
Review Article
Austin Oncol. 2016; 1(3): 1013.
Multiparametric- Mri and Targeted Prostate Biopsy in Prostate Cancer: What is Established and What is Expected?
Azevedo MQ¹, Almeida GL²*, Medronha EF³, Rêgo HC³, Júnior WB² and Cobelli OD4
¹Department of Urology, Moinhos de Vento Hospital, Porto Alegre, Rio Grande do Sul, Brazil
²Department of Urology, University of Vale do Itajaí, Itajaí, Santa Catarina, Brazil/Instituto Catarinense de Urologia (INCAU), Itajaí, Santa Catarina, Brazil
3Department of Radiology, Mãe de Deus Hospital, Porto Alegre, Rio Grande do Sul, Brazil
4Department of Urology, European Institute of Oncology (IEO), Milan, Lombardia, Italy/ University of Milan, Milan, Lombardia, Italy
*Corresponding author: Gilberto Laurino Almeida, Avenida Marcos Konder 1120, Centro Zip Code 88301- 302, Itajaí - Santa Catarina, Brazil
Received: October 21, 2016; Accepted: December 13, 2016; Published: December 15, 2016
Abstract
Transrectal ultrasonography–guided biopsy (TRUS-Bx) is the standard of care for diagnosis of Prostate Cancer (PCa). However, TRUS-Bx has some limitations as frequently fail to detect aggressive tumors or provide reliable parameters for pretreatment risk stratification, had a false-negative around 21- 47% to PCa diagnosis, detection of clinically insignificant PCa around 17% in the first biopsy), and frequently necessity of re-biopsies with consequently morbidity and infection risk. Multiparametric Magnetic Resonance Imaging (mpMRI) has recently emerged as the most accurate imaging technique for PCa detection and staging. Recently a biopsy guided by fusion mp MRI and TRUS has being developed. Techniques of MRI-targeted biopsy include in-gantry MRI guided biopsy, TRUS-guided visual estimation biopsy, and software co-registered MRI-US guided biopsy (MRI-TRUS fusion). Clinical applications for which MRITRUS fusion biopsies of the prostate include patients with suspected PCa and previous negative biopsy, patients with known PCa for whom active surveillance is an option, patients with known PCa to determine disease status during active surveillance, and candidates for focal therapy. We review and discuss these applications for mpMRI and MRI-TRUS fusion.
Keywords: Prostate cancer; Radical prostatectomy; Magnetic Resonance Imaging (MRI)
Introduction
Prostate Cancer (PCa) is the most commonly diagnosed malignant tumor among men, except non-melanoma skin tumors [1]. In the United States 180.890 new cases will be diagnosed in 2016, and 26.120 men will die from PCa [2]. It is the second greatest cause of death of cancer in the Western male population. The widespread use of PSA testing has resulted in a dramatic increase in the diagnosis. Transrectal ultrasonography–guided biopsy (TRUS-Bx) is the standard of care for diagnosis of PCa, based on alterations of the digital rectal exam or serum PSA measurement [2].
Approximately 1 million prostate biopsies are performed annually in the United States. However, TRUS-Bx frequently fails to detect aggressive tumors or provide reliable parameters for pretreatment risk stratification [3]. Some of TRUS-Bx disadvantages are falsenegative (21-47%), inadequate stratification of risk (46% upgrading patients candidates to active surveillance), detection of negligible clinical PCa (17% PCa indolent in the first biopsy), and the necessity of re-biopsies with consequently morbidity and infection risk [3,4]. More accurate targeting biopsy should reduce false-negative biopsies and improve accuracy in risk classification through better tumor sampling [4,5]. As result, a reduction in false-negative biopsies could reduce re-biopsies and, thus, decrease cost and side effects [5].
Magnetic Resonance Imaging (MRI) has recently emerged as the most accurate imaging technique for PCa detection and staging. Multiparametric-MRI (mpMRI) protocol consisting of T2-weighted imaging, diffusion-weighted imaging, and dynamic contrast enhanced imaging, and possibly MR spectroscopy has being proposed to increase accuracy. mpMRI has demonstrated a better specificity in PCa detection compared to conventional T2-weighted images alone. An mpMRI suspicion score has been developed and, more recently, a standardized reporting scale (Prostate Imaging–Reporting and Data System version 2) was updated [6]. Recently a biopsy guided by fusion mpMRI and TRUS has being developed. Techniques of MRI-targeted biopsy include in-gantry MRI guided biopsy, TRUS-guided visual estimation biopsy, and software co-registered MRI-US guided biopsy (MRI-TRUS fusion) [6].
In-bore MRI-guided biopsies were the first targeted biopsies performed using MRI identified lesions. The major advantage of the in-bore technique is that it offers the most accurate targeting of the MRI-identified lesions. However, there several drawbacks because requires significant additional training for the physician and there is increased costs associated with this method. For cognitive registration, the MRI of the prostate and its presentation on TRUS are mentally co-registered by the physician performing the biopsy. The major shortcoming is depending on practitioner experience and therefore confers a great deal of inter-operator variability and potential inaccuracies. Finally, software-based registration platforms like the MRI-TRUS fusion biopsy systems were conceived and developed in an attempt to offer a low-cost, accurate alternative to in-bore prostate biopsies that can be performed by any urologist in an office setting with minimal additional training [6,7].
Clinical applications for which MRI-TRUS fusion biopsies of the prostate include patients with suspected PCa and previous negative biopsy, patients with known PCa for whom active surveillance is an option, patients with known PCa to determine disease status during active surveillance, and candidates for focal therapy [6].
Special Situations to Consider Prostate Mri and Guided Biopsy
Patients with clinical suspicion of PCa
Haffner et al reported a series of 555 patients who underwent pre-biopsy MRI followed by systematic biopsy and visual estimation biopsy of MRI abnormalities. Although systematic biopsy detected 66 more cases of cancer, 53 were deemed clinically insignificant [4]. Schoots et al showed similar detection of overall PCa in men with an initial biopsy (MRI-TBx versus TRUS-Bx) (relative sensitivity 0.97, 95% CI 0.94–1.01). Furthermore they showed that MRI-TBx and TRUS-Bx did not differ in overall detection of prostate cancer in men with clinical suspicion of PCa and a suspicious lesion on mpMRI [7].
Delongchamps et al also examined the use of pre-biopsy mpMRI in 391 patients, and reported that targeted biopsy was significantly better in detecting high Gleason score (greater than 3+3) cancer in men without previous biopsy [8]. However, the cost-effectiveness and true benefit have yet to be determined through larger randomized studies and, as such, its use is currently investigational [9,10].
Pokorny et al compared the diagnostic efficacy of the MRI pathway with TRUS-Bx [11]. Although the lack of long follow-up for this study, the authors found that mpMRI and MRI-TBx reduces the detection of low-risk PCa and reduces the number of men requiring biopsy while improving the overall rate of detection of intermediate/ high-risk PCa. Panebianco et al [12] showed that the proportion of men with clinically significant PCa is higher among those randomized to mp-MRI/biopsy vs. those randomized to TRUS-guided biopsy. Moreover, mp-MRI is a very reliable tool to identify patients to schedule in active surveillance [13].
For asymptomatic men with elevated PSA, mpMRI followed by selective use of MRI biopsy compared with TRUSGB reduces the detection of low-risk PCa, and it reduces the need for biopsy while improving the overall detection of intermediate/high-risk PCa, but future studies with longer oncologic follow-up and comparison of the different targeted biopsy techniques are needed to assess which technique is preferable, also in terms of implementation and costs [11,13].
Patients with Suspected PCa and Previous Negative Biopsy
Hoeks et al reported a PCa detection rate of 41% (108 of 265 patients) using in-bore targeted biopsy and previous negative biopsy, with 87% (94 of 108 patients) of these cancers found to be clinically significant [14]. Vourganti et al reported 37% of PCa detection on 195 patients with a previous negative biopsy and suspicious mpMRI, using a combination of MRI-US fusion biopsy and systematic biopsy [15].
Sonn et al found 34% (36 of 105 patients) PCa detection rate in men with a previous negative biopsy and 72% (26 of 36 patients) was clinically significant [9]. MRI-US fusion biopsy detected clinically significant PCa in 21 of 23 (91%) men compared to only 15 of 28 (54%) with systematic biopsy. A highly suspicious MRI lesion was the most significant predictor of significant cancer on multivariate analysis. Labanaris et al found PCa detection of 56% with targeted biopsies compared to only 18% with systematic biopsies among 170 of 260 (65%) patients with a suspicious MRI [16].
Based on these studies, a change in diagnostic approach to MRITBx must be considered in men with a previous negative biopsy.
Patients with Known PCa and Active Surveillance Decision-making
Optimal stratification risk method for PCa patients and selection for Active Surveillance (AS) is still under judgment. Margel et al found, on confirmatory biopsy in 60 patients with PCa low-risk, 32.1% of reclassification as no longer fulfilling surveillance criteria [17].
Park et al found a suspicion of PCa in 88.3% on preoperative mpMRI before radical prostatectomy [18]. Patients with cancer suspected on imaging had a higher likelihood of upgrading at radical prostatectomy compared to those with no suspicion on MRI (49.8% vs 14.3%). Turkbey et al retrospectively analyzed 133 patients who underwent mpMRI before radical prostatectomy [19]. mpMRI had a 93% sensitivity, 57% positive predictive value and 92% overall accuracy for predicting the appropriate AS candidates.
Almeida et al evaluated the prognostic role of mpMRI in patients with clinically localized prostate cancer (PCa) eligible for AS according to PRIAS (Prostate Cancer Research International Active Surveillance) criteria [20]. In this study, the authors recommend mpMRI as an important tool to be added to clinical selection criteria for AS, because a visible lesion, especially PIRADS 5, on mp-MRI strongly predicts significant PCa in patients eligible for AS based on upstaging and unfavorable disease [20].
De Cobelli et al [21] analyzed 223 PCa patients who performed mpMRI staging and demonstrated a strong association between PIRADS score with upgrading (P<0.0001), extra capsular extension (P<0.0001), unfavorable prognosis (P<0.0001), and large tumor volume (P<0.002) at final histology. ROC curves (Figure 1) and Decision Curve Analysis (DCA) (Figure 2) of this study shows that mpMRI and PIRADS scoring could be used as decision-support systems for a more accurate selection of patients eligible for AS.
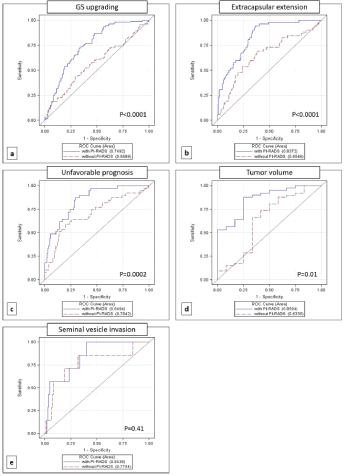
Figure 1: Reference: De Cobelli O, Terracciano D, Tagliabue E, et al. Predicting Pathological Features at Radical Prostatectomy in Patients with Prostate Cancer
Eligible for Active Surveillance by Multiparametric Magnetic Resonance Imaging. PLoS One. 2015; 10(10): e0139696.
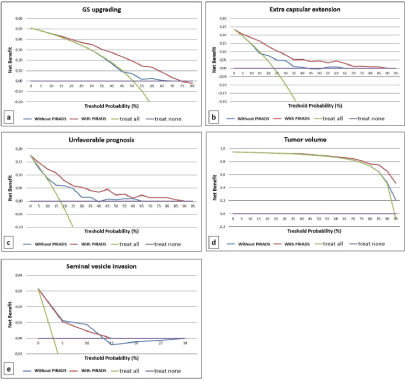
Figure 2: Reference: De Cobelli O, Terracciano D, Tagliabue E, et al. Predicting Pathological Features at Radical Prostatectomy in Patients with Prostate Cancer
Eligible for Active Surveillance by Multiparametric Magnetic Resonance Imaging. PLoS One. 2015; 10(10): e0139696.
By the other hand, Schoots et al postulated that there is no evidence for the use of MRI in men on AS program, and the reason for this affirmative is that MRI at the start of surveillance can detect clinically significant disease in one-third to half of men [22].
We need more data to assess the use of MRI as a monitoring tool during AS. Moreover, define significant PCa on MRI and significant changes over time are still under investigation.
Patients with Known PCa and Radical Prostatectomy with Nerve-sparing Decisionmaking
Petralia et al [23] investigated retrospectively whether mpMRI– directed Intraoperative Frozen-Section (IFS) analysis during nerve- Sparing Robot-Assisted Radical Prostatectomy (RALP) reduces the rate of positive surgical margins. The significantly lower rate of positive surgical margins compared with that in control patients provides preliminary evidence of the positive clinical effect of mpMRI–directed IFS analysis for patients who undergo nervesparing RALP.
Patients with suspicious of local recurrence after Radical Prostatectomy
Paneblanco et al validated the role of 3-T Diffusion-Weighted Imaging (DWI) in the detection of local PCa recurrence after Radical Prostatectomy (RP) [12]. For this purpose, T2-weighted imaging, DWI and Dynamic Contrast-Enhanced MRI (DCE-MRI) were performed with a 3-T magnet in 262 patients after RP. They found that DCE-MRI is the most reliable technique in detecting local PCa recurrence after RP, though DWI can be proposed as a reliable alternative [12].
American Urological Association-Society of Abdominal Radiology Consensus Statement [24]
- Prostate MRI and subsequent MRI-targeted cores facilitate the detection of clinically significant disease over standardized repeat biopsy if a biopsy is recommended after prior negative biopsy
- When high-quality prostate MRI is available should be strongly considered for any patient with a prior negative biopsy who has persistent clinical suspicion for prostate cancer and is undergoing a repeat biopsy.
- MRI should be performed, interpreted and reported in accordance with PI-RADS. Experience by the reporting radiologist and biopsy operator are required to achieve optimal results beyond the practices integrating prostate MRI into patient management are advised to implement quality assurance programs to monitor targeted biopsy results.
- Patients with PI-RADS assessment category 3-5 warrant repeat biopsy with image guided targeting.
- In the absence of such TRUS-MRI or in-bore MRItargeting, cognitive targeting remains a reasonable approach to be considered. At least two targeted cores should be obtained from each MRI-defined target.
- Patients with a negative or low-suspicion MRI (PI-RADS 1-2) should consider other ancillary (PSA, PCA3, 4K).
Questions to be answered
Moreover, some questions must remain to be clear: “TRUS-BxP should be omitted when negative mp-MRI?”
“Random biopsy should be omitted when the target biopsy is indicated?”
Some studies not concluded, as PRECISON and PROMIS trials, will try to answer these questions.
Conclusion
Introducing mpMRI and MRI targeted biopsy as modalities to evaluate men at risk of PCa may aid in better determining which men need a prostate biopsy and improve sampling in the performance of the biopsy, thereby allowing greater detection of clinically significant disease with fewer biopsy cores, more accurate risk stratification, and avoid detection of indolent disease.
It is recommended that an mpMRI-Prostate Biopsy approach should be undertaken only in men who are likely to benefit from any eventual treatment by balancing the risks of over diagnosis and/or overtreatment and the impact on quality of life and costeffectiveness. An mpMRI-PB does not completely eliminate the detection of clinically insignificant disease (with the potential to lead to overtreatment).
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60: 277-300.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66: 7-30.
- Costa DN, Pedrosa I, Donato F Jr, Roehrborn CG, Rofsky NM. MR Imaging–Transrectal US Fusion for Targeted Prostate Biopsies: Implications for Diagnosis and Clinical Management. Radiographics. 2015; 35: 696-708.
- Haffner J, Lemaitre L, Puech P, Haber GP, Leroy X, Jones JS. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011; 108: 171.
- Wysock JS, Rosenkrantz AB, Huang WC, Stifelman MD, Lepor H, Deng FM, et al. A prospective, blinded comparison of Magnetic Resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR targeted prostate biopsy: the Profus trial. Eur Urol. 2014; 66: 343-351.
- Bjurlin MA, Mendhiratta N, Wysock JS, Taneja SS. Multiparametric MRI and targeted prostate biopsy: Improvements in cancer detection, localization, and risk assessment. Cent European J Urol. 2016; 69: 9-18.
- Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG, et al. Magnetic Resonance Imaging–targeted Biopsy May Enhance the Diagnostic Accuracy of Significant Prostate Cancer Detection Compared to Standard Transrectal Ultrasound-guided Biopsy: A Systematic Review and Meta-analysis. Eur Urol. 2015; 68: 438-450.
- Delongchamps NB, Peyromaure M, Schull A, Beuvon F, Bouazza N, Flam T, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013; 189: 493.
- Puech P, Rouvièere O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate cancer diagnosis: multiparametric MR- targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy-prospective multicenter study. Radiology. 2013; 268: 461.
- Sonn GA, Natarajan S, Margolis DJ, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013; 189: 86.
- Pokorny MR, Duncan MRE, Schröder FH, Parkinson R, Barentsz JO, Thompson LC. Prospective Study of Diagnostic Accuracy Comparing Prostate Cancer Detection by Transrectal Ultrasound– Guided Biopsy Versus Magnetic Resonance (MR) Imaging with Subsequent MR-guided Biopsy in Men Without Previous Prostate Biopsies. Eur Urol. 2014; 66: 22-29.
- Panebianco V, Barchetti F, Sciarra A, Musio D, Forte V, Gentile V, et al. Prostate cancer recurrence after radical prostatectomy: the role of 3-T diffusion imaging in multi-parametric magnetic resonance imaging. Eur Radiol. 2013; 23: 1745-1752.
- Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: A randomized study. Urol Oncol. 2015; 33: 17. e1-7.
- Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, Hulsbergen-van de Kaa CA, Hambrock T, et al. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol. 2012; 62: 902.
- Vourganti S, Rastinehad A, Yerram NK, Nix J, Volkin D, Hoang A, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012; 188: 2152.
- Labanaris AP, Engelhard K, Zugor V, Nützel R, Kühn R. Prostate cancer detection using an extended prostate biopsy schema in combination with additional targeted cores from suspicious images in conventional and functional endorectal magnetic resonance imaging of the prostate. Prostate Cancer Prostatic Dis. 2010; 13: 65.
- Margel D, Yap SA, Lawrentschuk N, Klotz L, Haider M, Hersey K, et al. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: a prospective cohort study. J Urol. 2012; 187: 1247.
- Park BH, Jeon HG, Choo SH, Jeong BC, Seo SI, Jeon SS, et al. Role of multiparametric 3.0 tesla magnetic resonance imaging in prostate cancer patients eligible for active surveillance. BJU Int. 2014; 113: 864-870.
- Turkbey B, Mani H, Aras O, Ho J, Hoang A, Rastinehad AR, et al. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013; 268: 144.
- Almeida GL, Petralia G, Ferro M, Ribas CA, Detti S, Jereczek-Fossa BA, et al. Role of Multi-Parametric Magnetic Resonance Image and PIRADS Score in Patients with Prostate Cancer Eligible for Active Surveillance According PRIAS Criteria. Urol Int. 2016; 96: 459-469.
- De Cobelli O, Terracciano D, Tagliabue E, Raimondi S, Bottero D, Cioffi A et al. Predicting Pathological Features at Radical Prostatectomy in Patients with Prostate Cancer Eligible for Active Surveillance by Multiparametric Magnetic Resonance Imaging. PLoS One. 2015; 10: e0139696.
- Schoots IG, Petrides N, Giganti F, Bokhorst LP, Rannikko A, Klotz L, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. 2015; 67: 627-636.
- Petralia G, Musi G, Padhani AR, Summers P, Renne G, Alessi S et al. Robot-assisted radical prostatectomy: Multiparametric MR imaging-directed intraoperative frozen-section analysis to reduce the rate of positive surgical margins. Radiology. 2015; 274: 434-444.
- Rosenkrantz AB, Verma S, Choyke P, Eberhardt SC, Eggener SE, Gaitonde K, et al. Prostate MRI and MRI-targeted biopsy in patients with prior negative biopsy. American Urological Association and Society of Abdominal Radiology’s Prostate Cancer Disease-Focused Panel. J Urol. 2016.