
Review Article
Austin Oncol. 2016; 1(2):1009.
Progress of New Formulations of Paclitaxel
Shan Xu, Heng Zhang, Shaozhi Fu and Jingbo Wu*
Department of Oncology, The Affiliated Hospital of Southwest Medical University, Southwest Medical University, China
*Corresponding author: Jingbo Wu, Department of Oncology, The Affiliated Hospital of Southwest Medical University, Southwest Medical University, China
Received: June 21, 2016; Accepted: September 02, 2016; Published: September 06, 2016
Abstract
Over the past two decades, paclitaxel shows remarkable potency as an antineoplastic agent with a broad range of cancers, such as lung, ovarian, and pancreatic cancers, etc. However, traditional treatment regimens limited the clinical effectiveness of paclitaxel by high rates of toxicitiessuch as myalgia, hypersensitivity reactions and peripheral neuropathy. These effects mainly were caused by Cremophor EL (polyethoxylated castor oil), a delivery of paclitaxel due to its poor solubility, and ethanol. To overcome these problems, numerous advances have been made in dosing schedules and delivery strategies. This review describes existing and future novel formulations for delivery of paclitaxel. In addition, novel formulations of paclitaxel, their mechanisms of action, dose and administration and clinical efficacy will be discussed.
Keywords: Paclitaxel; Cancer; Chemotherapy
Introduction
Cancer had become the most common cause of death in China and many other parts of the world. Currently, the crude incidence rate in Chinese cancer registration areas was 285.91/100,000 (males 317.97/100,000, females 253.09/100,000) and the cancer mortality in urban areas was 181.86/100,000, whereas in rural areas, it was 177.83/100,000 [1]. A central aim in cancer therapy is to ‘‘cure’’ the cancer, or prolonging survival time of the patients, while improving the quality of life in both the long and short terms [2]. Over the decades, the treatment of cancer has relied primarily on the use of various forms of surgery, cytotoxic chemotherapy and radiation therapy, especially chemotherapy is an important part of treatment for many cancers by keeping the cancer cells from growing and dividing to make more cells [3]. Among the anti-cancer drugs, taxanes are the most researched agents that play important roles in oncotherapy through a unique mechanism, and paclitaxel is the first and most successful member of the taxane family to be used in cancer chemotherapy [4]. Paclitaxel have shown remarkable potency as an antineoplastic agent with a broad range of cancers activity against various cancers, such as lung, ovarian, and pancreatic cancers, etc. The history of development of paclitaxel is summarised in Figure 1. Their therapeutic effect is due to disrupt the tubulin-microtubule equilibrium and destroy the cancer cell division process by inducing cell cycle arrest and the programmed cell death [5-7]. So far, paclitaxel is widely used in the therapy of various cancers. Although crucial progress has been made in curing cancers, chemotherapy with paclitaxel can be associated with significant hypersensitivity reactions, hematologic toxicity, neurotoxicity, arthralgia, myalgia and skin changes that may offset the therapeutic benefits of paclitaxel use. These reactions were mediated by the direct release of histamine or other vasoactive substances, and these reactions have been caused by paclitaxel itself or Cremophor EL [8,9]. Recently, more and more evidences had to be found that those reactions mainly attributed to Cremophor EL, which induced histamine release and similar manifestations in dogs and other drugs formulated in Cremophor EL including cyclosporine and vitamin K, have been associated with similar reactions [10,11]. Therefore, novel formulations of paclitaxel become a hotspot in the treatment of tumors. This article reviews developments in therapeutic strategies of paclitaxel.
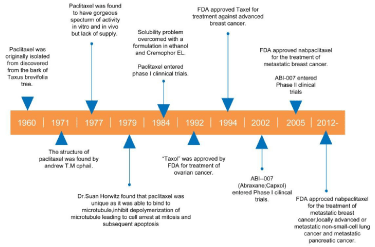
Figure 1: History of paclitaxel development.
Novel Formulations for Delivery of Paclitaxel
Paclitaxel, no doubt, is one of the most active antineoplastic agents with a broad spectrum of activity against several human cancers. However, because of the high lipophilicity and poor water solubility of paclitaxel, Cremophor EL had been used as solvents which can cause a number of adverse reactions, including acute hypersensitivity, neutropenia and peripheral neuropathy. In this setting, innovative approaches to treatment are welcome. So far, novel formulations of paclitaxel, including nab-paclitaxel, injectable hydrogel, etc. has showed marked improvement in the treatment of various cancers in the clinic or fundamental research.
Nab-paclitaxel
Nanoparticle albumin -bound (nab)-paclitaxel, a novel, biologically interactive, nanometer sized albumin-bound paclitaxel particle, is prepared by high-pressure homogenization of paclitaxel in the presence of serum albumin, resulting in a colloidal suspension comprising nanoparticles with an average size of 130nm, that prevent the risk of capillary blockage after intravenous infusion [12]. The mechanism of delivery of nab-paclitaxel has been proposed to be mediated by active transport of albumin into the interstitial space via gp60-mediated transcytosis and the association with the albuminbinding protein SPARC (secreted protein, acidic and rich in cysteine) in the tumor microenvironment [13,14]. Therefore, compared with the solvent-based paclitaxel, nab-paclitaxel demonstrates more efficient transport across endothelial cells, greater penetration, cell uptake , mitotic arrest induction in tumor xenografts, as well as to reduce solvent-associated toxicities ,especially polyoxyethylated castor oil solvent (Cremophor EL) [15]. In addition, nab-paclitaxel allows the safe infusion of significantly higher doses of paclitaxel than the doses used with standard paclitaxel therapy, with shorter infusion schedules (30minutes v 3hours, respectively) and no premedication [16]. This novel formulation of paclitaxel has been used in clinical trials at present and has gorgeous results in the treatment of cancers.
In the treatment of lung cancer, the initial nab-paclitaxel monotherapy study showed that patients with NSCLC were administrated with 260mg/m² every 3 weeks. As a result, the response rate (ORR) was 16%, disease control rate was 49%, median Time To Progression (TTP) of 6 months, and median Overall Survival (OS) of 11 months with no acute hypersensitivity reactions [17]. Following this, patients with NSCLC were received a combination therapy including nab-paclitaxel 100mg/m² intravenous infused over 30 minutes weekly and carboplatin AUC 6 every 3 weeks. Comparing with those patients were administrated with paclitaxel 200mg/m² intravenous infused over 3 hours once every cycle and carboplatin AUC 6 every 3 weeks, nab-paclitaxel group showed a 10% improvement in OS (12.1 months versus 11.2 months ) and 10% improvement in PFS (6.3 versus 5.8 months; P=0.214). In addition, grade 3 or 4 neutropenia was lower in the nab-paclitaxel group compared to paclitaxel group (47% versus 58%, respectively) [18]. In another study, a combination of nabpaclitaxel 300mg/m², carboplatin AUC 6, and bevacizumab 15mg/kg every 21 days showed that the response rate was 31%, the median OS of 16.8 months and median progression-free survival (PFS) of 9.8 months [19].
In the treatment of pancreatic adenocarcinoma, nab-paclitaxel also showed their excellent effect. A current clinical trial, patients with previously untreated advanced pancreatic cancer were treated with 100, 125, or 150mg/m² nab-paclitaxel followed by gemcitabine 1,000mg/m² on days 1, 8, and 15 every 28 days to identify the Maximum-Tolerated Dose (MTD) of first-line gemcitabine plus nab-paclitaxel. This study showed that the MTD was 1,000mg/m² of gemcitabine plus 125mg/m² of nab-paclitaxel once a week for 3 weeks, every 28 days, which caused the response rate was 48%, with 12.2 median months of Overall Survival (OS) and 48% 1-year survival, indicating this regimen has tolerable adverse effects with substantial antitumor activity [20]. The other current clinical trial, 431 patients with advanced pancreatic cancer were assigned to nab-paclitaxel plus gemcitabine. More specifically, patients were administrated with nabpaclitaxel at a dose of 125mg/m² by intravenous infusion, followed by an infusion of gemcitabine at a dose of 1000mg/m², on days 1, 8, 15 every 4 weeks and 430 patiens received gemcitabine alone at a dose of 1000mg/m² weekly for 7 of 8 weeks (cycle 1) and then on days 1, 8, and 15 every 4 weeks (cycle 2 and subsequent cycles). The result also showed that in patients with metastatic pancreatic adenocarcinoma, nab-paclitaxel plus gemcitabine obviously enhanced OS, PFS, and response rate. The rate of survival in nab-paclitaxel–gemcitabine group was significantly higher in the gemcitabine group by 59% at 1 year (35% vs. 22%) and by more than 100% at 2 years (9% vs. 4%). However, the rate of peripheral neuropathy and myelosuppression were increased [21].
Recently, nab-paclitaxel provided a promising activity against previously treated unresectable or recurrent gastric cancers and safer treatment to patients. In a study, patients were administrated nab-paclitaxel (260mg/m²) via the intravenous on day 1 of each 21- day cycle, resulting in the median PFS was 2.9 months, the median survival time was 9.2 months, grade 3 ⁄ 4 toxicities were neutropenia (49.1%), leucopenia (20.0%), lymphopenia (10.9%), and peripheral sensory neuropathy (23.6%) [22].
Experimentally, nab-paclitaxel has also significantly stronger antitumor effects on gastric cancer cell lines than cytotoxic agents oxaliplatin and epirubicin in vitro and in vivo, which increased expression of the mitotic-spindle associated phospho-stathmin irrespective of the baseline total or phosphorylated stathmin level, and induced mitotic cell death as confirmed through increased expression of cleaved-PARP and caspase-3. The average of local tumor growth inhibition rate in vivo for nab-paclitaxel, oxaliplatin or epirubicin treatment were 77, 17.2 and 21.4 percent respectively. The median animal survival was 93 days after nab-paclitaxel treatment, controls was 31 days, oxaliplatin was 40 days and docetaxel therapy 81 days [23]. What is more, nab-paclitaxel could enhance its antitumor activity by antiangiogenic agents like bevacizumab (Bev) or sunitinib (Su). Compared with controls (19 days), median animal survival was increased after Nab-Paclitaxel therapy (32 days), NPT+Bev was 38days, NPT+Su was 37days and NPT+Bev+Su was 49 days. This combination could decrease in angiogenesis, reduction of desmoplastic stroma formation, increased delivery of Nab-Paclitaxel to tumor, and greater efficacy in growth inhibition of multiple cell types within the tumor microenvironment [24]. Furthermore, combination of nab-Paclitaxel and Gemcitabine exhibits synergistic antitumor activity in a xenograft model of Pancreatic Ductal Adenocarcinoma (PDA). Mice were treated with established tumors of comparable size for 8 days with vehicle, gemcitabine, nabpaclitaxel, or nab-paclitaxel/gemcitabine, resulted in a significantly smaller tumors with nab-paclitaxel/gemcitabine (mean, 140%±15%) as compared with gemcitabine (mean, 234%±32%) , vehicle (mean, 278%±33%) and single–agent nab-paclitaxel (mean, 170%±15%). Importantly, some tumors in the nabpaclitaxel/gemcitabine cohort regressed after only 8 days of treatment [13]. Additionally, mice with lewis lung carcinoma (LLC) and H460 lung carcinoma (H460) were treated with nab-paclitaxel, radiation and HVGGSSV, a recombinant peptide was used to target nab-paclitaxel to irradiate tumors, achieving tumor-specificity and enhanced bioavailability of paclitaxel, showing a great bioavailability of paclitaxel, better tumor growth delay compared with controls, and a significantly greater loss in vasculature in irradiated tumors compared with unirradiated tumors. More specifically, controls showed no significant growth delay in LLC tumors and only 2 days’ delay in H460, radiation alone (9 Gy) achieved only a slight tumor growth delay (2 days) in LLC tumors and 6 days in H460, nabpaclitaxel alone produced tumor growth delay of 2 days (LLC) and 6 days (H460), and upon additional irradiation increased to 6 days (LLC) and 11 days (H460), HVGGSSV-nab-paclitaxel treatment achieved a growth delay of 3.4 days (LLC) and 8 days (H460). Additional irradiation increased this to 10 days (LLC) and 15 days (H460) [25].
The nab-paclitaxel showed more effectively uptake by tumour cells, as well as to reduce solvent-associated toxicities. The evidence reviewed above suggests this novel technology is achieving its aim of improving the therapeutic index of this well-established agent for the benefit of various cancers patients
Injectable hydrogel as carriers for paclitaxel
Injectable in situ hydrogels also play important role in the novel drug delivery systems, which ease of administration, biocompatibility, improve patient compliance, high regional drug concentration and low systemic toxicity [26]. Hydrogels are three-dimensional hydrophilic polymeric networks that can absorb and retain a considerable amount of water (from 10 to 20% up to thousands of times their dry weight in water) with maintenance of shape [27]. These material systems are flow able aqueous solutions before administration, but once injected, rapidly gel due to one or combination of different stimuli like pH change, temperature modulation and solvent exchange [28]. For use in drug/cell delivery and tissue engineering, hydrogels should be lowviscosity solutions (free flowing) prior to subcutaneous injection and should rapidly gel in the human body, where ultimate degradation of the hydrogels is desired.
To date, several reviews pertinent to injectable hydrogels have been published. Such as Poly (ethylene glycol)–b-polycaprolactone (MPEG–PCL) diblock copolymer gel serves as an injectable drug depot for paclitaxel. The copolymer solution remained liquid at room temperature and rapidly gelled in vivo at body temperature. This thermo sensitive hydrogel showed that intratumoral injection of Ptx-containing gels more effectively inhibited tumor growth than did Ptx, increasing the average tumor volume doubling time to 9 days, compared with 4.3 days in the PTX-treated group and 3.3 days in the saline control group and increase in necrotic tissue in tumors treated with Ptx-loaded gel [29]. In addition, combination of MPEG-PCL/ PTX with PEG-PCL-PEG/DDP (PDMP) as an in situ gel-based dual drug delivery also showed an effective at inhibiting tumor growth and prolong the survival time of tumor-bearing BALB/c nude mice after intratumoral injection. Mice with lung cancer were treated with PDMP hydrogel, free DDP+PTX, blank drug carrier and Normal Saline (NS), result in the median survival time in PDMP hydrogel composite group was significantly longer (53days) than those of the free DDP+PTX group (40 days, p < 0.05), the blank drug carrier group (26 days, p < 0.05), and NS (25 days, p < 0.05) group [30]. Likewise, thermosensitive in-situ forming hydrogels based on poly (g-ethyl-Lglutamate)- poly(ethylene glycol)-poly(g-ethyl-L-glutamate) triblock copolymers (PELG-PEG-PELG) for localized and sustained delivery of paclitaxel. The PELG-PEG-PELG triblock copolymer mixed with paclitaxel in the aqueous solutions and then rapidly gel as a drug depot with the employment of body temperature after subcutaneous injection into mice. This thermosensitive PTX-encapsulated hydrogels could efficiently suppress the tumor growth up to 21 days, and did not result in obvious organ damage [31]. Similarly, poly (organophosphazene), a novel thermosensitive hydrogel, is an injectable drug delivery system that transforms from sol to gel at body temperature. Paclitaxel encapsulated this hydrogel could sustain PTX release for 14 days in the tumor tissue after injecting intratumorally and reduce systemic exposure and toxicity by restricting its biodistribution to the tumor tissue [32]. Additionally, paclitaxel conjugated poly (3-caprolactone)– poly(ethylene glycol)–poly (3caprolactone) (PCEC/PTX) presented a thermosensitive reversible sol-to-gel transition property around body temperature. This thermosensitive hydrogel could sustain at least 28 days after subcutaneous injection in mice [33]. Furthermore, OSMPCGA- PEG-PCGA-OSM block copolymers poly, composed of poly (ethylene glycol) (PEG), polyglycolide (GA), ε-caprolactone (CL) and sulfamethazine oligomers (OSMs), is a pH and temperature sensitive hydrogel. This block copolymer solution showed a reversible solgel transition as a result of both a pH change in the pH 7.4, and a temperature change around body temperature. Following the loading this matrix and paclitaxel , more than 90% of the drug was released from this matrix after 20-22 days, indicating this pH/temperature sensitive hydrogel may use for drug delivery systems [34]. Further study found that this PTX-loaded block copolymer solution were injected subcutaneously to tumor-bearing mice and showed good anti-tumor effect for 2 weeks and induced strong apoptosis in tumor tissue. Therefore this OSM–PCLA–PEG–PCLA–OSM block copolymer hydrogel may be a effective injectable carrier of PTX [35].
Currently, hydrogel systems are commonly used as a local drug delivery system. This local delivery of antitumor drugs delivery system provides a high local concentration and decreases the incidence of side effects commonly observed with systemic therapy. Although much progress has been made in the fundamental research of injectable hydrogels, some shortage requires improving in order to use in clinical practice, such as drug in this system from the tumor site still subsequently reached systemic circulation, mechanically weak, difficult to sterilize.
Miscellaneous formulations for delivery of paclitaxel
Up to now, there are also many ways to formulate paclitaxel in a delivery system that does not require cremophor for solubilization, including micelles, nanoparticles, liposomes, emulsions and various conjugates.
For instance, polymeric Nano Particles (NPs) have attracted considerable attention as potential drug delivery, which can effectively deliver the drug to a target site, escape from the vasculature through the leaky endothelial tissue, increase the therapeutic benefit and minimize side effects. Nanoparticles generally vary in size from 10 to 1000 nm. The drug is dissolved, entrapped, encapsulated or attached to a NP matrix [36]. A case in point is that paclitaxel-loaded PLGA nanoparticles (Ptx-PLGA-Nps) could be considered promising systems for in vivo paclitaxel delivery. This formulation strongly enhances the cytotoxic effect of the drug as compared to paclitaxel in vitro. The release behaviour of paclitaxel from the polymer matrix exhibited a biphasic pattern characterized by a fast initial release during the first 24h, followed by a slower and continuous release [37]. Further study exhibited that PTX was released from the PLGA nanoparticles for a period of 15 days in vitro without obvious toxic effects and inhibited human breast cancer cell growth for a period of 168 h after intravenous injection in mice [38]. A recent study, addition of signal transducer and activator of transcription-3 (Stat3) to Ptx-PLGA-Nps could enhance antitumor efficacy and provide a new therapeutic strategy to control cancer cell growth. This novel formulation could induce more microtubule aggregation, decrease cell viability, increase apoptosis, and reduce cellular resistance to paclitaxel in vitro [39]. What’s more, paclitaxel-loaded poly (L-γ- glutamylglutamine) also demonstrated to be efficacious in antitumor activity in vivo and outperformed paclitaxel alone. Mice with the NCI-H460 human lung cancer cells were treated with free PTX, PTXloaded nanoparticles and NS, result in the PTX-loaded nanoparticles could efficiently suppress the tumor growth up to 27 days, free PTX group was 19 days and NS group was 16 days [40].
Liposomes are another delivery system for paclitaxel, those liposomes have microscopic phospholipid bubbles with a bilayered membrane structure, which can improve solubilization of lipophilic drugs as a versatile drug carrier [41]. Encapsulation in liposomes often results in distinct changes in the pharmacokinetic and the pharmacodynamic properties of the drugs, in some cases causing a marked decrease in toxicity or increase in potency. Such as, liposomes were subsequently coated with anionic Polymer Polyacrylic Acid (PAA) followed by coating of cationic polymer polyallylamine hydrochloride (PAH) to form multilayered liposomes and then paclitaxel encapsulated this layersome formulation. This formulation was able to sustain the drug release for 24h in vitro and also exhibited 4.07-fold oral bioavailability of PTX as compared to the free drug in vivo. The tumor growth also reduced significantly with safer toxicity profile compared to PTX [42]. Moreover, paclitaxel liposomes may serve as a lung-targeting drug delivery system. In a study, the liposome carrier markedly altered the tissue distribution profile of paclitaxel in dogs compared with the paclitaxel injection. After intravenous administration of paclitaxel liposomes in dogs, the drug concentration in the lungs was higher than in the other organs or tissues [43].
Additionally, micellisation is also an important approach capable of solubilizing a hydrophobic drug in a hydrophilic environment comprising of biodegradable drug carrier micelle and a hydrophobic drug wherein the drug is physically entrapped and not covalently bonded to the polymeric drug carrier micelle. Polymer micelles are convenient passive targeting carrier systems of anticancer drugs since they are structurally strong and unlike liposomes are not captured by the reticuloendothelial cell system. For instance, paclitaxel (PTX)- loaded polymeric micelles (M-PTX) could enhance the blood flow and oxygenation of tumors 24h after treatment. These changes in the tumor microenvironment could lead to an enhancement of the EPR (enhanced permeability and retention) effect. As result, M-PTX could increase in blood flow, permeability, induce EPR effect and enhance accumulation of anti-cancer drugs in tumors [44]. Furthermore, Disulfide Cross-Linked Micelles (DCMs) loaded paclitaxel (PTX), this formulation were found to be able to preferentially accumulate at the tumor site in nude mice and released PTX over 10days after injection. Compared with the control group and PTX group, mice in PTX-DCMs group showed significant inhibition of tumor growth. The median survival time was 21 days for control, 27 days for PTX, and 32.5 days for PTX-DCMs, respectively [45].
To improve the safety profile of paclitaxel, a number of advances have been made in dosing delivery strategies. These newer formulations use paclitaxel bound in a novel fashion to proteins, polypeptides or fatty acids to assist in solubilization, or form to polymer-based paclitaxel drug delivery systems, resulting maintain therapeutic cytotoxic tumor drug concentrations over extended periods of time and reduce drug’s toxicities. Those novel formulations for delivery of paclitaxel, to some extent, are superior to PTX indoor patients may benefit from the improved therapeutic effect of those modern drug formulations
Summary and Perspectives
Paclitaxel have played a significant role in the treatment of various malignancies over the past 40 years. Their therapeutic effect is due to disrupt the tubulin-microtubule equilibrium and destroy the cancer cell division process by inducing cell cycle arrest and the programmed cell death. However, traditional treatment regimens limited the clinical effectiveness of paclitaxel by high rates of toxicitiessuch as myalgias, hypersensitivity reactions and peripheral neuropathy. These effects mainly were caused by Cremophor EL (polyethoxylated castor oil), a delivery of paclitaxel due to its poor solubility, and ethanol. To overcome these problems, numerous advances have been made in dosing schedules and delivery strategies. Those different treatments, to some extent, produce an anti-tumor effect and reduce the toxic and side effects. In addition, combination of paclitaxel with other agents, including gemcitabine, cisplatin and cyclophosphamide etc., often gives synergistic effects, leading to reduced administered quantities of drugs, therefore, reduced side effects. However, despite those advancements aimed at optimizing paclitaxel therapy, a hard nut to crack remains unresolved, such as its poor selectivity towards tumour cells and inability to prevent, treat the toxicity of paclitaxel. Recently, novel formulations of paclitaxel, including injectable hydrogel, liposomes, micelles and particulate drug delivery systems, have circumvented many of the problems. Clinical trials and preclinical work has demonstrated that those formulations discussed above could enhance the cytotoxic effect of the drug, significantly reduce tumor growth and obviously prolong the survival time of patients or mice with safer toxicity profile compared to PTX, due to their small particle size and changes in tumor microenvironment of drug molecules, realizing a sustained, controlled and targeted delivery of PTX, while at the same time limiting PTX distribution to normal tissue or organ. Although it has been established that those novel formulations are feasible and promising in cancer therapy, there still exists a lot of room for improvement of paclitaxel. Firstly, the lack understanding of formulations stability and drug release mechanisms from formulations in vivo. Furthermore, biodistribution, elimination routes and the effect of degradation products on organs, are also of concern. What’s more, another potential for improvement is the development of more complex targeted systems, which can release therapeutics at a target site. Lastly, clinical and preclinical work should also explore additional applications of those formulations for the treatment of cancer. Thus, in the coming years, further efforts will require to provides better treatment regimen to delay relapse, promote remission, and most importantly improve the quality of life of patients.
References
- Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, et al. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Reserach. 2013; 24: 10-21.
- Siegel R, Desantis C, MBA KVP, Stein K, Mariotto A, Tenbroeck Smith MA, et al. Cancer treatment and survivorship statistics, 2012. Ca A Cancer Journal for Clinicians. 2012; 62: 220-241.
- O'Neill VJ, Twelves CJ. Oral cancer treatment: developments in chemotherapy and beyond. British Journal of Cancer. 2002; 87: 933-937.
- Ma P, Mumper RJ. Paclitaxel nano-delivery systems: a comprehensive review. Journal of nanomedicine & nanotechnology. 2013; 4: 1000164.
- Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. Journal of the National Cancer Institute. 1990; 82: 1247-1259.
- Dustin P. Microtubules. Scientific American. 1980; 243: 66-76.
- Wilson L. Microtubules as Drug Receptors: Pharmacological Properties of Microtubule Protein. Annals of the New York Academy of Sciences. 1975; 253: 213-231.
- Essayan DM, Kagey-Sobotka A, Colarusso PJ, Lichtenstein LM, Ozols RF, King ED. Successful parenteral desensitization to paclitaxel. Journal of Allergy & Clinical Immunology. 1996; 97: 42-46.
- Peereboom DM, Donehower RC, Eisenhauer EA, Mcguire WP, Onetto N, Hubbard JL, et al. Successful re-treatment with taxol after major hypersensitivity reactions. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology. 1993; 11: 885-890.
- Weiszhár Z, Czúcz J, Révész C, Rosivall L, Szebeni J, Rozsnyay Z. Complement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20. European Journal of Pharmaceutical Sciences. 2012; 45: 492-498.
- W L, HJ R, A S, P D, B S, E N, et al. Histamine release in dogs by Cremophor E1 and its derivatives: oxethylated oleic acid is the most effective constituent. Agents & Actions. 1977; 7: 63-67.
- Cecco S, Aliberti M, Baldo P, Giacomin E, Leone R. Safety and efficacy evaluation of albumin-bound paclitaxel. Expert Opinion on Drug Safety. 2014; 13: 511-520.
- Frese KK, Neesse A, Cook N, Bapiro TE, Lolkema MP, Jodrell DI, et al. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer discovery. 2012; 2: 260-269.
- Yardley DA. nab-Paclitaxel mechanisms of action and delivery. Journal of controlled release : official journal of the Controlled Release Society. 2013; 170: 365-372.
- Chen N, Brachmann C, Liu X, Pierce DW, Dey J, Kerwin WS, et al. Albumin-bound nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue distribution and tumor penetration. Cancer chemotherapy and pharmacology. 2015; 76: 699-712.
- Mrózek E, Layman R, Ramaswamy B, Lustberg M, Vecchione A, Knopp MV, et al. Phase II Trial of Neoadjuvant Weekly Nanoparticle Albumin-Bound Paclitaxel, Carboplatin, and Biweekly Bevacizumab Therapy in Women With Clinical Stage II or III HER2-Negative Breast Cancer. Clinical Breast Cancer. 2014; 14: 228-234.
- Green MR, Manikhas GS, Afanasyev B, Makhson AM, Bhar P, Hawkins MJ. Abraxane®, a novel Cremophor®-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Annals of Oncology. 2006; 17: 1263-1268.
- Green MR, Manikhas GM, Orlov S, Afanasyev B, Makhson AM, Bhar P, et al. Abraxane((R)), a novel Cremophor((R))-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Annals of Oncology. 2006; 17: 1263-1268.
- Reynolds C, Barrera D, Jotte R, Spira AI, Weissman C, Boehm KA, et al. Phase II trial of nanoparticle albumin-bound paclitaxel, carboplatin, and bevacizumab in first-line patients with advanced nonsquamous non-small cell lung cancer. Journal of Thoracic Oncology Official Publication of the International Association for the Study of Lung Cancer. 2009; 4: 1537-1543.
- Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011; 29: 4548-4554.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine. 2013; 369: 1691-1703.
- Yasutsuna S, Tomohiro N, Hirofumi Y, Masahiro G, Kei M, Akihito T, et al. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Science. 2014; 105: 812-817.
- Zhang C, Awasthi N, Schwarz MA, Hinz S, Schwarz RE. Superior antitumor activity of nanoparticle albumin-bound paclitaxel in experimental gastric cancer. PloS one. 2013; 8: 58037.
- Awasthi N, Zhang C, Schwarz AM, Hinz S, Schwarz MA, Schwarz RE. Enhancement of Nab-Paclitaxel Antitumor Activity through Addition of Multitargeting Antiangiogenic Agents in Experimental Pancreatic Cancer. Molecular cancer therapeutics. 2014; 13: 1032-1043.
- Hariri G, Yan H, Wang H, Han Z, Hallahan DE. Radiation-guided drug delivery to mouse models of lung cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010; 16: 4968-4977.
- Lin Z, Gao W, Hu H, Ma K, He B, Dai W, et al. Novel thermo-sensitive hydrogel system with paclitaxel nanocrystals: High drug-loading, sustained drug release and extended local retention guaranteeing better efficacy and lower toxicity. Journal of controlled release: official journal of the Controlled Release Society. 2014; 174: 161-170.
- Nguyen MK, Lee DS. Injectable Biodegradable Hydrogels. Macromolecular Bioscience. 2010; 10: 563-579.
- Nirmal HB, Bakliwal SR, Pawar SP. In-Situ gel: New trends in Controlled and Sustained Drug Delivery System. International Journal of Pharmtech Research. 2010: 1398-1408.
- Lee JY, Kim KS, Kang YM, Kim ES, Hwang S-J, Lee HB, et al. In vivo efficacy of paclitaxel-loaded injectable in situ-forming gel against subcutaneous tumor growth. International journal of pharmaceutics. 2010; 392: 51-56.
- Wu Z, Zou X, Yang L, Lin S, Fan J, Yang B, et al. Thermosensitive hydrogel used in dual drug delivery system with paclitaxel-loaded micelles for in situ treatment of lung cancer. Colloids and surfaces B, Biointerfaces. 2014; 122: 90-98.
- Cheng Y, He C, Ding J, Xiao C, Zhuang X, Chen X. Thermosensitive hydrogels based on polypeptides for localized and sustained delivery of anticancer drugs. Biomaterials. 2013; 34: 10338-10347.
- Kim JH, Lee JH, Kim KS, Na K, Song SC, Lee J, et al. Intratumoral delivery of paclitaxel using a thermosensitive hydrogel in human tumor xenografts. Archives of pharmacal research. 2013; 36: 94-101.
- Lin X, Deng L, Xu Y, Dong A. Thermosensitive in situ hydrogel of paclitaxel conjugated poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone). Soft matter. 2012; 8: 3470.
- Huynh DP, Shim WS, Kim JH, Lee DS. PH/temperature sensitive poly(ethylene glycol)-based biodegradable polyester block copolymer hydrogels. Polymer. 2006; 47: 7918-7926.
- Shim W, Kim J, Kim K, Ys, Park R, Kim I, Kwon I, et al. pH- and temperature-sensitive, injectable, biodegradable block copolymer hydrogels as carriers for paclitaxel. International journal of pharmaceutics. 2007; 331: 11-18.
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. Journal of Controlled Release. 2001; 70: 1-20.
- Fonseca C, Simões S, Gaspar R. Paclitaxel-loaded PLGA nanoparticles: preparation, physicochemical characterization and in vitro anti-tumoral activity. Journal of Controlled Release. 2002; 83: 273-286.
- Averineni RK, Shavi GV, Gurram AK, Deshpande PB, Arumugam K, Maliyakkal N, et al. PLGA 50:50 nanoparticles of paclitaxel: Development, in vitro anti-tumor activity in BT-549 cells and in vivo evaluation. Bulletin of Materials Science. 2012; 35: 319-326.
- Su WP, Cheng FY, Shieh DB, Yeh CS, Su WC. PLGA nanoparticles codeliver paclitaxel and Stat3 siRNA to overcome cellular resistance in lung cancer cells. International journal of nanomedicine. 2012; 7: 4269-4283.
- Danbo Y, Sang V, Xinguo J, et al. Novel free paclitaxel-loaded poly(L-γ-glutamylglutamine)–paclitaxel nanoparticles. International journal of nanomedicine. 2011: 85-91.
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature reviews Drug discovery. 2005; 4: 145-60.
- Jain S, Kumar D, Swarnakar NK, Thanki K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials. 2012; 33: 6758-6768.
- Zhao L, Ye Y, Li J, Wei YM. Preparation and the in-vivo evaluation of paclitaxel liposomes for lung targeting delivery in dogs. The Journal of pharmacy and pharmacology. 2011; 63: 80-86.
- Danhier F, Danhier P, De Saedeleer CJ, Fruytier AC, Schleich N, des Rieux A, et al. Paclitaxel-loaded micelles enhance transvascular permeability and retention of nanomedicines in tumors. International journal of pharmaceutics. 2015; 479: 399-407.
- Li Y, Xiao K, Luo J, Xiao W, Lee JS, Gonik AM, et al. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials. 2011; 32: 6633-6645.