, Massard C, Lassau N, Armand JP, Le Cesne A, Soria JC. Hemangiopericytoma and antiangiogenic therapy: clinical benefit of antiangiogenic therapy (sorafenib and sunitinib) in relapsed malignant haeman-gioperyctoma/solitary fibrous tumour. Invest New Drugs. 2010; 28: 199-202.
- Fountas KN, Kapsalaki E, Kassam M, Feltes CH, Dimopoulos VG, Robinson JS, et al. Management of intracranial meningeal hemangiopericytomas: outcome and experience. See comment in PubMed Commons below Neurosurg Rev. 2006; 29: 145-153.
- Peters KB, McLendon R, Morse MA, Vredenburgh JJ. Treatment of recurrent intracranial hemangiopericytoma with SRC related tyrosine kinase targeted therapy: a case report. Case Rep Oncol. 2010; 3: 93-97.
- Tatar Z, Thivat E, Planchat E, Gimbergues P, Gadea E, Abrial C, et al. Temozolomide and unusual indications: review of literature. See comment in PubMed Commons below Cancer Treat Rev. 2013; 39: 125-135.
- Hatva E, Böhling T, Jääskeläinen J, Persico MG, Haltia M, Alitalo K. Vascular growth factors and receptors in capillary hemangioblastomas and hemangiopericytomas. See comment in PubMed Commons below Am J Pathol. 1996; 148: 763-775.
- Lackner H, Urban C, Dornbusch HJ, Schwinger W, Kerbl R, Sovinz P. Interferon alfa-2a in recurrent metastatic hemangiopericytoma. See comment in PubMed Commons below Med Pediatr Oncol. 2003; 40: 192-194.
- Rossi G, Schirosi L, Giovanardi F, Sartori G, Paci M, Cavazza A . Pleural malignant solitary fibrous tumor with sarcomatous overgrowth showing PDGFRbeta mutation. See comment in PubMed Commons below Chest. 2006; 130: 581-583.
- Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, et al. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. See comment in PubMed Commons below Acta Neuropathol. 2013; 125: 651-658.
- Chmielecki J, Crago AM, Rosenberg M, O'Connor R, Walker SR, Ambrogio L, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. See comment in PubMed Commons below Nat Genet. 2013; 45: 131-132.
- Lee SJ, Kim ST, Park SH, Choi YL, Park JB, Kim SJ, et al. Successful use of pazopanib for treatment of refractory metastatic hemangiopericytoma. See comment in PubMed Commons below Clin Sarcoma Res. 2014; 4: 13.
- De Pas T, Toffalorio F, Colombo P, Trifiro G, Pelosi G, Vigna PD, et al. Brief report: activity of imatinib in a patient with platelet-derived-growth-factor receptor positive malignant solitary fibrous tumor of the pleura. J Thorac Oncol 2008; 3: 938–941.
- Domont J, Massard C, Lassau N, Armand JP, Le Cesne A, Soria JC. Hemangiopericytoma and antiangiogenic therapy: clinical benefit of antiangiogenic therapy (sorafenib and sunitinib) in relapsed malignant haemangioperyctoma /solitary fibrous tumour. See comment in PubMed Commons below Invest New Drugs. 2010; 28: 199-202.
- Mulamalla K, Truskinovsky AM, Dudek AZ. Rare case of hemangiopericytoma responds to sunitinib. See comment in PubMed Commons below Transl Res. 2008; 151: 129-133.
- Rutkowski MJ, Sughrue ME, Kane AJ, Aranda D, Mills SA, Barani IJ, et al. Predictors of mortality following treatment of intracranial hemangiopericytoma. See comment in PubMed Commons below J Neurosurg. 2010; 113: 333-339.
- Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH. Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. See comment in PubMed Commons below Cancer. 2004; 100: 1491-1497.
- Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH . Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. See comment in PubMed Commons below Cancer. 2004; 100: 1491-1497.
- Galanis E, Buckner JC, Scheithauer BW, Kimmel DW, Schomberg PJ, Piepgras DG . Management of recurrent meningeal hemangiopericytoma. See comment in PubMed Commons below Cancer. 1998; 82: 1915-1920.
- Alén JF, Lobato RD, Gómez PA, Boto GR, Lagares A, Ramos A, et al. Intracranial hemangiopericytoma: study of 12 cases. See comment in PubMed Commons below Acta Neurochir (Wien). 2001; 143: 575-586.
- Dufour H, Metellus P, Fuentes S, Murracciole X, Regis J, Figarella-Branger D, et al. Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of postoperative external radiotherapy. Neurosurgery 2001; 48: 756-763.
- Tashjian VS, Khanlou N, Vinters HV, Canalis RF, Becker DP. Hemangiopericytoma of the cerebellopontine angle: a case report and review of the literature. See comment in PubMed Commons below Surg Neurol. 2009; 72: 290-295.