
Review Article
Austin Oncol. 2016; 1(2): 1010.
Quercetin and Ursolic Acid: Dietary Moieties with Promising Role in Tumor Cell Cycle Arrest
Kashyap D¹, Sharma A², Mukherjee TK³, Tuli HS³* and Sak K4
¹Department of Histopathology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, Punjab, India
²Department of Chemistry, Career Point University, Tikker-kharwarian, Hamirpur, Himachal Pradesh, India
³Department of Biotechnology, M.M. University, Mulana, Ambala, Haryana, India
4Department of Hematology and Oncology, Institute of Clinical Medicine, University of Tartu, Tartu, Estonia
*Corresponding author: Hardeep Singh Tuli, Assistant Professor, Department of Biotechnology, M.M University, Mullana-Ambala, Haryana, India
Received: September 05, 2016; Accepted: October 05, 2016; Published: October 07, 2016
Abstract
Despite extensive efforts done in the recent decades, cancer has still remained an incurable disorder. On the other hand, there is no doubt that different natural compounds possess a huge potential to suppress the promotion and progression of tumorigenesis, and numerous studies have described the possible molecular mechanisms of such substances. Probably one of the most efficient ways to hinder the multiplication of cancer cells is to arrest their cell cycle progression. Therefore, in the current article, a detailed review is presented about the arrest of cell cycle in different phases followed by exposure of cancer cells to two natural dietary agents, quercetin, and ursolic acid. Both these compounds have previously been shown to exert anticancer properties, whereas pleiotropic action mechanisms were proposed. The current work describes a variety of molecules occupied in regulation of cell cycle progression and transition between different phases initiated by treatment of cancer cells with the respective flavonoid and triterpenoid. It is clear that better knowledge about the processes and molecules involved in cell cycle, as well as possibilities to modulate such mechanisms by natural compounds, may lead to the development of more efficient and targeted chemopreventive and chemotherapeutic strategies in the future.
Keywords: Cancer treatment and prevention; Cell cycle arrest; Natural dietary agents; Quercetin; Ursolic acid
Introduction
Studies in last few decades utilizing phytochemicals have ameliorated and emerged as an incipient platform for cancer treatment [1-3]. Several phytochemicals with anti-cancer properties have superseded the synthetic chemotherapeutic molecules due to lesser side effects [4]. Among the variety of health benign phytochemicals, quercetin (Quer, flavonoid) and ursolic acid (UA, triterpenoid) are emerging as promising therapeutics [5-15]. Being pharmacologically active, these phytochemicals are intensively utilized in chemoprevention and treatment. UA and Quer are kenned to modulate the sundry anti-cancer cell signaling pathways, such as induction of apoptosis, anti-angiogenesis, anti-metastasis, and cell cycle inhibition [16-20]. Especially in the cell cycle, a variety of different transcriptional factors and regulatory proteins are kenned to play a paramount role that is being targeted for cancer treatment and prevention.
In most eukaryotes, cell cycle-regulated transcription can be grouped into three main waves that coincide with the different transition points during the cell cycle, namely G1-to-S, G2-to-M and M-to-G1 [21,22]. Following mitotic division, a somatic cell undergoes an interim reposing phase called G0 phase which is followed by preparation of cell division through G1, S, and G2 phases which are further followed by next mitotic division [21,23] This cyclic order of physiological events comprising the mitotic division, reposing phase and preparation of cell division followed by next mitotic division is referred as the cell cycle. The transition from G0 to G1 phase of a cell cycle is tightly regulated by the balancing activity of positive signal engendered from mitogenic signals like magnification factors, hormones, amino acids and negative signals originated from Cyclin Inhibitory Proteins (CIPs) and Kinase Inhibitory Proteins (KIPs) [24,25]. At physiological state, positive signals subsist at their basal level and negative signals at their peak level in undivided somatic cells at G0 phase whereas the cells enter G1 phase only when negative signals minimize and positive signals reach their peak [26]. The length of the G0 phase varies from cell type to cell type. For example, while neuronal cells may have perennial G0 phase, the White Blood Cells (WBC) may have G0 phase for the only a couple of days. In contrast, cancer cells may have a very short span of G0 phase or none at all [26,27]. This designates that cancer cells are always in active cell cycle and cell division occurs perpetually irrespective of the presence of magnification promoting positive signals or absence of negative signals in the cellular environment. Mutation in the positive signaling molecules (e.g. magnification factor receptors) or negative signaling molecules (e.g P53) may be one of the vital reasons for this kind of uninterrupted, perpetual cell division of cancer cells [26,28].
Consequently, regulation of cell cycle is most paramount to regulate uncontrolled and aberrant proliferation of cancer cells. Quer and UA have been studied in sundry cancer cell lines and in in-vivo cancer models and declared as cell cycle inhibitors [29,30]. Up- and down-regulation of sundry cell cycle inhibitors and other regulatory proteins in the presence of these phytochemicals have been noted in various experiments. The present review summarizes the all known mechanisms of cell cycle inhibition by Quer and UA.
Chemistry of Quercetin and Ursolic Acid
Quer (3,5,7,3’,4’–Penta hydroxy flavone) is a flavonol belonging to the class of polyphenolic flavonoids which is characterized by the presence of five hydroxyl groups on C6-C3-C6 backbone structure especially a 3-OH group on the pyrone ring (ring C) [31]. It is the most important and abundant flavonol being found in apples, onions, and blueberries at a higher level while in form of glycoside [32-34]. It is synthesized by two methods: (i) By the cyclization of chalcone of 2,4-dimethoxy-6-hydroxyacetophenone and 3,4-dimethoxybenzaldehyde; (ii) The condensation of ω-Methoxyphloroacetophenone with a veratric anhydride in the presence of the potassium salt of 3,4-dimethoxybenzoic acid [35].
UA (3β-hydroxy-urs-12-en-28-oic acid) is a pentacyclic triterpenoid (C30) of ursane (α-amyrin) and it consists five cycles of six-membered rings with trans junction of rings like A/B, B/C, C/D and the cis junction of rings such as D/E. In this molecule, one hydroxyl group is attached at the C-3 position, one carboxylic group at C-17 and a double bond is present at C-12 while the seven methyl groups are linked at C-4, C-4, C-8, C-10, C-14 C-19 and C-20. The main sources of UA are reported to be berries and peels of fruits and its acetate derivative was synthesized from α-amyrinbenzoate [36,37] (Figure 1).
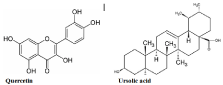
Figure 1: Chemical structure of quercetin and ursolic acid.
Mechanism of Cancer Cell Cycle Inhibition by Quercetin and Ursolic Acid
G0/G1 checkpoint regulation by quercetin and ursolic acid
In the cell cycle, G0 is the quiescent phase. The most nonproliferative cells of multicellular organism entering into this phase from G1 state and remain in it till no further stimuli come [21]. Signals in the form of DNA damage or degradation and presence of carcinogen stimulate the cell from the G0→G1 state. Next, G1 (magnification phase) is the first phase within interphase which is characterized by most biosynthetic activities including preparation of DNA replication to fortify precise cell division. The G0/G1 checkpoint is most tightly regulated by sundry proteins and transcriptional factors. In mammalian cells, the molecules including cyclins D and E form active protein kinase complexes with Cdk proteins (CDK4/ CDK6, CDK2) and are required for progression of cells through G→S phase. Overexpression of cyclin D has been associated with sundry human cancers that push the cell from G to S phase [38-41]. The cyclin D/Cdk4, Cdk6 complexes function by phosphorylating the pRb protein [42,43] which in unphosphorylated state suppresses the G1→S progression [44]. Rb (retinoblastoma), a negative regulator of cell cycle, binds to and inhibits transcription factors of the E2F family, which are composed of dimers of an E2F protein and a Dimerization Partner (DP) protein and control the G1→S phase [45,46]. pRb molecule additionally regulates the cyclin A and cdc2 expression and put these genes product under G1 cyclin control like cyclins D and E. Another cyclin/Cdk complex that play a crucial role in the G1/S phase transition is cyclin E/Cdk2. Most of the synthetic and natural anti-cancer agents target this checkpoint to contravene the cancer cell proliferation. Results suggest that flavonoids and triterpenoids exhibit consequential anti-tumor effects by suppressing cell proliferation, promoting apoptosis and inducing cell cycle arrest in both in-vitro and in vivo tumor models [47]. Cell cycle study of UA-treated HepG2 cell line explored the G0/G1 cell cycle arrest induction via upregulation of p21 (WAF1) expression [29,48]. An in-vitro experiment utilizing HCT15 (human colon cancer cell line) cells found the more accumulation of cancer cells in G0/G1 phase after 36h treatment of UA [49]. Inhibition of MCF-7 breast cancer cells demonstrated the pro-apoptotic effect of UA with cyclin D1/CDK4 inactivation, which is kenned to play a crucial role in cell cycle progression [50]. Similarly, utilizing SNG-2 and HEC108 (endometrial adenocarcinoma cancer cell lines) cells, studies showed the cell cycle inhibitory effect of UA in G1 phase via modulating MAPK signaling pathways [51] (Figure 2).
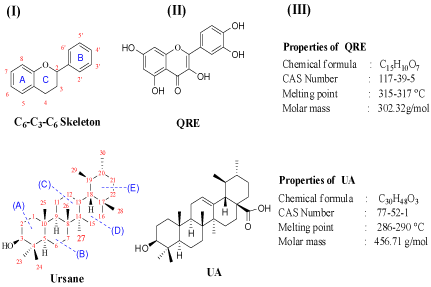
Figure 2: Chemistry of Quercetin and Ursolic acid.
Quer, a flavonoid molecule was also kenned to block cell cycle progression at G1 phase and exert its magnification inhibitory effect through the incrementation of Cdk inhibitors p21 and p27 and tumor suppressor p53 in HepG2 cells [52]. The down-regulation of cyclin D1 level linked to the G1/S phase alteration was found in Quertreated human ovarian carcinoma and osteosarcoma cell lines [53]. In a homogeneous way, Quer mediated G1 cell cycle arrest by downregulation of cyclin D1/Cdk4 and cyclin E/Cdk2 and up-regulation of p21 proteins in vascular smooth endothelial cells [54,55]. Again, it was found that Quer incremented the expression of Rb gene and subsequently arrested HK1 and CNE2 cells in G2/M or G0/G1 cell cycle phase (Ong et al. 2004). Further, the proliferation of ovarian cancer SKOV-3 cells and Malignant Mesothelioma (MM) MSTO- 211H and H2452 cells has been inhibited by treatment of Quer which blocked cell cycle progression from G0/G1 to G2/M and also induced cell apoptosis [56,57]. Quer suppressed HSP27-mediated cyclin D1 expression and ergo obstructed the U937 leukemia cell proliferation in the G1 phase [58,59]. Withal, the reduction of cyclin D level was found in Quer-treated SKOV3 and U2OSPt cells which could be linked to G1/S phase alteration.
S-phase arrest by quercetin and ursolic acid
S-phase (synthesis phase) represents DNA replication occurring between G1 and G2 phases and responds the precise and consummate DNA replication of all chromosomes to obviate any genetic abnormalities. Several magnification-dependent Cyclin-Dependent Kinases (CDKs) are required to promote DNA replication and to initiate G1→S phase transition. Cyclin E which is engendered during cell division binds to CDK2, forming the cyclin E-CDK2 complex and pushes the cell from G1→S phase (G1/S, which initiates the G2/M transition) [60,61]. Recently, Wang and his colleagues found that the S-phase cell cycle arrest of GBC-SD and SGC-996 (gall bladder cancer cell lines) cells by UA was associated with mitochondrial apoptotic cell death [47].
Quer has also been proven a mediator of S-phase arrest [62]. The dose- and time-dependent inhibition of DNA synthesis and Thymidylate Synthase (TS), a key S-phase enzyme, has been associated with Quer exposure to SCC-9 cells, which thereafter arrested the cells in S phase [63]. Additionally, the arrest of MCF-7 cells in the S phase by down-regulation of Cdk2 and cyclins A and B and up-regulation of p53 and p57 proteins has also been found to be subjected to the Quer treatment [64,65].
G2/M checkpoint regulation by quercetin and ursolic acid
The G2/M DNA damage checkpoint is a consequential point that ascertains that only normal cell has to initiate the mitosis after proofreading [66]. Treatment of tumor cells with either flavonoid or triterpenoid or in combination enlightens the way of anti-tumor therapy that causes cell arrest in G2/M phase. Anti-cancer effect of Quer through the inhibition of the cell proliferation and an incremented arrest of the cells in G2/M phase was indeed described in an in vitro study [67]. Individually, as well as synergistically in combination with another chemotherapy drug, Quer induced G2/M arrest in the HT29 cells [68]. In adult male Wistar rats, the Quer-mediated arrest of cancer cells in the G2 phase with reduced expression of cyclin D1, cyclin A, cyclin B, and CDK1 has also been described [69]. Similarly, Quer elicited a spectacular extent of G2/M phase cell cycle arrest in HepG2 tumor cell line [70]. The reduction of cyclin D1 level that could be linked to the G1/S phase alteration has been found in Quer-treated human ovarian carcinoma and osteosarcoma cell lines [53]. Furthermore, results also demonstrated that Quer suppressed the viability of HeLa cells by inducing G2/M phase cell cycle arrest and mitochondrial apoptosis through a p53- dependent mechanism [71].
Regulation of other targets by quercetin and ursolic acid
Several regularity proteins other than cyclins and CDK indispensable during cell division are functionally vigorously organized in the mammalian cells [72-74]. Tumor suppressor protein/ transcription factor p53 functions by binding to the regulatory sequences and trans-activating a number of genes, including p21, Mdm2, and GADD45 [75-78]. Another protein, p21, called also Cip1/Wafl, binds directly to cyclin-CDK2, -CDK1, and -CDK4/6 complexes and thus functions as a regulator of cell cycle progression at G1 and S phases [79,80]. The expression of p21 is tightly controlled by the tumor suppressor protein p53, through which this protein mediates the p53-dependent G1 phase arrest in replication to a variety of stress stimuli [81-83]. A study revealed that UA was able to inhibit Murine Double Minute-2 Protein (MDM2) and T-LAK Cell- Originated Protein Kinase (TOPK), the two negative regulators of p53, which in turn contributed to UA-induced p53 activation [84,85]. In addition, Quer-mediated up-regulation of p21, p27, p53, and Chk2, down-regulation of Cdk1 and cyclin B1, and phosphorylation of pRb followed by the arrest of the cell cycle in the G1 and G2/M phase have been found in a variety of cancer cell lines [52,86,87]. Furthermore, dose- and time-dependent treatment with Quer led to the arrest of MCF-7 breast cancer cells in the S phase as a result of the down-regulation of Cdk2 and cyclins A and B and the upregulation of p53 and p57 [64,65]. The study done with HepG2 cells suggested that the anti-carcinogenic action of Quer was mediated by up-regulation of p53 and BAX and via down-regulation of Reactive Oxygen Species (ROS), PKC, P(I) 3K and COX [88,89]. Furthermore, Quer in combination with ellagic acid elevated the expression of p53 and p21 and MAP kinases, JNK1/2 and p38, in a more than additive manner, thus suggesting a synergistic mechanism of tumor inhibition [90] (Figure 3).
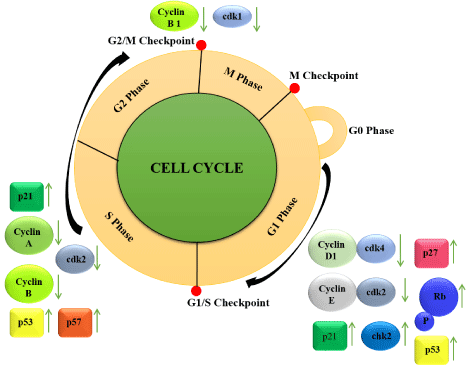
Figure 3: Mechanism of tumor cell cycle inhibition through modulation of
several regulatory proteins under the influence of quer and UA (adopted from
kashyap et. al. 2016-[91]).
Conclusion and Future Perspectives
The data presented above clearly show that the more insight into the function of all the regularity proteins of cell cycle could ensure the good treatment and better prognosis of cancer patients. Although most of the chemotherapeutic molecules are effective against the proliferation, development of resistance and use of alternative pathways biased their anti-proliferation effect [92,93]. The complete detail regarding all other targets of flavonoids and triterpenoids in tumor cell division is therefore urgently required to further extend their molecular mechanisms of cancer inhibition [1,47,91,94]. An organized up-regulation/down-regulation of several transcriptional factors or other regulatory proteins, cyclins, and cdks in the cancer cells is intentionally necessary to develop an effective way of treatment and prevention of malignant manifestations [41,95- 97]. The targeted delivery of natural anti-cancer molecules with the help of nanoparticles might be used to design an ensured therapy for cancer prevention in future.
Acknowledgement
The authors would like to acknowledge department of Histopathology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh (Punjab) India for platform provided to perform this study.
References
- Kashyap D, Tuli HS, Sharma AK. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016; 146: 201-213.
- Kashyap D, Mondal R, Tuli HS, Kumar G, Sharma AK. Molecular targets of gambogic acid in cancer: recent trends and advancements. Tumor Biol. 2016.
- Kashyap D, Mondal R, Tuli HS, Kumar G, Sharma AK. Molecular targets of gambogic acid in cancer: recent trends and advancements. Tumor Biol. 2016; 3: 208-215.
- Tuli HS, Sandhu SS, Sharma AK. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3 Biotech. 2013; 4: 1-12.
- Yeh CT, Wu CH, Yen GC. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol. Nutr. Food Res. 2010; 54: 1285-1295.
- Shanmugam MK, Manu KA, Ong TH, Ramachandran L, Surana R, Bist P, et al. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int. J. Cancer. 2011; 129: 1552-1563.
- Lin CC, Huang CY, Mong MC, Chan CY, Yin MC. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J. Agric. Food Chem. 2011; 59: 755-762.
- Martin-Aragón S, de las Heras B, Sanchez-Reus MI, Benedi J. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp. Toxicol. Pathol. 2001; 53: 199-206.
- Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Ye Q, et al. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-??B pathway activation. Cereb. Cortex. 2010; 20: 2540-2548.
- Nascimento PGG, Lemos TLG, Bizerra AMC, Arriaga ÂMC, Ferreira DA, Santiago GMP, et al. Antibacterial and Antioxidant Activities of Ursolic Acid. Molecules. 2014; 19: 1317-1327.
- Lee YJ, Lee DM, Leev SH. Nrf2 Expression and Apoptosis in Quercetin-treated Malignant Mesothelioma Cells. Mol. Cells. 2015; 38: 416-425.
- Senggunprai L, Kukongviriyapan V, Prawan A, Kukongviriyapan U. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phyther. Res. 2014; 28: 841-848.
- Pratheeshkumar P, Budhraja A, Son YO, Wang X, Zhang Z, Ding S, et al. Quercetin Inhibits Angiogenesis Mediated Human Prostate Tumor Growth by Targeting VEGFR- 2 Regulated AKT/mTOR/P70S6K Signaling Pathways. PLoS One. 2012; 7.
- Wilkinson K, Boyd JD, Glicksman M, Moore KJ, El Khoury J. A high content drug screen identifies ursolic acid as an inhibitor of amyloid ?? protein interactions with its receptor CD36. J. Biol. Chem. 2011; 286: 34914-34922.
- Jang SM, Kim MJ, Choi MS, Kwon EY, Lee MK. Inhibitory effects of ursolic acid on hepatic polyol pathway and glucose production in streptozotocin-induced diabetic mice. Metabolism. 2010; 59: 512-519.
- Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J. Nutr. 2006; 136: 2715-2721.
- Igura K, Ohta T, Kuroda Y, Kaji K. Resveratrol and quercetin inhibit angiogenesis in vitro. Cancer Lett. 2001; 171: 11-16.
- Wu B, Wang X, Chi ZF, Hu R, Zhang R, Yang W, et al. Ursolic acid-induced apoptosis in K562 cells involving upregulation of PTEN gene expression and inactivation of the PI3K/Akt pathway. Arch. Pharm. Res. 2012; 35: 543-548.
- Kim ES, Moon A. Ursolic acid inhibits the invasive phenotype of SNU-484 human gastric cancer cells. Oncol. Lett. 2015; 9: 897-902.
- Lin J, Chen Y, Wei L, Hong Z, Sferra TJ, Peng J. Ursolic acid inhibits colorectal cancer angiogenesis through suppression of multiple signaling pathways. Int. J. Oncol. 2013; 43: 1666-1674.
- Liu JD, Wang YJ, Chen CH, Yu CF, Chen LC, Lin JK, et al. Molecular mechanisms of G0/G1 cell-cycle arrest and apoptosis induced by terfenadine in human cancer cells. Mol. Carcinog. 37: 2003; 39-50.
- Shackelford RE, Kaufmann WK, Paules RS. Cell Cycle Control, Checkpoint Mechanisms and Genotoxic Stress Biology of the Cell Cycle. Environ. Heal. 1999; 107: 5-24.
- Collins K, Jacks T, Pavletich NP. The cell cycle and cancer. Proc. Natl. Acad. Sci. U.S.A. 1997; 94: 2776-2778.
- Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994; 266: 1821-1828.
- G??rard C1, Tyson JJ, Coudreuse D, Nov??k B. Cell Cycle Control by a Minimal Cdk Network, PLoS Comput. Biol. 2015; 11: 1004056.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Components of the Cell-Cycle Control System. in: Mol. Biol. Cell, Garland Science, 2014; 2002.
- Bastians H, Müller R. Cell-cycle Control, in: Encycl. Mol. Pharmacol., Springer Berlin Heidelberg, Berlin, Heidelberg. 2008; 340-345.
- Bretones G, Delgado MD, León J. Myc and cell cycle control, Biochim. Biophys. Acta. 2015; 1849: 506-516.
- Kim DK, Baek JH, Kang CM, Yoo MA, Sung JW, Kim DK, et al. Apoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replication, Int. J. Cancer. 2000; 87: 629-636.
- Ong CS, Tran E, Nguyen TTT, Ong CK, Lee SK, Lee JJ, et al. Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Oncol. Rep. 2004; 11: 727-733.
- Marais JPJ, Deavours B, Dixon RA, Ferreira D. The stereochemistry of flavonoids, in: Sci. Flavonoids. Springer New York, New York, NY. 2006; 1-46.
- Sampson L, Rimm E, Hollman PCH, De Vries JHM, Katan MB. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc. 2002; 102: 1414-1420.
- Hollman PCH, Van Trijp JMP, Buysman MNCP, Martijn MS, Mengelers MJB, De Vries JHM, et al. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997; 418: 152-156.
- J. Buttriss, Dietary Intake and Bioavailability of Plant Bioactive Compounds, in: Plants Diet Heal. Blackwell Science Ltd, Oxford, UK, n.d. 86-106.
- Sharma, H. Gupta, Quercetin a flavanoid, Chronicles Young Sci. 2010; 1: 10-15.
- D. Kashyap, H.S. Tuli, A.K. Sharma, Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016; 146: 201-13.
- Kashyap D, Sharma A, Tuli HS, Punia S, Sharma AK. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-Inflammatory Activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016.
- Lammie GA, Fantl V, Smith R, Schuuring E, Brookes S, Michalides R, et al. D11S287, a putative oncogene on chromosome 11q13, is amplified and expressed in squamous cell and mammary carcinomas and linked to BCL-1. 1991; 6: 439-444.
- Motokura T, Bloom T, Kim HG, Jüppner H, Ruderman JV, Kronenberg HM, et al. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991; 350: 512-515.
- Seto M, Yamamoto K, Iida S, Akao Y, Utsumi KR, Kubonishi I, et al. Gene rearrangement and overexpression of PRAD1 in lymphoid malignancy with t(11;14)(q13;q32) translocation. Oncogene. 1992; 7: 1401-1406.
- Dharambir Kashyap HST, Ajay Sharma, Manoj Kumar, Katrin Sak. Molecular targets of natural metabolites in cancer: recent trends and advancements, J. Biol. Chem. Sci. 2016; 3: 208-215.
- Matsushime H, Ewen ME, Strom DK, Kato JY, Hanks SK, Roussel MF, et al. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins, Cell. 1992; 71: 323-334.
- Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner., Mol. Cell. Biol. 1994; 14: 2077-2086.
- Herwig S, Strauss M. The retinoblastoma protein: a master regulator of cell cycle, differentiation and apoptosis., Eur. J. Biochem. 1997; 246: 581-601.
- Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA. Inhibition of CDK activity and pcna-dependent DNA replication p21 is blocked by interaction with the HPV-16 E7 oncoprotein, Genes Dev. 1997; 11: 2090-2100.
- De Veylder L, Joubès J, Inzé D. Plant cell cycle transitions, Curr. Opin. Plant Biol. 2003; 6: 536-543.
- Weng H, Tan ZJ, Hu YP, Shu YJ, Bao RF, Jiang L, et al. Ursolic acid induces cell cycle arrest and apoptosis of gallbladder carcinoma cells. Cancer Cell Int. 2014; 14: 96.
- Leal AS, Wang R, Salvador JAR, Jing Y. Semisynthetic Ursolic Acid Fluorolactone Derivatives Inhibit Growth with Induction of p21waf1 and Induce Apoptosis with Upregulation of NOXA and Downregulation of c-FLIP in Cancer Cells, ChemMedChem. 2012; 7: 1635-1646.
- Li J, Guo WJ, Yang QY. Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World J. Gastroenterol. 2002; 8: 493-495.
- Wang J, Ren T, Xi T. Ursolic acid induces apoptosis by suppressing the expression of FoxM1 in MCF-7 human breast cancer cells., Med. Oncol. 2012; 29: 10-15.
- Achiwa Y, Hasegawa K, Udagawa Y. Effect of ursolic acid on MAPK in cyclin D1 signaling and RING-type E3 ligase (SCF E3s) in two endometrial cancer cell lines. Nutr. Cancer. 2013; 65: 1026-1033.
- Mu C, Jia P, Yan Z, Liu X, Li X, Liu H. Quercetin induces cell cycle G1 arrest through elevating Cdk inhibitors p21 and p27 in human hepatoma cell line (HepG2), Methods Find Exp Clin Pharmacol. 2007; 29: 179-183.
- Catanzaro D, Ragazzi E, Vianello C, Caparrottav L, Montopoli M. Effect of Quercetin on Cell Cycle and Cyclin Expression in Ovarian Carcinoma and Osteosarcoma Cell Lines. Nat. Prod. Commun. 2015; 10: 1365-1368.
- Moon SK, Cho GO, Jung SY, Gal SW, Kwon TK, Lee YC, Madamanchi NR, Kim CH. Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: role of ERK1/2, cell-cycle regulation, and matrix metallopteinase-9. Biochem Biophys Res Commun. 2003; 30: 1069-1078.
- Zhou J, Li L, Fang L, Xie H, Yao W, Zhou X, et al. Quercetin reduces cyclin D1 activity and induces G1 phase arrest in HepG2 cells. Oncol. Lett. 2016; 12: 516-522.
- Lee YJ, Lee DM, Lee SH. Nrf2 Expression and Apoptosis in Quercetin-treated Malignant Mesothelioma Cells. Mol. Cells. 38: 416-425.
- Ren MX, Deng XH, Ai F, Yuan GY, Song HY. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp. Ther. Med. 2015; 10: 579-583.
- Lou G, Liu Y, Wu S, Xue J, Yang F, Fu H, et al. The p53/miR-34a/SIRT1 positive feedback loop in quercetin-induced apoptosis. Cell. Physiol. Biochem. 2015; 35: 2192-2202.
- Wang Y, Han A, Chen E, Singh RK, Chichester CO, Moore RG, et al. The cranberry flavonoids PAC DP-9 and quercetin aglycone induce cytotoxicity and cell cycle arrest and increase cisplatin sensitivity in ovarian cancer cells. Int. J. Oncol. 2015; 46: 1924-1934.
- Tuli HS, Kashyap D, Sharma AK. Cordycepin: A Cordyceps Metabolite with Promising Therapeutic Potential. in: Fungal Metab. Springer International Publishing, Cham. 2015; 1-22.
- Tuli HS, Sharma AK, Sandhu SS, Kashyap D. Cordycepin: A bioactive metabolite with therapeutic potential. Life Sci. 2013; 93: 863-869.
- Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, et al. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016; 6: 24049.
- Haghiac M, Walle T. Quercetin induces necrosis and apoptosis in SCC-9 oral cancer cells. Nutr. Cancer. 2005; 53: 220-231.
- Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu CC, et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch. Pharm. Res. 2010; 33: 1181-1191.
- Duo J, Ying GG, Wang GW, Zhang L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 2012; 5: 1453-1456.
- Kosaka C, Sasaguri T, Ishida A, Ogata J. Cell cycle arrest in the G2 phase induced by phorbol ester and diacylglycerol in vascular endothelial cells. Am. J. Physiol. 1996; 270: 170-178.
- Kuo PC, Liu HF, Chao JI. Survivin and p53 modulate quercetin-induced cell growth inhibition and apoptosis in human lung carcinoma cells. J. Biol. Chem. 2004; 279: 55875-55885.
- Atashpour S, Fouladdel S, Movahhed TK, Barzegar E, Ghahremani MH, Ostad SN, et al. Quercetin induces cell cycle arrest and apoptosis in CD133 + cancer stem cells of human colorectal HT29 cancer cell line and enhances anticancer effects of doxorubicin. Iran. J. Basic Med. Sci. 2015; 18: 635-643.
- Casella ML, Parody JP, Ceballos MP, Quiroga AD, Ronco MT, Franc??s DE, et al. Quercetin prevents liver carcinogenesis by inducing cell cycle arrest, decreasing cell proliferation and enhancing apoptosis. Mol. Nutr. Food Res. 2014; 58: 289-300.
- Poór M, Zrínyi Z, Kőszegi T. Structure related effects of flavonoid aglycones on cell cycle progression of HepG2 cells: Metabolic activation of fisetin and quercetin by catechol-O-methyltransferase (COMT). Biomed. Pharmacother. 2016; 83: 998-1005.
- Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-??B inhibition. Eur. J. Pharmacol. 2010; 649: 84-91.
- Tuli HS, Kashyap D, Sharma AK, Sandhu SS. Molecular aspects of melatonin (MLT)-mediated the rapeutic effects. Life Sci. 2015; 135: 147-57.
- Tuli HS, Kashyap D, Bedi SK, Kumar P, Kumar G, Sandhu SS, et al. Molecular aspects of metal oxide nanoparticle (MO-NPs) mediated pharmacological effects. Life Sci. 2015; 143: 71-79.
- Mukherjee P, Winter SL, Alexandrow MG. Cell cycle arrest by transforming growth factor beta1 near G1/S is mediated by acute abrogation of prereplication complex activation involving an Rb-MCM interaction. Mol. Cell. Biol. 2010; 30: 845-856.
- El-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994; 54: 1169-1174.
- Funk WD, Pak DT, Karas RH, Wright WE, Shay JW. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol. Cell. Biol. 1992; 12: 2866-2871.
- Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992; 71: 587-597.
- Kern SE, Kinzler KW, Bruskin A, Jarosz D, Friedman P, Prives C, et al. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991; 252: 1708-1711.
- Wade Harper J, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993; 75: 805-816.
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D, et al. P21 Is a Universal Inhibitor of Cyclin Kinases. Nature. 1993; 366: 701-704.
- Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 65: 2005; 3980-3985.
- Xavier CPR, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother. Pharmacol. 2011; 68: 1449-1457.
- Wang S, El-deiry WS, El Deiry WS. P53, Cell Cycle Arrest and Apoptosis. in: 25 Years p53 Res. Springer Netherlands, Dordrecht. 2007: 141-163.
- Zhang X, Song X, Yin S, Zhao C, Fan L, Hu H. p21 induction plays a dual role in anti-cancer activity of ursolic acid. Exp. Biol. Med. (Maywood). 2016; 24: 501-508.
- Pietenpol JA, Stewart ZA. Cell cycle checkpoint signaling: Cell cycle arrest versus apoptosis. Toxicology. 181-182 2002; 475-481.
- Bender–Sigel BJ, Schrenk D, Flügel D, Kietzmann T. The Antioxidant Quercetin Inhibits Cellular Proliferation via HIF-1-Dependent Induction of p21WAF. Antioxid. Redox Signal. 2010; 13: 437-448.
- Jeong JH, An JY, Kwon YT, Rhee JG, Lee YJ. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009; 106: 73-82.
- Maurya AK, Vinayak M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol. Biol. Rep. 2015; 42: 1419-1429.
- Tanigawa S, Fujii M, Hou DX. Stabilization of p53 is involved in quercetin-induced cell cycle arrest and apoptosis in HepG2 cells. Biosci. Biotechnol. Biochem. 2008; 72: 797-804.
- Mertens-Talcott SU, Bomser J, Romero C, Talcott ST, Percival SS. Ellagic acid potentiates the effect of quercetin on p21waf1/cip1, p53, and MAP-kinases without affecting intracellular generation of reactive oxygen species in vitro. J. Nutr. 2005; 135: 609-614.
- Lindley C, McCune JS, Thomason TE, Lauder D, Sauls A, Adkins S, et al. Perception of chemotherapy side effects: Cancer versus noncancer patients. Cancer Pract. 1999; 7: 59-65.
- Azim HA, de Azambuja E, Colozza M, Bines J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann. Oncol. 22: 2011; 1939-1947.
- Kashyap D, Mittal S, Sak K, Singhal P, Tuli HS. Molecular mechanisms of action of quercetin in cancer: recent advances. Tumor Biol. 2016.
- Es-saady D, Simon A, Ollier M, Maurizis JC, Chulia AJ, Delage C. Inhibitory effect of usolic acid on B16 proliferation through cell cycle arrest. Cancer Lett. 1996; 106: 193-197.
- Shapiro GI, Harper JW. Anticancer drug targets: cell cycle and checkpoint control. J. Clin. Invest. 104: 1999; 1645-1653.
- Kohn KW, Jackman J, O’Connor PM. Cell cycle control and cancer chemotherapy. J. Cell. Biochem. 1994; 54: 440-452.
- Schwartz GK, Shah MA. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005; 23: 9408-9421.